The Giant Panda Transferrin Receptor Facilitates Feline Parvovirus Infection to Drive Cross-Species Transmission
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Cells, Virus, and Antibodies
2.2. Sequence Analysis and Plasmid Construction
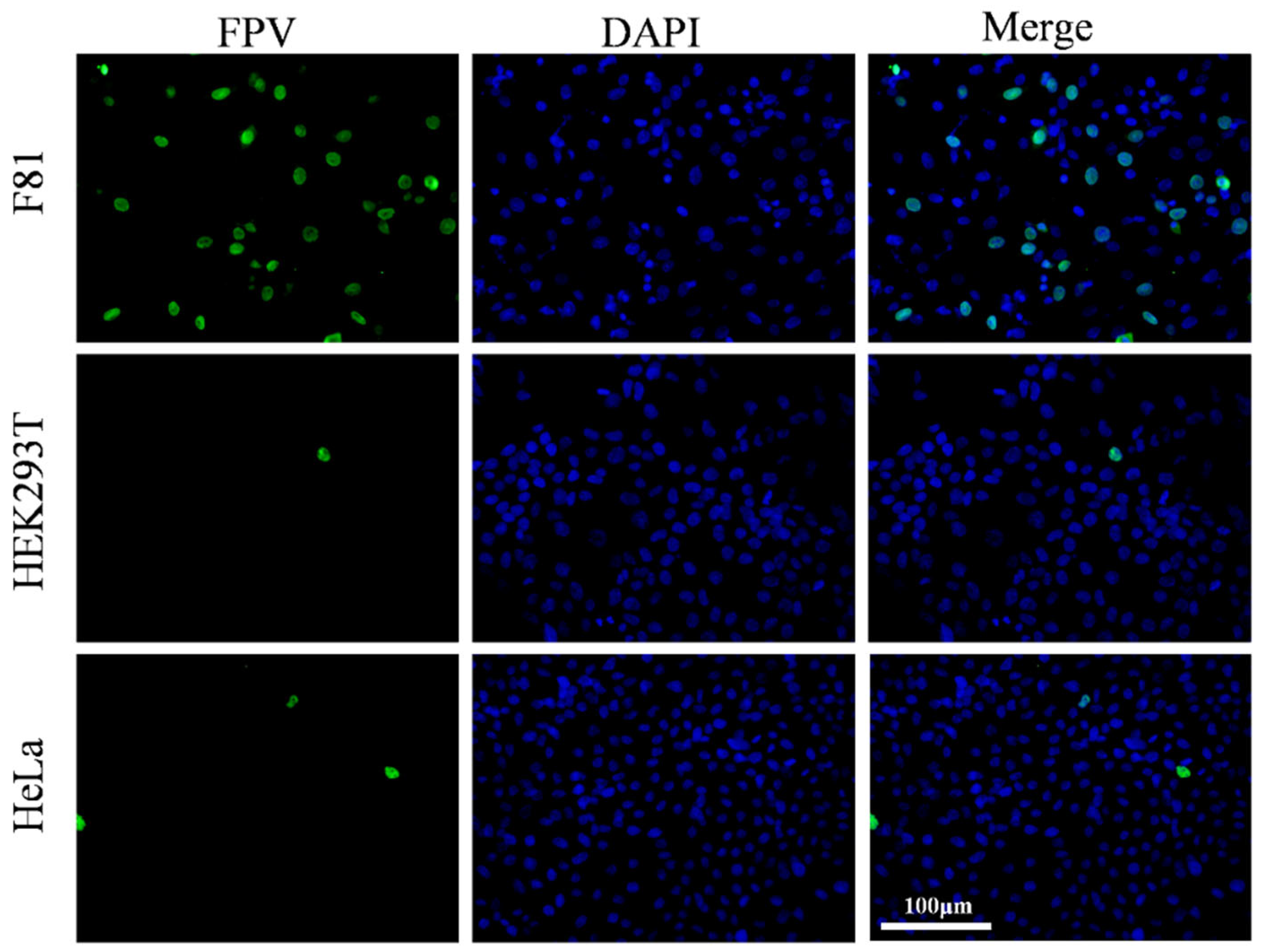
2.3. Indirect Immunofluorescence Detection of pFPV-sc Infection in Different Cells
2.4. qPCR Verification of TfR1 Overexpression and FPV Copy Number
2.5. Indirect Immunofluorescence Verification of TfR1 Overexpression and pFPV-sc Replication Levels
2.6. Effect of gpTfR1 Expression on pFPV-sc Adsorption and Internalization
2.7. Colocalization Analysis of gpTfR1 Expression and pFPV-sc Infection
2.8. Examination of Cell Viability Detection by CCK8 Assay
2.9. Data Analysis
3. Results
3.1. Validation of Cell Tropism of the Giant Panda Derived FPV (pFPV-sc)
3.2. Conformation of Expression of Recombinant fTfR1 and gpTfR1
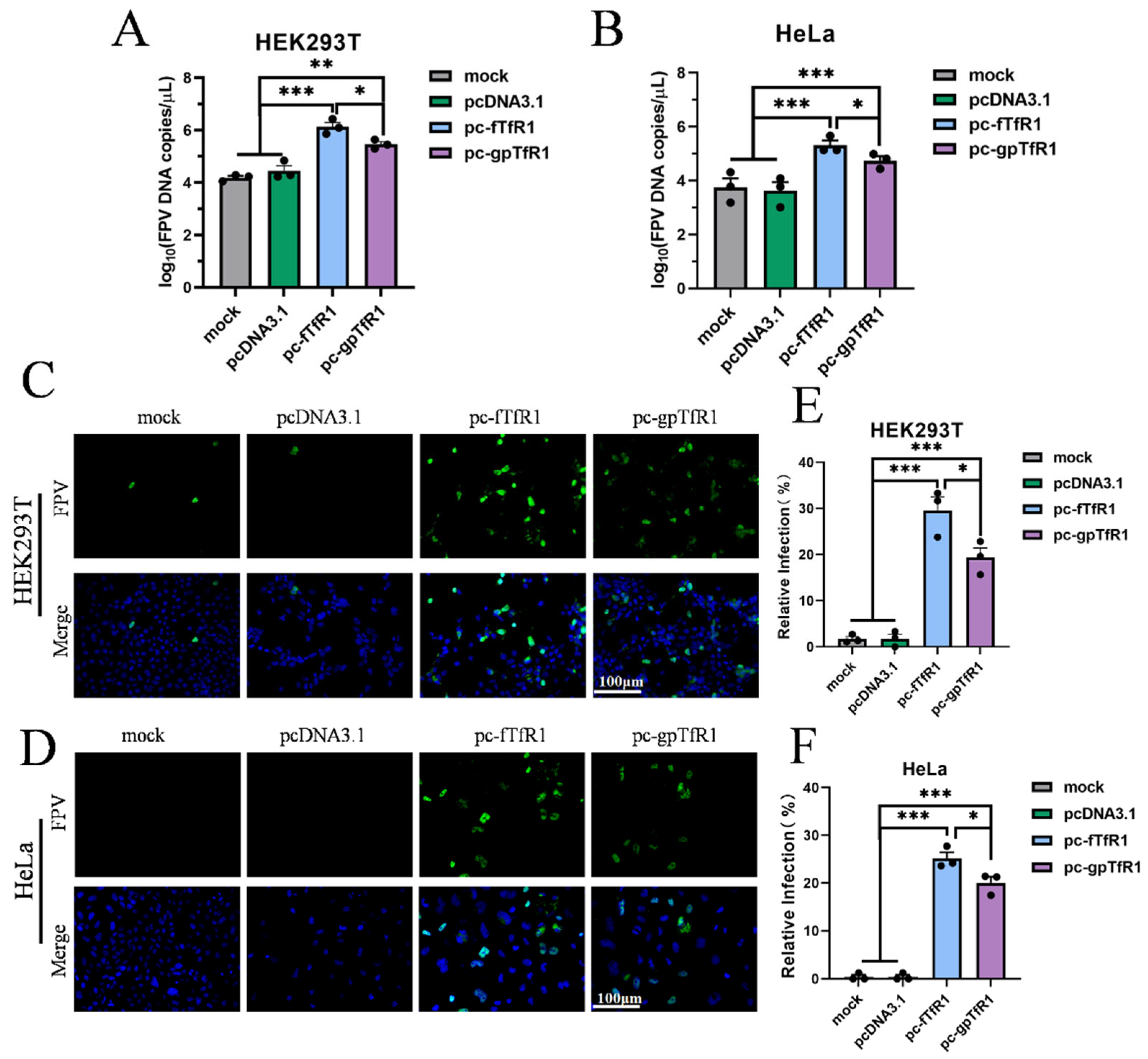
3.3. Giant Panda TfR1 Promotes pFPV-sc Replication
3.4. Overexpression of gpTfR1 Promotes pFPV-sc Adsorption and Internalization
3.5. pFPV-sc Infection in Unsusceptible Cells Requires gpTfR1 Expression
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jager, M.C.; Tomlinson, J.E.; Lopez-Astacio, R.A.; Parrish, C.R.; Van de Walle, G.R. Small but mighty: Old and new parvoviruses of veterinary significance. Virol. J. 2021, 18, 210. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Du, S.S.; Wang, Y.Q.; Wang, S.Y.; Luo, X.Q.; Zhang, Y.Y.; Liu, C.F.; Wang, H.J.; Pei, Z.H.; Hu, G.X. Isolation and identification of tiger parvovirus in captive siberian tigers and phylogenetic analysis of VP2 gene. Infect. Genet. Evol. 2019, 75, 103957. [Google Scholar] [CrossRef] [PubMed]
- Studdert, M.; Kelly, C.; Harrigan, K. Isolation of panleucopaenia virus from lions. Vet. Rec. 1973, 93, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Li, X.; Xie, W.; Guo, L.; You, D.; Xu, H.; Liu, D.; Wang, Y.; Hou, Z.; Zeng, X.; et al. Molecular detection of parvovirus in captive Siberian tigers and lions in Northeastern China from 2019 to 2021. Front. Microbiol. 2022, 13, 898184. [Google Scholar] [CrossRef] [PubMed]
- Barker, I.K.; Povey, R.C.; Voigt, D.R. Response of mink, skunk, red fox and raccoon to inoculation with mink virus enteritis, feline panleukopenia and canine parvovirus and prevalence of antibody to parvovirus in wild carnivores in Ontario. Can. J. Comp. Med. 1983, 47, 188. [Google Scholar] [PubMed]
- Yang, S.T.; Wang, S.J.; Feng, H.; Zeng, L.; Xia, Z.P.; Zhang, R.Z.; Zou, X.H.; Wang, C.Y.; Liu, Q.A.; Xia, X.Z. Isolation and characterization of feline panleukopenia virus from a diarrheic monkey. Vet. Microbiol. 2010, 143, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Liu, S.; Meng, X.; Huang, P.; Cao, Z.; Jin, H.; Wang, J.; Hu, G.; Lan, J.; Zhang, D.; et al. Feline panleukopenia virus with G299E substitution in the VP2 protein first identified from a captive giant panda in China. Front. Cell. Infect. Microbiol. 2022, 11, 820144. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Hu, H.Y.; Lan, J.C.; Yang, Z.S.; Peng, Q.L.; Yan, L.H.; Luo, L.; Wu, L.; Lang, Y.F.; Yan, Q.G. Characterization of a fatal feline panleukopenia virus derived from giant panda with broad cell tropism and zoonotic potential. Front. Immunol. 2023, 14, 1237630. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Woods, L.; Gerstenberg, G.; Deng, X.; Delwart, E. A Common Parvovirus in Deer from California, USA. J. Wildl. Dis. 2016, 52, 962–964. [Google Scholar] [CrossRef] [PubMed]
- Cotmore, S.F.; Agbandje-McKenna, M.; Chiorini, J.A.; Mukha, D.V.; Pintel, D.J.; Qiu, J.; Soderlund-Venermo, M.; Tattersall, P.; Tijssen, P.; Gatherer, D.; et al. The family Parvoviridae. Arch. Virol. 2013, 159, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Callaway, H.M.; Cifuente, J.O.; Bator, C.M.; Parrish, C.R.; Hafenstein, S.L. Transferrin receptor binds virus capsid with dynamic motion. Proc. Natl. Acad. Sci. USA 2019, 116, 20462–20471. [Google Scholar] [CrossRef] [PubMed]
- Hueffer, K.; Govindasamy, L.; Agbandje-McKenna, M.; Parrish, C.R. Combinations of two capsid regions controlling canine host range determine Canine transferrin receptor binding by canine and feline parvoviruses. J. Virol. 2003, 77, 10099–10105. [Google Scholar] [CrossRef] [PubMed]
- Callaway, H.M.; Welsch, K.; Weichert, W.; Allison, A.B.; Hafenstein, S.L.; Huang, K.; Iketani, S.; Parrish, C.R. Complex and dynamic interactions between parvovirus capsids, transferrin receptors, and antibodies control cell infection and host range. J. Virol. 2018, 92, e00460-18. [Google Scholar] [CrossRef] [PubMed]
- Harbison, C.E.; Chiorini, J.A.; Parrish, C.R. The parvovirus capsid odyssey: From the cell surface to the nucleus. Trends Microbiol. 2008, 16, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Hueffer, K.; Parker, J.; Weichert, W.S.; Geisel, R.E.; Sgro, J.Y.; Parrish, C.R. The natural host range shift and subsequent evolution of canine parvovirus resulted from virus-specific binding to the canine transferrin receptor. J. Virol. 2003, 77, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- Chi, P.-I.; Liu, H.-J. Molecular signaling and cellular pathways for virus entry. ISRN Virol. 2013, 2013, 306595. [Google Scholar] [CrossRef]
- Harbison, C.E.; Lyi, S.M.; Weichert, W.S.; Parrish, C.R. Early steps in cell infection by parvoviruses: Host-specific differences in cell receptor binding but similar endosomal trafficking. J. Virol. 2009, 83, 10504–10514. [Google Scholar] [CrossRef] [PubMed]
- Hueffer, K.; Parrish, C.R. Parvovirus host range, cell tropism and evolution. Curr. Opin. Microbiol. 2003, 6, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Cureton, D.K.; Harbison, C.E.; Cocucci, E.; Parrish, C.R.; Kirchhausen, T. Limited transferrin receptor clustering allows rapid diffusion of canine parvovirus into clathrin endocytic structures. J. Virol. 2012, 86, 5330–5340. [Google Scholar] [CrossRef] [PubMed]
- Palermo, L.M.; Hueffer, K.; Parrish, C.R. Residues in the apical domain of the feline and canine transferrin receptors control host-specific binding and cell infection of canine and feline parvoviruses. J. Virol. 2003, 77, 8915–8923. [Google Scholar] [CrossRef] [PubMed]


| Primer | Sequence (5′-3′) | PCR Products/(bp) |
|---|---|---|
| F81-fTfR1-F | aaaaaGGATCCATGATGGATCAAGCCAGAT | 2310 |
| F81-fTfR1-R | aaaaaCTCGAGAAACTCATTGTCAATATCCCA | |
| TfR1-mRNA-F | TGGCTGTATTCTGCTCGTGG | 189 |
| TfR1-mRNA-R | GCCCCAAAAGATATGTCGG | |
| F81-RPL17-F | CTCTGGTCATTGAGCACATCC | 123 |
| F81-RPL17-R | TCAATGTGGCAGGGAGAGC | |
| 293-βactin-F | CAAAAGGCGGGGTCGCAAT | 108 |
| 293-βactin-R | CGACGATGGAAGGAAACACG | |
| Hela-PPIA-F | GAGGAAAACCGTGTACTATTAGC | 86 |
| Hela-PPIA-R | GGGACCTTGTCTGCAAAC | |
| VP2-qPCR-F | ACGGGTACTTTCAATAATCAGAC | 179 |
| VP2-qPCR-R | AATATCATCTAAAGCCATGTTTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Q.; Hu, H.; Zhao, S.; Zhao, Q.; Wu, R.; Huang, X.; Wang, Y.; Wen, Y.; Zheng, Y.; Zhao, F.; et al. The Giant Panda Transferrin Receptor Facilitates Feline Parvovirus Infection to Drive Cross-Species Transmission. Vet. Sci. 2025, 12, 602. https://doi.org/10.3390/vetsci12070602
Yan Q, Hu H, Zhao S, Zhao Q, Wu R, Huang X, Wang Y, Wen Y, Zheng Y, Zhao F, et al. The Giant Panda Transferrin Receptor Facilitates Feline Parvovirus Infection to Drive Cross-Species Transmission. Veterinary Sciences. 2025; 12(7):602. https://doi.org/10.3390/vetsci12070602
Chicago/Turabian StyleYan, Qigui, Huanyuan Hu, Shan Zhao, Qin Zhao, Rui Wu, Xiaobo Huang, Yiping Wang, Yiping Wen, Yi Zheng, Fei Zhao, and et al. 2025. "The Giant Panda Transferrin Receptor Facilitates Feline Parvovirus Infection to Drive Cross-Species Transmission" Veterinary Sciences 12, no. 7: 602. https://doi.org/10.3390/vetsci12070602
APA StyleYan, Q., Hu, H., Zhao, S., Zhao, Q., Wu, R., Huang, X., Wang, Y., Wen, Y., Zheng, Y., Zhao, F., Cao, S., Du, S., & Lang, Y. (2025). The Giant Panda Transferrin Receptor Facilitates Feline Parvovirus Infection to Drive Cross-Species Transmission. Veterinary Sciences, 12(7), 602. https://doi.org/10.3390/vetsci12070602







