Preparation of Monoclonal Antibodies Against the gD Protein of Feline Herpesvirus Type-1 by mRNA Immunization
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Viral Infection
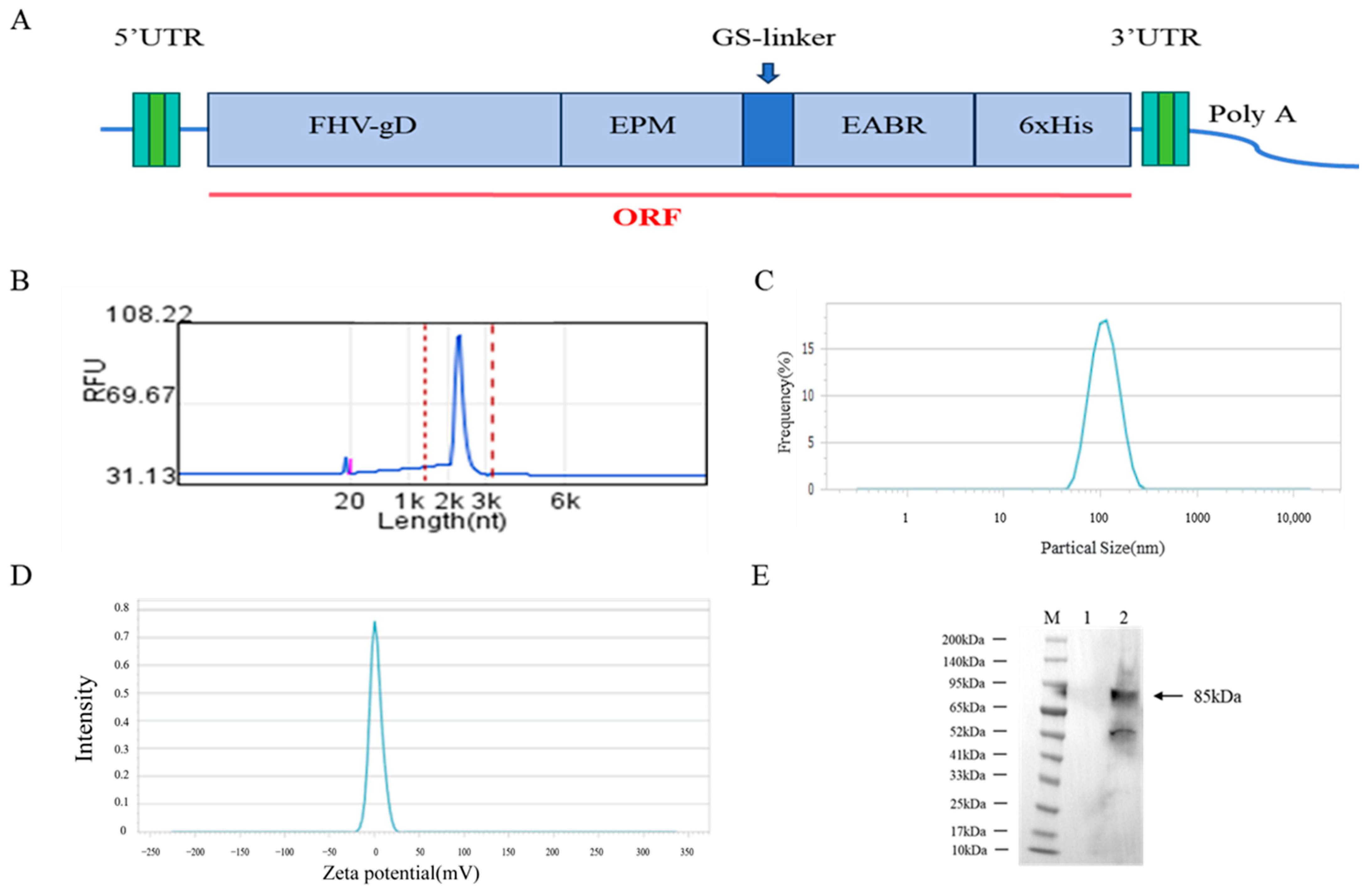
2.2. Design and Preparation of mRNA Vaccines
2.3. Preparation of mRNA–Lipid Nanoparticles
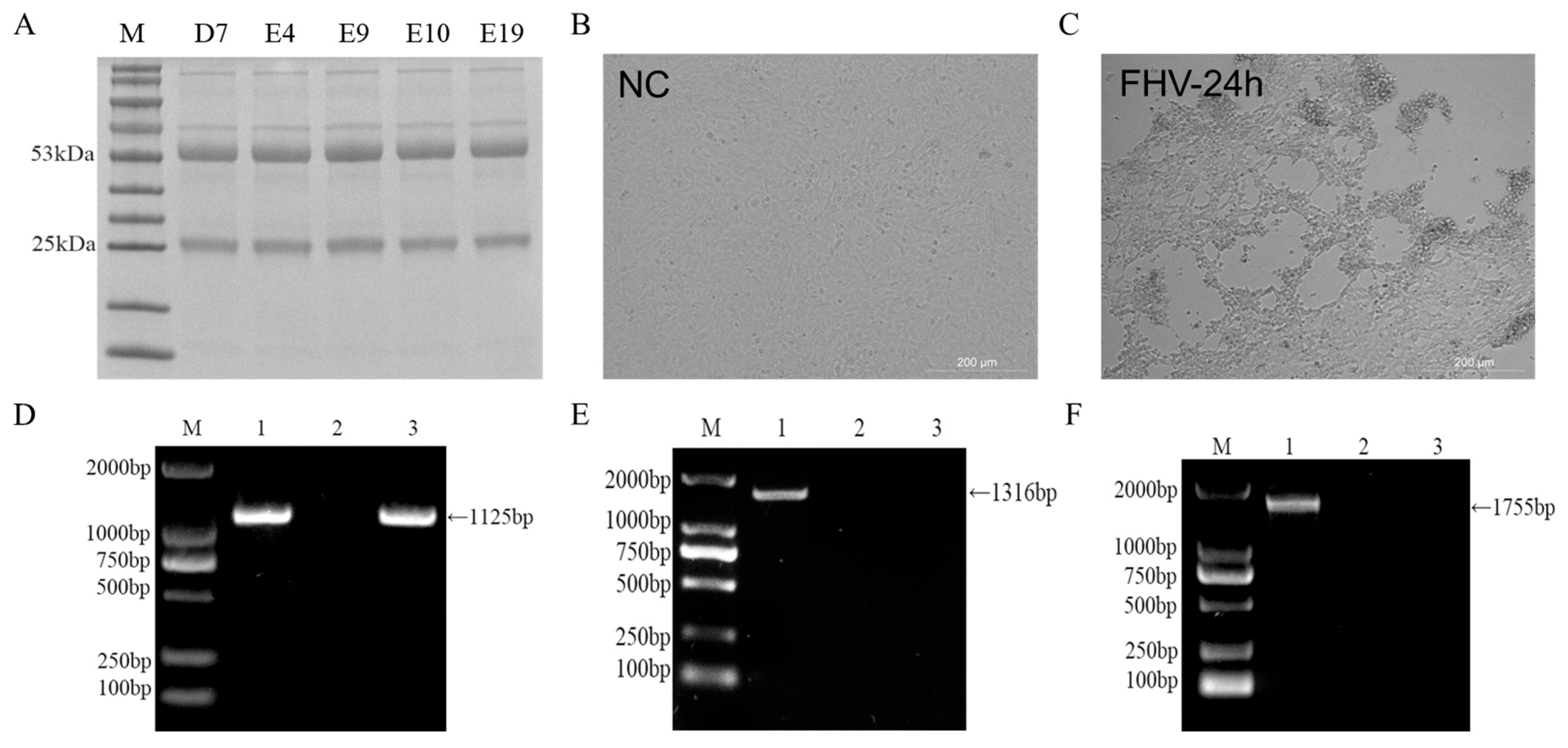
2.4. Preparation of FHV Strains and Detection of Exogenous Viral Nucleic Acids
2.5. Detection of gD Protein Expression After mRNA Vaccine Transfection into HEK293T Cells
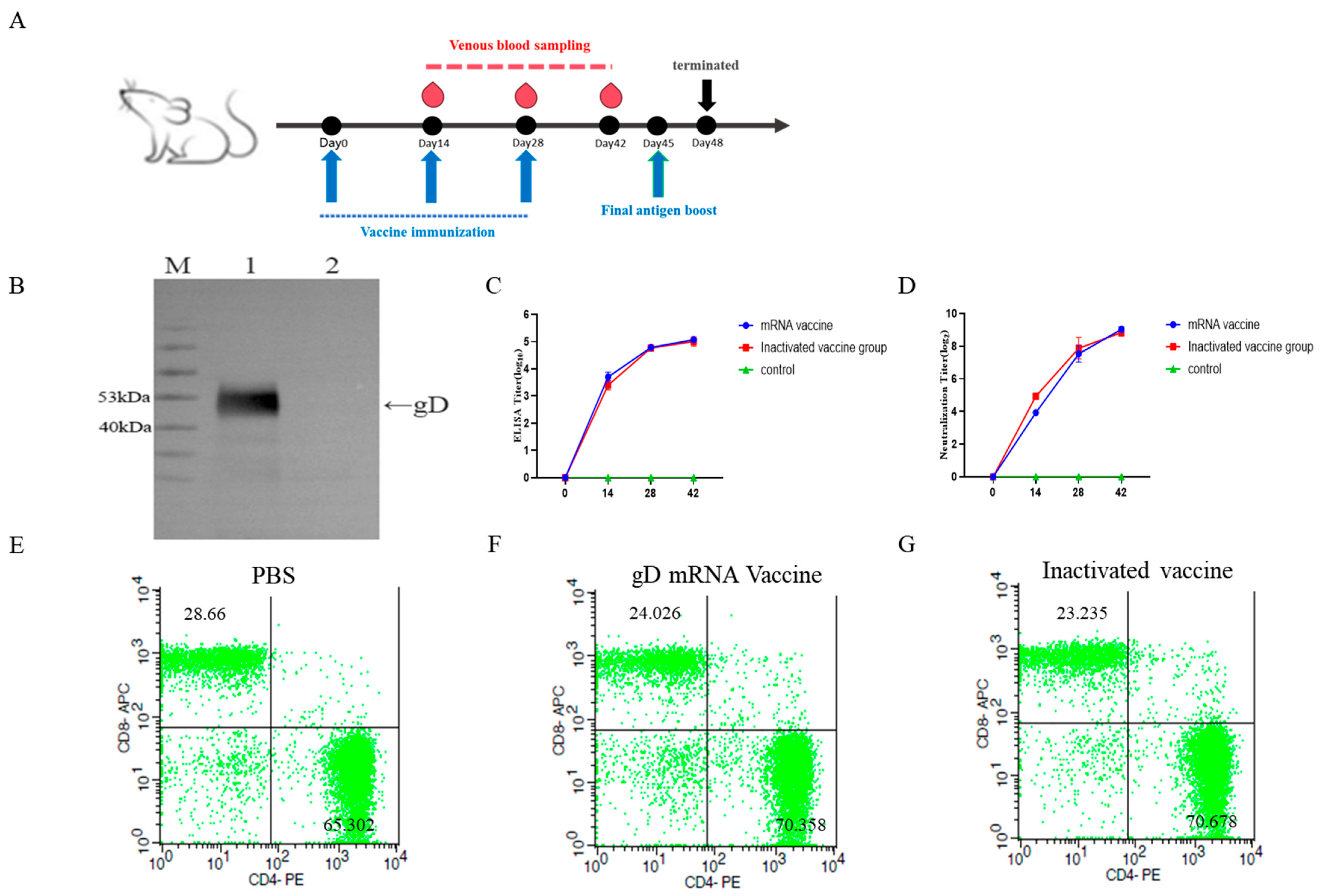
2.6. Detection of Serum Antibody Levels and Neutralizing Antibody Titers in Mice After mRNA Vaccine Immunization
2.7. Expression and Purification of gD Protein for Monoclonal Antibody Screening
2.8. Flow Cytometry Detection of Mouse Lymphocyte Activation
2.9. Screening of Hybridoma Cells and Preparation of Ascites Antibodies
2.10. Evaluation of the Binding Activity of Monoclonal Antibodies and Antibody Subtype Identification
2.11. Virus Titration
2.12. Statistical Analysis
3. Results
3.1. Characterization of the Physicochemical Properties of mRNA Vaccines
3.2. Evaluation of mRNA Vaccine Immunization Effects and Expression of gD Protein for Monoclonal Antibody Screening
3.3. Detection of gD Protein Expression for Monoclonal Antibody Screening
3.4. Detection of gD Protein-Specific Antibody Levels and Neutralizing Antibody Titers in Mouse Serum
3.5. Flow Cytometric Detection of Splenocyte Activation
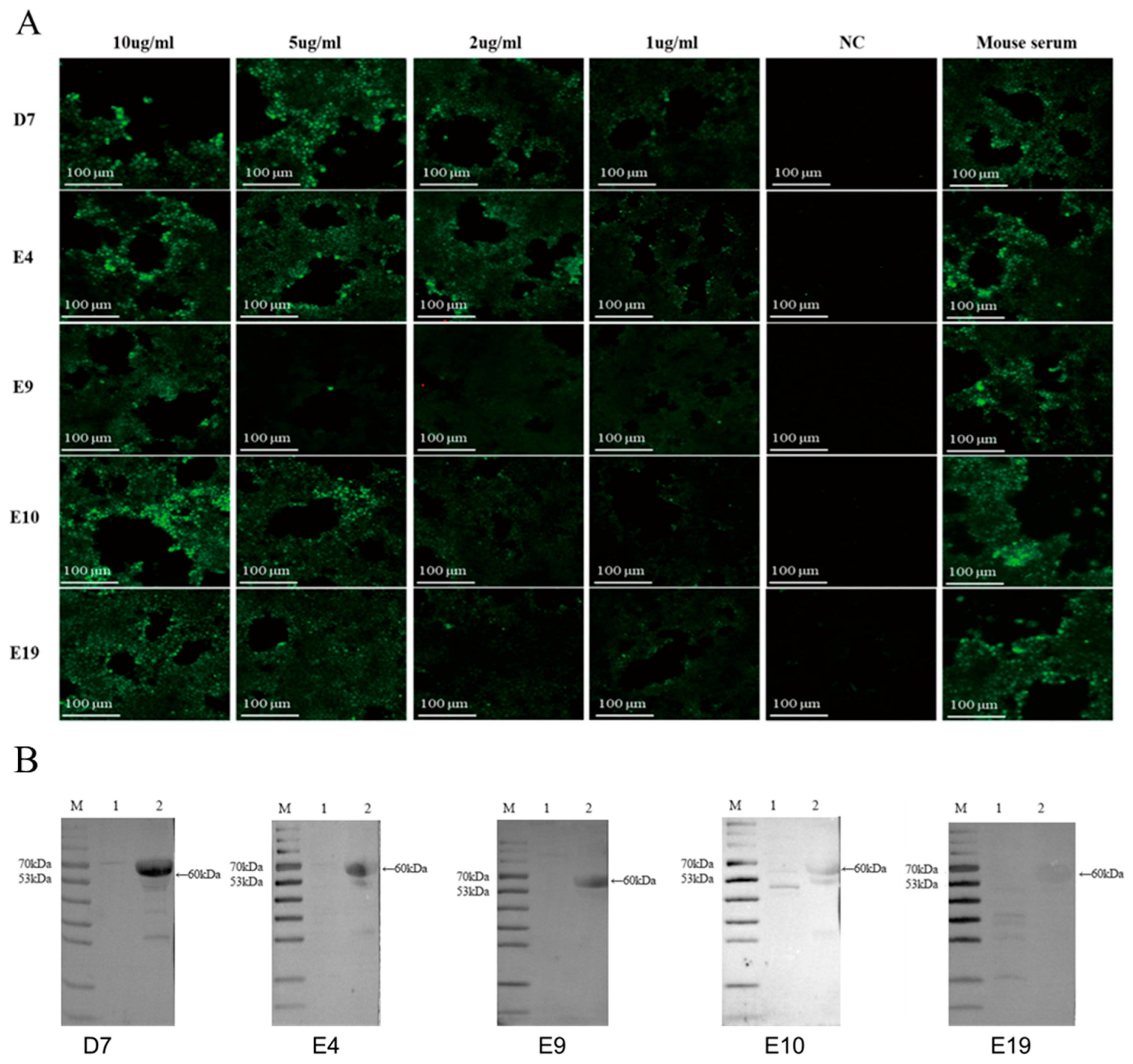
3.6. Preparation and Screening of Hybridoma Cells
3.7. Preparation of FHV-1 Virus and Detection of Extraneous Viral Contamination
3.8. Characterization of Monoclonal Antibody Binding Properties by IFA
3.9. Characterization of Monoclonal Antibody Binding Properties by Western Blotting
3.10. Monoclonal Antibody Subtype Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Synowiec, A.; Dąbrowska, A.; Pachota, M.; Baouche, M.; Owczarek, K.; Niżański, W.; Pyrc, K. Feline herpesvirus 1 (FHV-1) enters the cell by receptor-mediated endocytosis. J. Virol. 2023, 97, e0068123. [Google Scholar] [CrossRef] [PubMed]
- Gaskell, R.; Dawson, S.; Radford, A.; Thiry, E. Feline herpesvirus. Vet. Res. 2007, 38, 337–354. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.E.; Mironovich, M.A.; Ineck, N.E.; Citino, S.B.; Emerson, J.A.; Maggs, D.J.; Coghill, L.M.; Dubovi, E.J.; Turner, R.C.; Carter, R.T.; et al. Full Viral Genome Sequencing and Phylogenomic Analysis of Feline Herpesvirus Type 1 (FHV-1) in Cheetahs (Acinonyx jubatus). Viruses 2021, 13, 2307. [Google Scholar] [CrossRef] [PubMed]
- Thiry, E.; Addie, D.; Belák, S.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Gruffydd-Jones, T.; Hartmann, K.; Hosie, M.J.; Lloret, A.; et al. Feline herpesvirus infection. ABCD guidelines on prevention and management. J. Feline Med. Surg. 2009, 11, 547–555. [Google Scholar] [CrossRef]
- Jiao, C.; Jin, H.; Zhang, M.; Liu, D.; Huang, P.; Bai, Y.; Dai, J.; Zhang, H.; Li, Y.; Wang, H. A bacterium-like particle vaccine displaying protective feline herpesvirus 1 antigens can induce an immune response in mice and cats. Vet. Microbiol. 2023, 287, 109898. [Google Scholar] [CrossRef]
- Maes, R. Felid herpesvirus type 1 infection in cats: A natural host model for alphaherpesvirus pathogenesis. ISRN Vet. Sci. 2012, 2012, 495830. [Google Scholar] [CrossRef]
- Spatz, S.J.; Rota, P.A.; Maes, R.K. Identification of the feline herpesvirus type 1 (FHV-1) genes encoding glycoproteins G, D, I and E: Expression of FHV-1 glycoprotein D in vaccinia and raccoon poxviruses. J. Gen. Virol. 1994, 75 Pt 6, 1235–1244. [Google Scholar] [CrossRef]
- Rizvi, S.M.; Raghavan, M. Responses of herpes simplex virus type 1-infected cells to the presence of extracellular antibodies: gE-dependent glycoprotein capping and enhancement in cell-to-cell spread. J. Virol. 2003, 77, 701–708. [Google Scholar] [CrossRef]
- Tang, A.; Zhu, M.; Zhu, J.; Zhang, D.; Zhu, S.; Wang, X.; Meng, C.; Li, C.; Liu, G. Pathogenicity and immunogenicity of gI/gE/TK-gene-deleted Felid herpesvirus 1 variants in cats. Virol. J. 2023, 20, 87. [Google Scholar] [CrossRef]
- Yang, M.; Mu, B.; Ma, H.; Xue, H.; Song, Y.; Zhu, K.; Hao, J.; Liu, D.; Li, W.; Zhang, Y.; et al. The Latest Prevalence, Isolation, and Molecular Characteristics of Feline Herpesvirus Type 1 in Yanji City, China. Vet. Sci. 2024, 11, 417. [Google Scholar] [CrossRef]
- Harder, T.C.; Harder, M.; de Swart, R.L.; Osterhaus, A.D.; Liess, B. Major immunogenic proteins of phocid herpes-viruses and their relationships to proteins of canine and feline herpesviruses. Vet. Q. 1998, 20, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Ono, M.; Kawaguchi, Y.; Niikura, M.; Okazaki, K.; Yokoyama, N.; Tokiyoshi, Y.; Tohya, Y.; Mikami, T. Expression and properties of feline herpesvirus type 1 gD (hemagglutinin) by a recombinant baculovirus. Virus Res. 1996, 46, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Cheng, Y.; Fang, Z.; Zhongqi, Q.; Weidong, Y.; Yilmaz, A.; Yilmaz, H.; Umar, S. First report of molecular epidemiology and phylogenetic characteristics of feline herpesvirus (FHV-1) from naturally infected cats in Kunshan, China. Virol. J. 2024, 21, 115. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Bang, Y.J.; Kwon, S.P.; Kwak, W.; Park, S.I.; Roh, G.; Bae, S.H.; Kim, J.Y.; Kwak, H.W.; Kim, Y.; et al. Analyzing immune responses to varied mRNA and protein vaccine sequences. npj Vaccines 2023, 8, 84. [Google Scholar] [CrossRef]
- Ul Munamm, S.A.; Nadeem, I.; Mahdi, N.; Saqlain, M.; Rana, Z.K.; Khatana, U.F.; Bhatty, U.M.; Navayogaarajah, V.; Khan, F.M.; Ur Rasool, M. Comparative analysis of mRNA and inactivated COVID-19 vaccines: A study from Faisalabad district of Pakistan. J. R. Coll. Physicians Edinb. 2022, 52, 240–246. [Google Scholar] [CrossRef]
- Hoffmann, M.A.G.; Yang, Z.; Huey-Tubman, K.E.; Cohen, A.A.; Gnanapragasam, P.N.P.; Nakatomi, L.M.; Storm, K.N.; Moon, W.J.; Lin, P.J.C.; West, A.P., Jr.; et al. ESCRT recruitment to SARS-CoV-2 spike induces virus-like particles that improve mRNA vaccines. Cell 2023, 186, 2380–2391. [Google Scholar] [CrossRef]
- Mishra, M.; Tiwari, S.; Gomes, A.V. Protein purification and analysis: Next generation Western blotting techniques. Expert Rev. Proteom. 2017, 14, 1037–1053. [Google Scholar] [CrossRef]
- Tomita, M.; Tsumoto, K. Hybridoma technologies for antibody production. Immunotherapy 2011, 3, 371–380. [Google Scholar] [CrossRef]
- Hoskins, J.D. Feline respiratory diseases. Vet. Clin. N. Am. Small Anim. Pract. 1999, 29, 945–958. [Google Scholar] [CrossRef]
- Burns, R.E.; Wagner, D.C.; Leutenegger, C.M.; Pesavento, P.A. Histologic and molecular correlation in shelter cats with acute upper respiratory infection. J. Clin. Microbiol. 2011, 49, 2454–2460. [Google Scholar] [CrossRef]
- Tan, Y.; Dong, G.; Niu, J.; Guo, Y.; Yi, S.; Sun, M.; Wang, K.; Hu, G. Development of an indirect ELISA based on glycoprotein B gene for detecting of Feline herpesvirus type 1. Pol. J. Vet. Sci. 2019, 22, 631–633. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhu, Y.; Li, N.; Aishanjiang, K.; Zhu, S.; Tang, A.; Li, G.; Liu, G. Development of a monoclonal antibody-based colloidal gold immunochromatographic strip for rapid detection of feline coronavirus. Int. J. Biol. Macromol. 2025, 309, 142683. [Google Scholar] [CrossRef]
- Yang, L.; Wang, M.; Cheng, A.; Yang, Q.; Wu, Y.; Jia, R.; Liu, M.; Zhu, D.; Chen, S.; Zhang, S.; et al. Innate Immune Evasion of Alphaherpesvirus Tegument Proteins. Front. Immunol. 2019, 10, 2196. [Google Scholar] [CrossRef]
- Hull, M.A.; Pritchard, S.M.; Nicola, A.V. Herpes Simplex Virus 1 Envelope Glycoprotein C Shields Glycoprotein D to Protect Virions from Entry-Blocking Antibodies. J. Virol. 2025, 99, e00090-25. [Google Scholar] [CrossRef] [PubMed]
- Limcumpao, J.A.; Horimoto, T.; Xuan, X.; Takahashi, E.; Mikami, T. Immunological relationship between feline herpesvirus type 1 (FHV-1) and canine herpesvirus (CHV) as revealed by polyvalent and monoclonal antibodies. Arch. Virol. 1990, 111, 165–176. [Google Scholar] [CrossRef]
- Wu, H.; Qiao, P.; Chen, Y.; Liu, C.; Huo, N.; Ding, H.; Wang, X.; Wang, L.; Xi, X.; Liu, Y.; et al. Cellular and humoral immune responses in cats vaccinated with feline herpesvirus 1 modified live virus vaccine. Front. Vet. Sci. 2024, 11, 1516850. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Elia, N.; Ghirlando, R.; Lippincott-Schwartz, J.; Hurley, J.H. Midbody targeting of the ESCRT machinery by a noncanonical coiled coil in CEP55. Science 2008, 322, 576–580. [Google Scholar] [CrossRef]
- McCullough, J.; Frost, A.; Sundquist, W.I. Structures, Functions, and Dynamics of ESCRT-III/Vps4 Membrane Remodeling and Fission Complexes. Annu. Rev. Cell Dev. Biol. 2018, 34, 85–109. [Google Scholar] [CrossRef]
- Uversky, V.N.; Redwan, E.M.; Makis, W.; Rubio-Casillas, A. IgG4 Antibodies Induced by Repeated Vaccination May Generate Immune Tolerance to the SARS-CoV-2 Spike Protein. Vaccines 2023, 11, 991. [Google Scholar] [CrossRef]
- Su, Z.; Cheshmehzangi, A.; McDonnell, D.; da Veiga, C.P.; Xiang, Y.T. Mind the “Vaccine Fatigue”. Front. Immunol. 2022, 13, 839433. [Google Scholar] [CrossRef]
- Zhu, J.; Paul, W.E. CD4 T cells: Fates, functions, and faults. Blood 2008, 112, 1557–1569. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.G.; Cho, M.J.; Choi, J.M. Bystander CD4+ T cells: Crossroads between innate and adaptive immunity. Exp. Mol. Med. 2020, 52, 1255–1263. [Google Scholar] [CrossRef]
- Kervevan, J.; Chakrabarti, L.A. Role of CD4+ T Cells in the Control of Viral Infections: Recent Advances and Open Questions. Int. J. Mol. Sci. 2021, 22, 523. [Google Scholar] [CrossRef] [PubMed]
- Sopp, J.M.; Peters, S.J.; Rowley, T.F.; Oldham, R.J.; James, S.; Mockridge, I.; French, R.R.; Turner, A.; Beers, S.A.; Humphreys, D.P.; et al. On-target IgG hexamerisation driven by a C-terminal IgM tail-piece fusion variant confers augmented complement activation. Commun. Biol. 2021, 4, 1031. [Google Scholar] [CrossRef] [PubMed]
- Buchner, J.; Sitia, R.; Svilenov, H.L. Understanding IgM Structure and Biology to Engineer New Antibody Therapeutics. BioDrugs 2025, 39, 347–357. [Google Scholar] [CrossRef]
- Kaveri, S.V.; Silverman, G.J.; Bayry, J. Natural IgM in immune equilibrium and harnessing their therapeutic potential. J. Immunol. 2012, 188, 939–945. [Google Scholar] [CrossRef]
- Fan, G.; Li, J. Engineering Antibodies for the Treatment of Infectious Diseases. Adv. Exp. Med. Biol. 2017, 1053, 207–220. [Google Scholar] [CrossRef]
| Primer Name | Primer Sequence (5′→3′) | Amplified Size |
|---|---|---|
| FHV-1-F | ATGCATCATCATCATCATCATATGACACGTCTACATTTTT | 1125 bp |
| FHV-1-R | TTAAGGATGGTGAGTTGTATGTATTATAGGAAGTTG | |
| FCV-F | CTCTGAGCTTCGTGCTTAAAACTCACA | 1316 bp |
| FCV-R | GGGTTGTATGATTGCAT | |
| FPV-F | GAATTCGCCACCATGCATCATCATCATCATCATAGTGATGGAGCAGTTCAA | 1755 bp |
| FPV-R | GAGCTCTTAATATAATTTTCTAGGTGCTAG |
| Hybridoma Cell Line | D7 | E4 | E9 | E10 | E19 | Negative (OD450nm) | |
|---|---|---|---|---|---|---|---|
| P/N | First subcloning | 12.37818 | 5.418182 | 5.454545 | 3.841105 | 2.414669 | 0.1095 |
| Secondary subcloning | 12.05802 | 14.5534 | 14.52913 | 10.35922 | 11.59021 | 0.1065 | |
| Tertiary subcloning | 32.25746 | 15.12088 | 20.53333 | 23.48571 | 36.83769 | 0.0965 | |
| E4 | E9 | E10 | E19 | D7 | |
|---|---|---|---|---|---|
| Heavy chain | mu chain | mu chain | mu chain | mu chain | mu chain |
| Light chain | kappa | kappa | kappa | kappa | kappa |
| Antibody isotypes | IgM | IgM | IgM | IgM | IgM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Liu, Y.; Zhao, G.; Hu, B.; Xu, L.; Liu, J.; Sun, Y.; Guo, X.; Deng, X.; Lian, S.; et al. Preparation of Monoclonal Antibodies Against the gD Protein of Feline Herpesvirus Type-1 by mRNA Immunization. Vet. Sci. 2025, 12, 601. https://doi.org/10.3390/vetsci12070601
Zhang C, Liu Y, Zhao G, Hu B, Xu L, Liu J, Sun Y, Guo X, Deng X, Lian S, et al. Preparation of Monoclonal Antibodies Against the gD Protein of Feline Herpesvirus Type-1 by mRNA Immunization. Veterinary Sciences. 2025; 12(7):601. https://doi.org/10.3390/vetsci12070601
Chicago/Turabian StyleZhang, Chengqi, Yawen Liu, Guangrong Zhao, Bo Hu, Liwen Xu, Jiajia Liu, Yajie Sun, Xiaolan Guo, Xiaoyu Deng, Shizhen Lian, and et al. 2025. "Preparation of Monoclonal Antibodies Against the gD Protein of Feline Herpesvirus Type-1 by mRNA Immunization" Veterinary Sciences 12, no. 7: 601. https://doi.org/10.3390/vetsci12070601
APA StyleZhang, C., Liu, Y., Zhao, G., Hu, B., Xu, L., Liu, J., Sun, Y., Guo, X., Deng, X., Lian, S., Han, T., Xu, M., Xu, S., & Bai, X. (2025). Preparation of Monoclonal Antibodies Against the gD Protein of Feline Herpesvirus Type-1 by mRNA Immunization. Veterinary Sciences, 12(7), 601. https://doi.org/10.3390/vetsci12070601








