Selective Dry Cow Therapy in Modern Dairy Management: Balancing Udder Health and Antimicrobial Stewardship
Simple Summary
Abstract
1. Introduction
2. Study Eligibility Criteria
3. International Adoption of SDCT
4. Selection Protocols
4.1. Cow-Level Versus Quarter-Level Selection
4.2. Methods Applied for Cow Selection
4.2.1. Bacteriological Culture
4.2.2. Somatic Cell Count
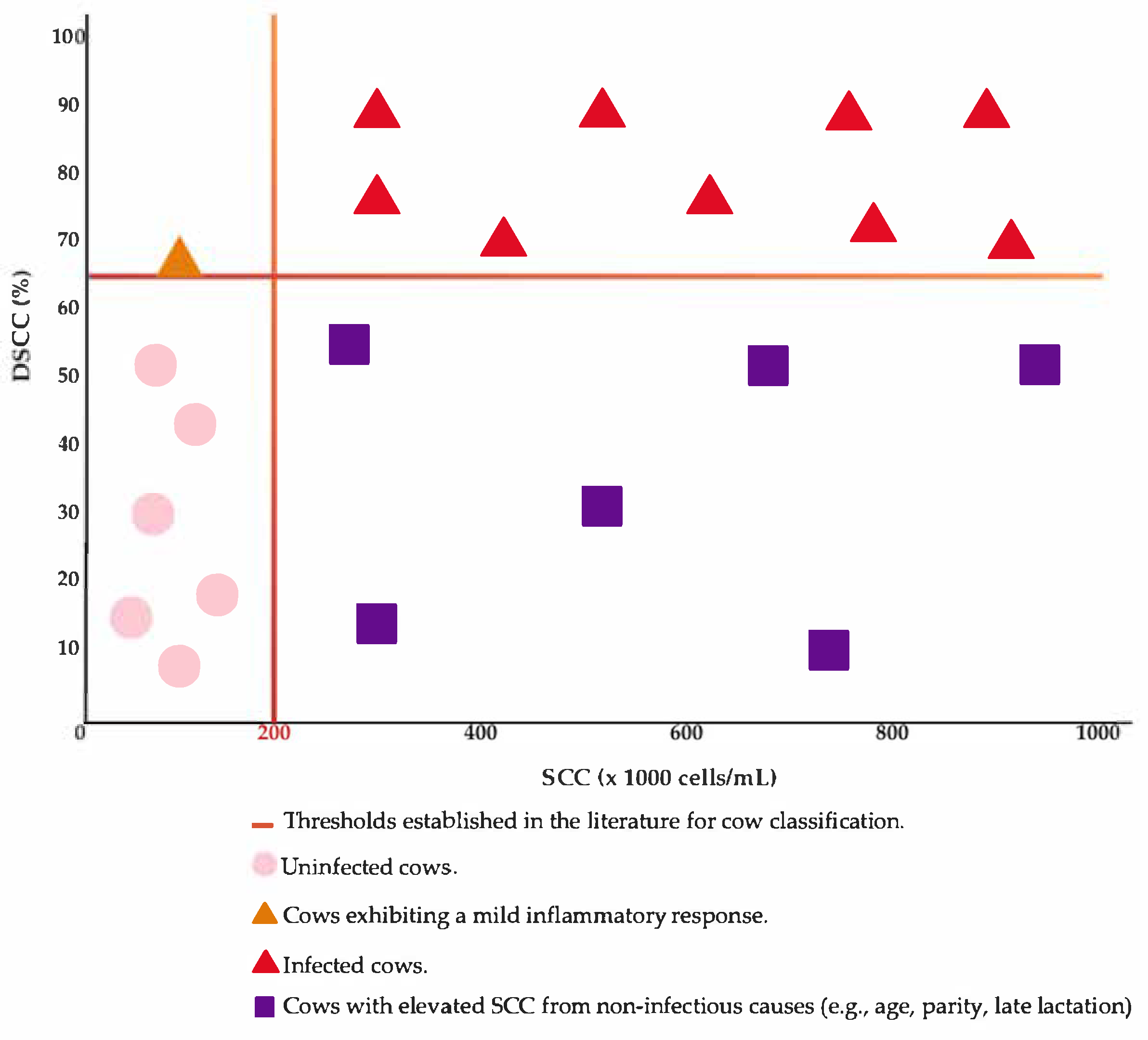
4.2.3. Differential Somatic Cell Count
4.2.4. California Mastitis Test
5. Teat Sealants
| Bibliographic References | Method | Internal Teat Sealants | Results |
|---|---|---|---|
| Rindsig et al. [1] | Infusion in all quarters only if: History of 1 CM during the current lactation. A score of +2 or +3 on the 2 CMT (in any quarter on the day of drying-off). 3 BTSCC > 500,000 cells/mL. | no | Similar cure rate of existing infections: BDCT—85.4%; SDCT—88.2%. Slightly higher new IMI rate during the dry period in SDCT (6.5%) compared to BDCT (3.1%). Higher incidence of CM after calving in SDCT (7.8%) than in BDCT (4.6%). |
| Browning et al. [24] | BTSCC: 100–400,000 cells/mL. Quarters were considered infected if 2 out of 3 consecutive bacteriological samples contained the same pathogen. Uninfected cows: randomly assigned to receive either treatment in all quarters or no treatment. Infected cows: allocated to receive treatment either in all quarters or only in the affected quarter. | no | Significantly higher new IMI rate during the dry period in cows treated only in infected quarters (15.3%) compared to those treated in all quarters (4.3%). Streptococcus uberis was more frequently isolated in the quarter-treated group, both at calving and mid-lactation. No significant differences in the incidence of CM, although group-level variations were observed in early lactation. |
| Browning et al. [2] | Refer to Browning et al. [24] | no | Cows treated selectively at the quarter level had a higher percentage of infected quarters at calving (10.0%) compared to those treated selectively at the cow level (7.9%) or those treated universally (6.8%), though no significant differences were observed between strategies. By mid-lactation, the prevalence of infection was similar across all treatment strategies. |
| Østerås & Sandvik [87] | Cows with >100,000 cells/mL in the last 2 tests and a positive CMT or a major pathogen identified in 1+ quarters. Randomized study: Group A = Control. Group B = Placebo. Group C = Long-acting antibiotic in infected quarters. Group D = Short-acting antibiotic administered every two days for 8 days in infected quarters. Treatment at the quarter level (except if >3 quarters were infected). | no | No effects on the culling rate. Cows in the control group exhibited a higher incidence of clinical mastitis, elevated SCC, and a lower average milk yield per lactation. |
| Berry & Hillerton [4] | Quarter-level AB administration when: the same pathogen was isolated in two consecutive samples, in two out of three samples, or in a single sample with SCC > 200,000 cells/mL and at least twice as high as in the other quarters. | no | Untreated groups showed a higher incidence of new IMIs. Treatment was effective in eliminating infections caused by Streptococcus uberis and partially effective against Staphylococcus aureus. The absence of treatment increased the risk of coinfections (S. uberis and coliform infections). |
| Bradley et al. [82] | BTSCC < 250,000 cells/mL. Uninfected cows: SCC < 200,000 cells/mL in the last three monthly tests and no CM during this period—only ITS or AB + ITS (at the quarter level). Infected cows: all other animals—only ITS or ITS + AB (at the quarter level). | yes | Infected cows: The combined treatment ITS + AB increased the chances of pathogen elimination and reduced the risk of CM in the first 100 days of lactation compared to AB alone. Uninfected cows with low SCC: The combination of ITS + AB did not significantly reduce the risk of infection compared to ITS alone, but was associated with a higher prevalence of IMI with coagulase-positive staphylococci and Streptococcus spp. |
| Rajala-Schultz et al. [68] | Low risk: SCC < 200,000 cells/mL in the last 3 monthly tests and no CM during this period; CM in the first 90 days of the previous lactation but SCC < 100,000 cells/mL during the rest of lactation randomly assigned to receive treatment or not. High risk: All other animals—all cows were treated. | no | The MY of cows with low SCC, whether treated or untreated, did not differ significantly in the following lactation. Treated cows with low SCC had a 16% lower SCC (35,000 cells/mL), but the effect varied between farms. |
| Scherpenzeel et al. [33] | SCC < 150,000 cells/mL for primiparous cows. SCC < 250,000 cells/mL for multiparous cows. No CM present. Quarter-level analysis (split by quarters). | no | The incidence of CM was 1.7 times higher in untreated quarters, especially during the first 21 days after calving. Significantly higher SCC was observed in untreated quarters at calving and during the first 14 days postpartum. |
| Cameron et al. [12] | BTSCC < 250,000 cells/mL. On-farm Petrifilm culture for cows with SCC < 200,000 cells/mL in the last three tests and no CM during this period. Negative culture: SDCT –ITS only. Positive culture: BDCT—AB + ITS. | yes | No differences were observed between the BDCT and SDCT groups in terms of bacteriological cure (per quarter), new IMIs (during the dry period), IMIs at calving, or CM (during the first 120 days of lactation). |
| Cameron et al. [22] | Refer to Cameron et al. [12] | yes | Similar MY between groups (BDCT: 39.3 kg, SDCT: 39.0 kg). The natural logarithm of SCC was also similar (BDCT: 3.95 vs. SDCT: 3.97). |
| Tho Seeth et al. [25] | Group A: On-farm Petrifilm culture. (+) Positive: AB + ITS. (–) Negative: ITS only. Group S: SCC < 200,000 cells/mL at last test and no CM in the previous lactation –ITS only. SCC ≥ 200,000 cells/mL or CM in the previous lactation AB + ITS. Group C (Control, BDCT): AB + ITS. | yes | Group C achieved the best results in terms of udder health, especially bacteriological cure during the dry period. Groups S and A showed only marginally weaker results. A significant difference in bacteriological cure was observed between Group S and Group C. The risk of new IMIs was similar across all three groups. |
| Vasquez et al. [19] | Low risk: SCC ≤ 200,000 cells/mL at the last test; Average SCC over the last 3 monthly tests ≤ 200,000 cells/mL; No signs of CM at dry-off. A maximum of one CM episode during the current lactation—randomly assigned to receive BDCT or SDCT. High risk: Treated with BDCT. | external sealant | No significant differences between treatment groups in terms of risk of new IMI, milk production, linear scores, culling events, or CM. Slightly higher bacteriological cure rates in animals treated with AB. |
| McParland et al. [85] | Low risk: SCC < 200,000 cells/mL throughout the previous lactation and no CM—randomly assigned to BDCT + ITS or SDCT. High risk: BDCT + ITS. | yes | Cows treated only with ITSs produced, on average, 0.67 kg/day more milk, but had a slightly higher SCC throughout lactation compared to cows treated with ITS + AB. Weekly SCC did not differ between cows with SDCT and those in the high-risk group. Cows with ITSs had a 2.7- times higher risk of bacterial presence in early lactation, compared to those in the low-risk group treated with the combined approach, and 1.6 times higher compared to high-risk cows. |
| Rowe et al. [36] | Culture-SDCT: Treatment AB + ITS only for bacteriologically positive quarters. SCC-SDCT: Treatment with AB + ITS only for cows with SCC > 200,000 cells/mL in any monthly test during the current lactation and ≥2 cases of CM during the same lactation. BDCT: All quarters treated with Ab + ITS. | yes | The risk of bacteriological cure, new IMIs during the dry period, and new IMIs after calving were similar across the three groups. |
| Rowe et al. [31] | Refer to Rowe et al. [36] | yes | The risk of culling, CM in the first 120 days of lactation, SCC, and MY were similar across the treatment groups. |
| Kabera et al. [35] | Petrifilm used for on-farm culture SDCT: Positive culture: AB + ITS; Negative culture: ITS only. BDCT: AB for infected quarters, ITS for healthy quarters, AB + ITS for infected quarters, sealant only for healthy quarters. | yes | There were no significant differences between groups in terms of acquisition of new IMIs, persistence of existing IMIs, incidence of clinical mastitis in the following lactation, average SCC score, and milk production. |
| Zecconi et al. [63] | Antibiotic treatment was administered when: the SCC at the last test exceeded 100,000 cells/mL in primiparous cows and 200,000 cells/mL in multiparous cows. | yes | The use of an ITS resulted in a higher bacteriological cure rate and significantly reduced the incidence of new IMIs. The proportions of negative cows (49.1% vs. 49.3%), transient infections (24.8% vs. 27.3%), and persistent IMI (26.1% vs. 23.5%) were very similar at dry-off and after calving. |
| Clabby et al. [11] | BTSCC < 250,000 cells/mL. Low risk: SCC < 200,000 cells/mL in the previous lactation—treated with ITS or ITS + AB. High risk: all other cows—treated with ITS + AB. | The logarithmic value of SCC in cows from the low-risk group treated only with ITSs was significantly higher compared to cows from the group treated with ITS + AB but showed no significant differences compared to cows from the high-risk group in the following lactation. The response to treatment varied depending on the herd studied. | |
| Goncalves et al. [16] | Healthy cows: no bacteria isolated, no CM, and SCC < 200,000 cells/mL in the last 3 monthly tests –ITSs only. Cows with subclinical mastitis: positive culture in one quarter and SCC > 200,000 cells/mL—AB + ITSs. | yes | The bacterial diversity was similar between healthy quarters and those that were cured, regardless of the dry-off protocol. Healthy cows treated only with ITS showed a higher abundance of beneficial or commensal bacteria in the mammary gland. |
| Lipkens et al. [88] | BTSCC < 250,000 cells/mL BDCT: cows with an odd ear tag number—treated with AB + ITSs. SDCT: cows with an even ear tag number—received AB only if the infection assessment algorithm indicated by Lipkens et al. [53] required it. | yes | The differences in SCC between the SDCT and BDCT groups were minimal, with slightly better values for the SDCT cows. Cows in the SDCT group had a higher average daily milk yield during the first 100 of lactation compared to those in the BDCT group. |
| Pavesi et al. [89] | No CM and SCC < 200,000 cells/mL throughout lactation—2 groups: cows treated with ITSs only; cows treated with ITSs + AB. | yes | SDCT did not negatively affect milk production or udder health, with SCC values being similar in both groups. No CM was observed in the first 100 days, even in the group treated with ITSs only. Milk microbiota remained stable. |
| Paiva et al. [69] | SDCT1: AB administered if SCC > 200,000 cells/mL at any of the monthly tests or if the cow had ≥2 cases of CM during the current lactation. SDCT2: AB administered if SCC > 200,000 cells/mL at the last test or if there was any case of CM during the current lactation. BDCT: control group. | no | SDCT did not affect the risk of new IMIs or the cure of existing ones. There were no significant differences between SDCT and BDCT in terms of CM, SCC, MY, or culling risk during the first 180 days of lactation. |
| D’Amico et al. [90] | 3 groups. S-SDCT: Quarter-level treatment only if SCC ≥ 200,000 cells/mL. C-SDCT: Quarter-level treatment only if bacterial growth was detected. BDCT: Treatment applied to all quarters. | yes | Antimicrobial use: Cows in both SDCT groups received fewer antimicrobial treatments than those in the BDCT group; C-SDCT cows were treated less than S-SDCT cows. Linear score at first test: Higher in SDCT groups (BDCT: 1.8; S-SDCT: 2.2; C-SDCT: 2.2). Other outcomes: No significant differences between groups. |
6. The Impact of SDCT
6.1. New Intramammary Infections, SCC, and Clinical Mastitis Rate
6.2. Milk Yield
6.3. Antibiotic Consumption
6.4. Economy
7. Influential Factors in Udder Health and SDCT Outcomes
7.1. Cow-Level Factors
7.2. Farm-Level Factors
7.3. Other Factors
8. Application of SDCT in Other Species
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rindsig, R.B.; Rodewald, R.G.; Smith, A.R.; Spahr, S.L. Complete Versus Selective Dry Cow Therapy for Mastitis Control. J. Dairy Sci. 1978, 61, 1483–1497. [Google Scholar] [CrossRef] [PubMed]
- Browning, J.W.; Meint, G.A.; Brightlingt, P.; Nichollst, T.J.; Bartont, M. Strategies for Mastitis Control: Dry Cow Therapy and Culling. Aust. Vet. J. 1994, 71, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.; Nitz, J.; Tellen, A.; Klocke, D.; Krömker, V. Effect of Antibiotic Compared to Non-Antibiotic Dry Cow Treatment on the Bacteriological Cure of Intramammary Infections during the Dry Period—A Retrospective Cross-Sectional Study. Antibiotics 2023, 12, 429. [Google Scholar] [CrossRef] [PubMed]
- Berry, E.A.; Hillerton, J.E. The Effect of Selective Dry Cow Treatment on New Intramammary Infections. J. Dairy Sci. 2002, 85, 112–121. [Google Scholar] [CrossRef]
- Green, M.J.; Green, L.E.; Medley, G.F.; Schukken, Y.H.; Bradley, A.J. Influence of Dry Period Bacterial Intramammary Infection on Clinical Mastitis in Dairy Cows. J. Dairy Sci. 2002, 85, 2589–2599. [Google Scholar] [CrossRef]
- Neave, F.K.; Dodd, F.H.; Kingwill, R.G.; Westgarth, D.R. Control of Mastitis in the Dairy Herd by Hygiene and Management. J. Dairy Sci. 1969, 52, 696–707. [Google Scholar] [CrossRef]
- O’Rourke, D. Assessment of Cows for Use of a Nonantimicrobial Dry Cow Product. J. Appl. Microbiol. 2005, 98, 1256–1260. [Google Scholar] [CrossRef]
- Dufour, S.; Dohoo, I.R.; Barkema, H.W.; DesCôteaux, L.; DeVries, T.J.; Reyher, K.K.; Roy, J.P.; Scholl, D.T. Manageable Risk Factors Associated with the Lactational Incidence, Elimination, and Prevalence of Staphylococcus Aureus Intramammary Infections in Dairy Cows. J. Dairy Sci. 2012, 95, 1283–1300. [Google Scholar] [CrossRef]
- USDA United States Department of Agriculture; Animal and Plant Health Inspection Service; Veterinary Services; National Animal Health Monitoring System. Dairy 2007 Part III: Reference of Dairy Cattle Health and Management Practices in the United States; USDA: Washington, DC, USA, 2008.
- Scherpenzeel, C.G.M.; den Uijl, I.E.M.; van Schaik, G.; Riekerink, R.G.M.O.; Hogeveen, H.; Lam, T.J.G.M. Effect of Different Scenarios for Selective Dry-Cow Therapy on Udder Health, Antimicrobial Usage, and Economics. J. Dairy Sci. 2016, 99, 3753–3764. [Google Scholar] [CrossRef]
- Clabby, C.; McParland, S.; Dillon, P.; Arkins, S.; Flynn, J.; Murphy, J.; Boloña, P.S. Internal Teat Sealants Alone or in Combination with Antibiotics at Dry-off—The Effect on Udder Health in Dairy Cows in Five Commercial Herds. Animal 2022, 16, 100449. [Google Scholar] [CrossRef]
- Cameron, M.; McKenna, S.L.; MacDonald, K.A.; Dohoo, I.R.; Roy, J.P.; Keefe, G.P. Evaluation of Selective Dry Cow Treatment Following On-Farm Culture: Risk of Postcalving Intramammary Infection and Clinical Mastitis in the Subsequent Lactation. J. Dairy Sci. 2014, 97, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Halasa, T.; Nielen, M.; Whist, A.C.; Østerås, O. Meta-Analysis of Dry Cow Management for Dairy Cattle. Part 2. Cure of Existing Intramammary Infections. J. Dairy Sci. 2009, 92, 3150–3157. [Google Scholar] [CrossRef] [PubMed]
- Firth, C.L.; Käsbohrer, A.; Egger-Danner, C.; Fuchs, K.; Pinior, B.; Roch, F.F.; Obritzhauser, W. Comparison of Defined Course Doses (Dcdvet) for Blanket and Selective Antimicrobial Dry Cow Therapy on Conventional and Organic Farms. Animals 2019, 9, 707. [Google Scholar] [CrossRef]
- Tijs, S.H.W.; Holstege, M.M.C.; Scherpenzeel, C.G.M.; Santman-Berends, I.M.G.A.; Velthuis, A.G.J.; Lam, T.J.G.M. Effect of Selective Dry Cow Treatment on Udder Health and Antimicrobial Usage on Dutch Dairy Farms. J. Dairy Sci. 2022, 105, 5381–5392. [Google Scholar] [CrossRef]
- Goncalves, J.L.; Young, J.; Leite, R.d.F.; Fidelis, C.E.; Trevisoli, P.A.; Coutinho, L.L.; Silva, N.C.C.; Cue, R.I.; Rall, V.L.M.; dos Santos, M.V. The Impact of Selective Dry Cow Therapy Adopted in a Brazilian Farm on Bacterial Diversity and the Abundance of Quarter Milk. Vet. Sci. 2022, 9, 550. [Google Scholar] [CrossRef]
- Wittek, T.; Tichy, A.; Grassauer, B.; Egger-Danner, C. Retrospective Analysis of Austrian Health Recording Data of Antibiotic or Nonantibiotic Dry-off Treatment on Milk Yield, Somatic Cell Count, and Frequency of Mastitis in Subsequent Lactation. J. Dairy Sci. 2018, 101, 1456–1463. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling Drug-resistant Infections Globally: Final Report and Recommendations the Review on Antimicrobial Resistance Chaired. Rev. Antimicrob. Resist. 2016, 1, 69–75. [Google Scholar]
- Vasquez, A.K.; Nydam, D.V.; Foditsch, C.; Wieland, M.; Lynch, R.; Eicker, S.; Virkler, P.D. Use of a Culture-Independent on-Farm Algorithm to Guide the Use of Selective Dry-Cow Antibiotic Therapy. J. Dairy Sci. 2018, 101, 5345–5361. [Google Scholar] [CrossRef]
- Clabby, C.; Valldecabres, A.; Dillon, P.; O’Sullivan, K.; Arkins, S.; Flynn, J.; McCarthy, S.; Silva Boloña, P. The Association Between Somatic Cell Count and Selective Dry Cow Therapy, Milking Routine, and Dry Cow Management Practices in Early-Lactation Cows from 21 Commercial Grazing Dairy Herds. J. Dairy Sci. 2024, 107, 7106–7120. [Google Scholar] [CrossRef]
- Mondini, S.; Gislon, G.; Zucali, M.; Sandrucci, A.; Tamburini, A.; Bava, L. Risk Factors of High Somatic Cell Count and Differential Somatic Cells in Early Lactation Associated with Selective Dry Cow Therapy. Animal 2023, 17, 100982. [Google Scholar] [CrossRef]
- Cameron, M.; Keefe, G.P.; Roy, J.P.; Stryhn, H.; Dohoo, I.R.; McKenna, S.L. Evaluation of Selective Dry Cow Treatment Following On-Farm Culture: Milk Yield and Somatic Cell Count in the Subsequent Lactation. J. Dairy Sci. 2015, 98, 2427–2436. [Google Scholar] [CrossRef] [PubMed]
- Biggs, A. Update on Dry Cow Therapy 1. Antibiotic v Non-Antibiotic Approaches. Practice 2017, 39, 328–333. [Google Scholar] [CrossRef]
- Browning, J.W.; Mein, G.A.; Barton, M.; Nicholls, T.J.; Brightling, P. Effects of Antibiotic Therapy at Drying off on Mastitis in the Dry Period and Early Lactation. Aust. Vet. J. 1990, 67, 440–442. [Google Scholar] [CrossRef]
- Tho Seeth, M.; Wente, N.; Paduch, J.H.; Klocke, D.; Mansion-De Vries, E.; Hoedemaker, M.; Krömker, V. Different Selective Dry Cow Therapy Concepts Compared to Blanket Antibiotic Dry Cow Treatment. Tierarztl. Prax. Ausg. G Grosstiere-Nutztiere 2017, 45, 343–349. [Google Scholar] [CrossRef]
- Østerås, O.; Sølverød, L. Norwegian mastitis control programme. Ir. Vet. J. 2009, 62, S26. [Google Scholar] [CrossRef]
- Vanhoudt, A.; van Hees-Huijps, K.; van Knegsel, A.T.M.; Sampimon, O.C.; Vernooij, J.C.M.; Nielen, M.; van Werven, T. Effects of Reduced Intramammary Antimicrobial Use during the Dry Period on Udder Health in Dutch Dairy Herds. J. Dairy Sci. 2018, 101, 3248–3260. [Google Scholar] [CrossRef]
- Santman-Berends, I.M.G.A.; van den Heuvel, K.W.H.; Lam, T.J.G.M.; Scherpenzeel, C.G.M.; van Schaik, G. Monitoring Udder Health on Routinely Collected Census Data: Evaluating the Short- to Mid-Term Consequences of Implementing Selective Dry Cow Treatment. J. Dairy Sci. 2021, 104, 2280–2289. [Google Scholar] [CrossRef]
- Krattley-Roodenburg, B.; Huybens, L.J.; Nielen, M.; van Werven, T. Dry Period Management and New High Somatic Cell Count during the Dry Period in Dutch Dairy Herds under Selective Dry Cow Therapy. J. Dairy Sci. 2021, 104, 6975–6984. [Google Scholar] [CrossRef]
- Ferreira, F.C.; Martínez-López, B.; Okello, E. Potential Impacts to Antibiotics Use around the Dry Period If Selective Dry Cow Therapy Is Adopted by Dairy Herds: An Example of the Western US. Prev. Vet. Med. 2022, 206, 105709. [Google Scholar] [CrossRef]
- Rowe, S.M.; Godden, S.M.; Nydam, D.V.; Gorden, P.J.; Lago, A.; Vasquez, A.K.; Royster, E.; Timmerman, J.; Thomas, M.J. Randomized Controlled Trial Investigating the Effect of 2 Selective Dry-Cow Therapy Protocols on Udder Health and Performance in the Subsequent Lactation. J. Dairy Sci. 2020, 103, 6493–6503. [Google Scholar] [CrossRef]
- McDougall, S. A Randomised, Non-Inferiority Trial of a New Cephalonium Dry-Cow Therapy. N. Z. Vet. J. 2010, 58, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Scherpenzeel, C.G.M.; Den Uijl, I.E.M.; Van Schaik, G.; Olde Riekerink, R.G.M.; Keurentjes, J.M.; Lam, T.J.G.M. Evaluation of the Use of Dry Cow Antibiotics in Low Somatic Cell Count Cows. J. Dairy Sci. 2014, 97, 3606–3614. [Google Scholar] [CrossRef] [PubMed]
- McDougall, S.; Williamson, J.; Gohary, K.; Lacy-Hulbert, J. Detecting Intramammary Infection at the End of Lactation in Dairy Cows. J. Dairy Sci. 2021, 104, 10232–10249. [Google Scholar] [CrossRef] [PubMed]
- Kabera, F.; Dufour, S.; Keefe, G.; Cameron, M.; Roy, J.P. Evaluation of Quarter-Based Selective Dry Cow Therapy Using Petrifilm on-Farm Milk Culture: A Randomized Controlled Trial. J. Dairy Sci. 2020, 103, 7276–7287. [Google Scholar] [CrossRef]
- Rowe, S.M.; Godden, S.M.; Nydam, D.V.; Gorden, P.J.; Lago, A.; Vasquez, A.K.; Royster, E.; Timmerman, J.; Thomas, M.J. Randomized Controlled Non-Inferiority Trial Investigating the Effect of 2 Selective Dry-Cow Therapy Protocols on Antibiotic Use at Dry-off and Dry Period Intramammary Infection Dynamics. J. Dairy Sci. 2020, 103, 6473–6492. [Google Scholar] [CrossRef]
- Berry, E.A.; Johnston, W.T.; Hillerton, J.E. Prophylactic Effects of Two Selective Dry Cow Strategies Accounting for Interdependence of Quarter. J. Dairy Sci. 2003, 86, 3912–3919. [Google Scholar] [CrossRef]
- Robert, A.; Bareille, N.; Roussel, P.; Poutrel, B.; Heuchel, V.; Seegers, H. Interdependence of Udder Quarters for New Intramammary Infection during the Dry Period in Cows Submitted to Selective Antibiotic Therapy. J. Dairy Res. 2006, 73, 345–352. [Google Scholar] [CrossRef]
- Ward, G.E.; Schultz, L.H. Incidence and Control of Mastitis During the Dry Period1. J. Dairy Sci. 1974, 57, 1341–1349. [Google Scholar] [CrossRef]
- Williamson, J.H.; Woolford, M.W.; Day, A.M. The Prophylactic Effect of a Dry-Cow Antibiotic against Streptococcus uberis. N. Z. Vet. J. 1995, 43, 228–234. [Google Scholar] [CrossRef]
- Swinkels, J.M.; Leach, K.A.; Breen, J.E.; Payne, B.; White, V.; Green, M.J.; Bradley, A.J. Randomized Controlled Field Trial Comparing Quarter and Cow Level Selective Dry Cow Treatment Using the California Mastitis Test. J. Dairy Sci. 2021, 104, 9063–9081. [Google Scholar] [CrossRef]
- Kabera, F.; Roy, J.P.; Keefe, G.; Dufour, S. Bayesian Estimation of Diagnostic Accuracy of Somatic Cell Counts History and On-Farm Milk Culture Using Petrifilm® to Identify Quarters or Cows That Should Be Treated with Antimicrobials in Selective Treatment Protocols at Dry Off. Prev. Vet. Med. 2021, 195, 105452. [Google Scholar] [CrossRef] [PubMed]
- Winder, C.B.; Sargeant, J.M.; Hu, D.; Wang, C.; Kelton, D.F.; Leblanc, S.J.; Duffield, T.F.; Glanville, J.; Wood, H.; Churchill, K.J.; et al. Comparative Efficacy of Antimicrobial Treatments in Dairy Cows at Dry-off to Prevent New Intramammary Infections during the Dry Period or Clinical Mastitis during Early Lactation: A Systematic Review and Network Meta-Analysis. Anim. Health Res. Rev. 2019, 20, 199–216. [Google Scholar] [CrossRef]
- Torres, A.H.; Rajala-Schultz, P.J.; DeGraves, F.J.; Hoblet, K.H. Using Dairy Herd Improvement Records and Clinical Mastitis History to Identify Subclinical Mastitis Infections at Dry-Off. J. Dairy Res. 2008, 75, 240–247. [Google Scholar] [CrossRef]
- Guadagnini, M.; Gogna, C.; Tolasi, C.; Tolasi, G.; Gnali, G.; Freu, G.; Masroure, A.J.; Moroni, P. Approach to Selective Dry Cow Therapy in Early Adopter Italian Dairy Farms: Why Compliance Is So Important. Animals 2023, 13, 3485. [Google Scholar] [CrossRef]
- McCubbin, K.D.; de Jong, E.; Lam, T.J.G.M.; Kelton, D.F.; Middleton, J.R.; McDougall, S.; De Vliegher, S.; Godden, S.; Rajala-Schultz, P.J.; Rowe, S.; et al. Invited Review: Selective Use of Antimicrobials in Dairy Cattle at Drying-Off. J. Dairy Sci. 2022, 105, 7161–7189. [Google Scholar] [CrossRef]
- Down, P.M.; Bradley, A.J.; Breen, J.E.; Green, M.J. Factors Affecting the Cost-Effectiveness of on-Farm Culture Prior to the Treatment of Clinical Mastitis in Dairy Cows. Prev. Vet. Med. 2017, 145, 91–99. [Google Scholar] [CrossRef]
- Robert, A.; Roussel, P.; Bareille, N.; Ribaud, D.; Sérieys, F.; Heuchel, V.; Seegers, H. Risk Factors for New Intramammary Infections during the Dry Period in Untreated Dairy Cows from Herds Using Selective Dry Cow Therapy. Animal 2008, 2, 247–254. [Google Scholar] [CrossRef]
- Rendos, J.J.; Eberhart, R.J.; Kesler, E.M. Microbial Populations of Teat Ends of Dairy Cows, and Bedding Materials1. J. Dairy Sci. 1975, 58, 1492–1500. [Google Scholar] [CrossRef]
- Buelow, K.L.; Goodger, W.J.; Collinsb, M.T.; Clayton, M.K.; Nordlund, K.V.; Thomasb, C.B. A Model to Determine Sampling Strategies and Milk Inoculum Volume for Detection of Intramammary Staphylococcus Aureus Infections in Dairy Cattle by Bacteriological Culture. Prev. Vet. Med. 1996, 25, 343–355. [Google Scholar] [CrossRef]
- Cameron, M.; Keefe, G.P.; Roy, J.P.; Dohoo, I.R.; MacDonald, K.A.; McKenna, S.L. Evaluation of a 3M Petrifilm On-Farm Culture System for the Detection of Intramammary Infection at the End of Lactation. Prev. Vet. Med. 2013, 111, 1–9. [Google Scholar] [CrossRef]
- Kiesner, K.; Wente, N.; Volling, O.; Krömker, V. Selection of Cows for Treatment at Dry-off on Organic Dairy Farms. J. Dairy Res. 2016, 83, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Lipkens, Z.; Piepers, S.; De Visscher, A.; De Vliegher, S. Evaluation of Test-Day Milk Somatic Cell Count Information to Predict Intramammary Infection with Major Pathogens in Dairy Cattle at Drying Off. J. Dairy Sci. 2019, 102, 4309–4321. [Google Scholar] [CrossRef] [PubMed]
- Harmon, R.J. Physiology of Mastitis and Factors Affecting Somatic Cell Counts. J. Dairy Sci. 1994, 77, 2103–2112. [Google Scholar] [CrossRef] [PubMed]
- Sanford, C.J.; Keefe, G.P.; Sanchez, J.; Dingwell, R.T.; Barkema, H.W.; Leslie, K.E.; Dohoo, I.R. Test Characteristics from Latent-Class Models of the California Mastitis Test. Prev. Vet. Med. 2006, 77, 96–108. [Google Scholar] [CrossRef]
- Schukken, Y.H.; Wilson, D.J.; Welcome, F.; Garrison-Tikofsky, L.; Gonzalez, R.N. Monitoring Udder Health and Milk Quality Using Somatic Cell Counts. Vet. Res. 2003, 34, 579–596. [Google Scholar] [CrossRef]
- Stocco, G.; Summer, A.; Cipolat-Gotet, C.; Zanini, L.; Vairani, D.; Dadousis, C.; Zecconi, A. Differential Somatic Cell Count as a Novel Indicator of Milk Quality in Dairy Cows. Animals 2020, 10, 753. [Google Scholar] [CrossRef]
- Biggs, A. Update on Dry Cow Therapy 2. Measuring Dry Period Performance. Practice 2017, 39, 363–371. [Google Scholar] [CrossRef]
- Cook, N.B.; Bennett, T.B.; Emery, K.M.; Nordlund, K.V. Monitoring Nonlactating Cow Intramammary Infection Dynamics Using DHI Somatic Cell Count Data. J. Dairy Sci. 2002, 85, 1119–1126. [Google Scholar] [CrossRef]
- Dohoo, I.R.; Leslie, K.E. Evaluation of Changes in Somatic Cell Counts as Indicators of New Intramammary Infections. Prev. Vet. Med. 1991, 10, 225–237. [Google Scholar] [CrossRef]
- Merle, R.; Schröder, A.; Hamann, J. Cell Function in the Bovine Mammary Gland: A Preliminary Study on Interdependence of Healthy and Infected Udder Quarters. J. Dairy Res. 2007, 74, 174–179. [Google Scholar] [CrossRef]
- Piccinini, R.; Binda, E.; Belotti, M.; Daprà, V.; Zecconi, A. Evaluation of Milk Components during Whole Lactation in Healthy Quarters. J. Dairy Res. 2007, 74, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Zecconi, A.; Dell’orco, F.; Vairani, D.; Rizzi, N.; Cipolla, M.; Zanini, L. Differential Somatic Cell Count as a Marker for Changes of Milk Composition in Cows with Very Low Somatic Cell Count. Animals 2020, 10, 604. [Google Scholar] [CrossRef] [PubMed]
- Zecconi, A. Staphylococcus Aureus Mastitis: What we need to know to control them. Isr. J. Vet. Med. 2010, 65, 93–99. [Google Scholar]
- Djabri, B.; Bareille, N.; Beaudeau, F.; Seegers, H. Quarter Milk Somatic Cell Count in Infected Dairy Cows: A Meta-Analysis. Vet. Res. 2002, 33, 335–357. [Google Scholar] [CrossRef] [PubMed]
- Pantoja, J.C.F.; Hulland, C.; Ruegg, P.L. Dynamics of Somatic Cell Counts and Intramammary Infections across the Dry Period. Prev. Vet. Med. 2009, 90, 43–54. [Google Scholar] [CrossRef]
- Østerås, O.; Edge, V.L.; Martin, S.W. Determinants of Success or Failure in the Elimination of Major Mastitis Pathogens in Selective Dry Cow Therapy. J. Dairy Sci. 1999, 82, 1221–1231. [Google Scholar] [CrossRef]
- Rajala-Schultz, P.J.; Torres, A.H.; Degraves, F.J. Milk Yield and Somatic Cell Count during the Following Lactation after Selective Treatment of Cows at Dry-Off. J. Dairy Res. 2011, 78, 489–499. [Google Scholar] [CrossRef]
- Paiva, D.; Menta, P.; Bielamowicz, L.P.; Machado, V.S. The Effect of Selective Dry Cow Therapies Based on Two Different Algorithms on Antimicrobial Use, Udder Health, Milk Production, and Culling in the Absence of Internal Teat Sealant Use at Dry-Off. J. Dairy. Sci. 2024, 107, 8259–8270. [Google Scholar] [CrossRef]
- Paape, M.J.; Wergin, W.P.; Guidry, A.J.; Pearson, R.E. Leukocytes–Second Line of Defense Against Invading Mastitis Pathogens. J. Dairy Sci. 1979, 62, 135–153. [Google Scholar] [CrossRef]
- Damm, M.; Holm, C.; Blaabjerg, M.; Bro, M.N.; Schwarz, D. Differential Somatic Cell Count—A Novel Method for Routine Mastitis Screening in the Frame of Dairy Herd Improvement Testing Programs. J. Dairy Sci. 2017, 100, 4926–4940. [Google Scholar] [CrossRef]
- Lozada-Soto, E.; Maltecca, C.; Anderson, K.; Tiezzi, F. Analysis of Milk Leukocyte Differential Measures for Use in Management Practices for Decreased Mastitis Incidence. J. Dairy Sci. 2020, 103, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Zecconi, A.; Meroni, G.; Sora, V.; Mattina, R.; Cipolla, M.; Zanini, L. Total and Differential Cell Counts as a Tool to Identify Intramammary Infections in Cows after Calving. Animals 2021, 11, 727. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, D.; Lipkens, Z.; Piepers, S.; De Vliegher, S. Investigation of Differential Somatic Cell Count as a Potential New Supplementary Indicator to Somatic Cell Count for Identification of Intramammary Infection in Dairy Cows at the End of the Lactation Period. Prev. Vet. Med. 2019, 172, 104803. [Google Scholar] [CrossRef]
- Kirkeby, C.; Toft, N.; Schwarz, D.; Farre, M.; Nielsen, S.S.; Zervens, L.; Hechinger, S.; Halasa, T. Differential Somatic Cell Count as an Additional Indicator for Intramammary Infections in Dairy Cows. J. Dairy Sci. 2020, 103, 1759–1775. [Google Scholar] [CrossRef]
- Dal Prà, A.; Biscarini, F.; Cavani, G.L.; Bacchelli, S.; Iotti, A.; Borghi, S.; Nocetti, M.; Moroni, P. Relationship between Total and Differential Quarter Somatic Cell Counts at Dry-off and Early Lactation. PLoS ONE 2022, 17, e0275755. [Google Scholar] [CrossRef]
- Poutrel, B.; Rainard, P. California Mastitis Test Guide of Selective Dry Cow Therapy. J. Dairy Sci. 1981, 64, 241–248. [Google Scholar] [CrossRef]
- Godden, S.M.; Royster, E.; Timmerman, J.; Rapnicki, P.; Green, H. Evaluation of an Automated Milk Leukocyte Differential Test and the California Mastitis Test for Detecting Intramammary Infection in Early- and Late-Lactation Quarters and Cows. J. Dairy Sci. 2017, 100, 6527–6544. [Google Scholar] [CrossRef]
- Parker, K.I.; Compton, C.; Anniss, F.M.; Weir, A.; Heuer, C.; McOougall, S. Subclinical and Clinical Mastitis in Heifers Following the Use of a Teat Sealant Precalving. J. Dairy Sci. 2007, 90, 207–218. [Google Scholar] [CrossRef]
- Huxley, J.N.; Green, M.J.; Green, L.E.; Bradley, A.J. Evaluation of the Efficacy of an Internal Teat Sealer during the Dry Period. J. Dairy Sci. 2002, 85, 551–561. [Google Scholar] [CrossRef]
- Biscarini, F.; Cremonesi, P.; Castiglioni, B.; Stella, A.; Bronzo, V.; Locatelli, C.; Moroni, P. A Randomized Controlled Trial of Teat-Sealant and Antibiotic Dry-Cow Treatments for Mastitis Prevention Shows Similar Effect on the Healthy Milk Microbiome. Front. Vet. Sci. 2020, 7, 581. [Google Scholar] [CrossRef]
- Bradley, A.J.; Breen, J.E.; Payne, B.; Williams, P.; Green, M.J. The Use of a Cephalonium Containing Dry Cow Therapy and an Internal Teat Sealant, Both Alone and in Combination. J. Dairy Sci. 2010, 93, 1566–1577. [Google Scholar] [CrossRef] [PubMed]
- Dufour, S.; Wellemans, V.; Roy, J.-P.; Lacasse, P.; Ordonez-Iturriaga, A.; Francoz, D. Non-Antimicrobial Approaches at Drying-off for Treating and Preventing Intramammary Infections in Dairy Cows. Part 1. Meta-Analyses of Efficacy of Using an Internal Teat Sealant Without a Concomitant Antimicrobial Treatment. Anim. Health Res. Rev. 2019, 20, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.B.; Pionek, D.A.; Sharp, P. An Assessment of the Benefits of Orbeseal® When Used in Combination with Dry Cow Antibiotic Therapy in Three Commercial Dairy Herds. Bov. Pract. 2005, 39, 83. [Google Scholar] [CrossRef]
- McParland, S.; Dillon, P.G.; Flynn, J.; Ryan, N.; Arkins, S.; Kennedy, A. Effect of Using Internal Teat Sealant with or without Antibiotic Therapy at Dry-off on Subsequent Somatic Cell Count and Milk Production. J. Dairy Sci. 2019, 102, 4464–4475. [Google Scholar] [CrossRef]
- McDougall, S.; Parker, K.I.; Heuer, C.; Compton, C.W.R. A Review of Prevention and Control of Heifer Mastitis via Non-Antibiotic Strategies. Vet. Microbiol. 2009, 134, 177–185. [Google Scholar] [CrossRef]
- Østerås, O.; Sandvik, L. Effects of Selective Dry-Cow Therapy on Culling Rate, Clinical Mastitis, Milk Yield and Cow Somatic Cell Count. A Randomized Clinical Field Study in Cows. J. Vet. Med. Ser. B 1996, 43, 555–575. [Google Scholar] [CrossRef]
- Lipkens, Z.; Piepers, S.; De Vliegher, S. Impact of Selective Dry Cow Therapy on Antimicrobial Consumption, Udder Health, Milk Yield, and Culling Hazard in Commercial Dairy Herds. Antibiotics 2023, 12, 901. [Google Scholar] [CrossRef]
- Filippone Pavesi, L.; Pollera, C.; Sala, G.; Cremonesi, P.; Monistero, V.; Biscarini, F.; Bronzo, V. Effect of the Selective Dry Cow Therapy on Udder Health and Milk Microbiota. Antibiotics 2023, 12, 1259. [Google Scholar] [CrossRef]
- D’Amico, K.; Neves, R.C.; Grantz, J.M.; Taechachokevivat, N.; Ueda, A.; Dorr, A.; Hubner, A. A Randomized, Controlled Trial Examining Quarter-Level Somatic Cell Count and Culture-Based Selective Dry Cow Therapy against Blanket Dry Cow Therapy on Early Lactation Production Outcomes. J. Dairy Sci. 2024, 107, 7201–7210. [Google Scholar] [CrossRef]
- Niemi, R.E.; Vilar, M.J.; Dohoo, I.R.; Hovinen, M.; Simojoki, H.; Rajala-Schultz, P.J. Antibiotic Dry Cow Therapy, Somatic Cell Count, and Milk Production: Retrospective Analysis of the Associations in Dairy Herd Recording Data Using Multilevel Growth Models. Prev. Vet. Med. 2020, 180, 105028. [Google Scholar] [CrossRef]
- Niemi, R.E.; Hovinen, M.; Rajala-Schultz, P.J. Selective Dry Cow Therapy Effect on Milk Yield and Somatic Cell Count: A Retrospective Cohort Study. J. Dairy Sci. 2022, 105, 1387–1401. [Google Scholar] [CrossRef] [PubMed]
- Vilar, M.J.; Rajala-Schultz, P.J. Dry-off and Dairy Cow Udder Health and Welfare: Effects of Different Milk Cessation Methods. Vet. J. 2020, 262, 105503. [Google Scholar] [CrossRef] [PubMed]
- Ruegg, P.L. A 100-Year Review: Mastitis Detection, Management, and Prevention. J. Dairy Sci. 2017, 100, 10381–10397. [Google Scholar] [CrossRef] [PubMed]
- Berry, E.A.; Hogeveen, H.; Hillerton, J.E. Decision Tree Analysis to Evaluate Dry Cow Strategies under UK Conditions. J. Dairy Res. 2004, 71, 409–418. [Google Scholar] [CrossRef]
- Berry, S.L.; Maas, J.; Kirk, J.H.; Reynolds, J.P.; Gardner, I.A.; Ahmadi, A. Effects of Antimicrobial Treatment at the End of Lactation on Milk Yield, Somatic Cell Count, and Incidence of Clinical Mastitis during the Subsequent Lactation in a Dairy Herd with a Low Prevalence of Contagious Mastitis. J. Am. Vet. Med. Assoc. 1997, 211, 207–211. [Google Scholar] [CrossRef]
- Bradley, A.J.; Green, M.J. A Study of the Incidence and Significance of Intramammary Enterobacterial Infections Acquired during the Dry Period. J. Dairy Sci. 2000, 83, 1957–1965. [Google Scholar] [CrossRef]
- Hogeveen, H.; Huijps, K.; Lam, T. Economic Aspects of Mastitis: New Developments. N. Z. Vet. J. 2011, 59, 16–23. [Google Scholar] [CrossRef]
- Susanna, L.K.; Tuomas, K. How Farmers Conceive and Cope with Megatrends: The Case of Finnish Dairy Farmers. Sustainability 2022, 14, 2265. [Google Scholar] [CrossRef]
- Rowe, S.M.; Nydam, D.V.; Godden, S.M.; Gorden, P.J.; Lago, A.; Vasquez, A.K.; Royster, E.; Timmerman, J.; Thomas, M.J.; Lynch, R.A. Partial Budget Analysis of Culture- and Algorithm-Guided Selective Dry Cow Therapy. J. Dairy Sci. 2021, 104, 5652–5664. [Google Scholar] [CrossRef]
- Rollin, E.; Dhuyvetter, K.C.; Overton, M.W. The Cost of Clinical Mastitis in the First 30 Days of Lactation: An Economic Modeling Tool. Prev. Vet. Med. 2015, 122, 257–264. [Google Scholar] [CrossRef]
- Gonçalves, J.L.; Kamphuis, C.; Martins, C.M.M.R.; Barreiro, J.R.; Tomazi, T.; Gameiro, A.H.; Hogeveen, H.; dos Santos, M.V. Bovine Subclinical Mastitis Reduces Milk Yield and Economic Return. Livest. Sci. 2018, 210, 25–32. [Google Scholar] [CrossRef]
- Patel, K.E.; Godden, S.M.; Royster, E.E.; Timmerman, J.A.; Crooker, B.A.; McDonald, N.E. Pilot Study: Impact of Using a Culture-Guided Selective Dry Cow Therapy Program Targeting Quarter-Level Treatment on Udder Health and Antibiotic Use. Bov. Pract. 2017, 51, 48–57. [Google Scholar] [CrossRef]
- Le Page, T.; Ferchiou, A.; Dufour, S.; Kabera, F.; Dubuc, J.; Lhermie, G.; Raboisson, D.; Roy, J.-P. Dairy Farmer’s Income, Working Time, and Antimicrobial Use under Different Dry Cow Therapy Protocols. J. Dairy Sci. 2024, 107, 8115–8129. [Google Scholar] [CrossRef] [PubMed]
- Hommels, N.M.C.; Ferreira, F.C.; van den Borne, B.H.P.; Hogeveen, H. Antibiotic Use and Potential Economic Impact of Implementing Selective Dry Cow Therapy in Large US Dairies. J. Dairy Sci. 2021, 104, 8931–8946. [Google Scholar] [CrossRef] [PubMed]
- Natzke, R.P.; Everett, R.W.; Bray, D.R. Effect of Drying Off Practices on Mastitis Infection. J. Dairy Sci. 1975, 58, 1828–1835. [Google Scholar] [CrossRef]
- Green, M.J.; Bradley, A.J.; Medley, G.F.; Browne, W.J. Cow, Farm, and Management Factors during the Dry Period That Determine the Rate of Clinical Mastitis after Calving. J. Dairy Sci. 2007, 90, 3764–3776. [Google Scholar] [CrossRef]
- Green, M.J.; Bradley, A.J.; Medley, G.F.; Browne, W.J. Cow, Farm, and Herd Management Factors in the Dry Period Associated with Raised Somatic Cell Counts in Early Lactation. J. Dairy Sci. 2008, 91, 1403–1415. [Google Scholar] [CrossRef]
- Niemi, R.E.; Hovinen, M.; Vilar, M.J.; Simojoki, H.; Rajala-Schultz, P.J. Dry Cow Therapy and Early Lactation Udder Health Problems—Associations and Risk Factors. Prev. Vet. Med. 2021, 188, 105268. [Google Scholar] [CrossRef]
- Rajala-Schultz, P.J.; Hogan, J.S.; Smith, K.L. Short Communication: Association between Milk Yield at Dry-off and Probability of Intramammary Infections at Calving. J. Dairy Sci. 2005, 88, 577–579. [Google Scholar] [CrossRef]
- Dingwell, R.T.; Leslie, K.E.; Schukken, Y.H.; Sargeant, J.M.; Timms, L.L.; Duffield, T.F.; Keefe, G.P.; Kelton, D.F.; Lissemore, K.D.; Conklin, J. Association of Cow and Quarter-Level Factors at Drying-off with New Intramammary Infections during the Dry Period. Prev. Vet. Med. 2004, 63, 75–89. [Google Scholar] [CrossRef]
- Barkema, H.W.; Schukken, Y.H.; Lam, T.J.G.M.; Galligan, D.T.; Beiboer, M.L.; Brand, A. Estimation of Interdependence among Quarters of the Bovine Udder with Subclinical Mastitis and Implications for Analysis. J. Dairy Sci. 1997, 80, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Van Knegsel, A.T.M.; van der Drift, S.G.A.; Čermáková, J.; Kemp, B. Effects of Shortening the Dry Period of Dairy Cows on Milk Production, Energy Balance, Health, and Fertility: A Systematic Review. Vet. J. 2013, 198, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Zecconi, A.; Gusmara, C.; Di Giusto, T.; Cipolla, M.; Marconi, P.; Zanini, L. Observational Study on Application of a Selective Dry-Cow Therapy Protocol Based on Individual Somatic Cell Count Thresholds. Ital. J. Anim. Sci. 2020, 19, 1341–1348. [Google Scholar] [CrossRef]
- Smith, K.L.; Todhunter, D.A.; Schoenberger, P.S. Environmental Mastitis: Cause, Prevalence, Prevention. J. Dairy Sci. 1985, 68, 1531–1553. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.P.; Yang, F.; Luo, J.Y.; Wang, X.R.; Liu, L.H.; Li, H.S. Influences of Season, Parity, Lactation, Udder Area, Milk Yield, and Clinical Symptoms on Intramammary Infection in Dairy Cows. J. Dairy Sci. 2016, 99, 6484–6493. [Google Scholar] [CrossRef]
- Godden, S.; Rapnicki, P.; Stewart, S.; Fetrow, J.; Johnson, A.; Bey, R.; Farnsworth, R. Effectiveness of an Internal Teat Seal in the Prevention of New Intramammary Infections during the Dry and Early-Lactation Periods in Dairy Cows When Used with a Dry Cow Intramammary Antibiotic. J. Dairy Sci. 2003, 86, 3899–3911. [Google Scholar] [CrossRef]
- Ariznabarreta, A.; Gonzalo, C.; San Primitivo, F. Microbiological Quality and Somatic Cell Count of Ewe Milk with Special Reference to Staphylococci. J. Dairy Sci. 2002, 85, 1370–1375. [Google Scholar] [CrossRef]
- Pengov, A. The Role of Coagulase-Negative Staphylococcus Spp. and Associated Somatic Cell Counts in the Ovine Mammary Gland. J. Dairy Sci. 2001, 84, 572–574. [Google Scholar] [CrossRef]
- Buswell, J.F.; Yeoman, G.H. Mastitis in Dry Ewes [Prevention with Cloxacillin, Correspondence]. Vet. Rec. 1976, 99, 221–222. [Google Scholar] [CrossRef]
- Chaffer, M.; Leitner, G.; Zamir, S.; Winkler, M.; Glickman, A.; Ziv, N.; Saran, A. Efficacy of Dry-off Treatment in Sheep. Small Rumin. Res. 2003, 47, 11–16. [Google Scholar] [CrossRef]
- Gonzalo, C.; Tardáguila, J.A.; De La Fuente, L.F.; San Primitivo, F. Effects of Selective and Complete Dry Therapy on Prevalence of Intramammary Infection and on Milk Yield in the Subsequent Lactation in Dairy Ewes. J. Dairy Res. 2004, 71, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Fox, L.K.; Hancock, D.D.; Homer, S.D. Selective Intramammary Antibiotic Therapy during the Nonlactating Period in Goats. Small Rumin. Res. 1992, 9, 313–318. [Google Scholar] [CrossRef]
- Rindsig, R.B.; Rodewald, R.G.; Smith, A.R.; Thomsen, N.K.; Spahr, S.L. Mastitis History, California Mastitis Test, and Somatic Cell Counts for Identifying Cows for Treatment in a Selective Dry Cow Therapy Program. J. Dairy Sci. 1979, 62, 1335–1339. [Google Scholar] [CrossRef] [PubMed]
- Dulinl, A.M.; Paapeh, M.J.; Wergin, W.P. Differentiation and Enumeration of Somatic Cells in Goat Milk. J. Food Prot. 1982, 45, 435–439. [Google Scholar] [CrossRef]
| Bibliographic References | Method | Sensitivity (%) | Specificity (%) |
|---|---|---|---|
| Torres et al. [44] | Three-month SCC recording | ||
| SCC < 200,000 cells/mL and no CM in the last 3 months or <100,000 cells/mL if there has been CM in the last 3 months. SCC < 100,000 cells/mL and no CM in the last 3 months SCC < 200,000 cells/mL and no CM in the last 3 months SCC < 300,000 cells/mL and no CM in the last 3 months | 69.7 (62.9–75.9) 85.1 (79.5–89.6) 71.2 (64.5–77.2) 62.5 (65.5–69.1) | 62.4 (57.7–66.9) 34.6 (30.2–39.3) 50.1 (45.3–54.9) 54.4 (49.7–59.2) | |
| Pantoja et al. [66] | SCC from the last monthly test: | ||
| 50,000 cells/mL 100,000 cells/mL 150,000 cells/mL 200,000 cells/mL 250,000 cells/mL 300,000 cells/mL | 86 63 51 40 34 30 | 40 63 69 80 86 88 | |
| SCC at dry-off | |||
| 50,000 cells/mL 100,000 cells/mL 150,000 cells/mL 200,000 cells/mL 250,000 cells/mL 300,000 cells/mL | 94 88 76 64 51 49 | 37 52 60 66 72 76 | |
| Kiesner et al. [52] | Three-month SCC recording | ||
| SCC < 200,000 cells/mL SCC < 100,000 cells/mL SCC < 100,000 cells/mL + CM SCC < 100,000 cells/mL + parity SCC < 100,000 cells/mL + CMT > 1 | 34.1 (27.8–40.5) 70.5 (64.5–76.7) 72.9 (66.9–78.9) 78.5 (73.0–84.0) 78.5 (73.0–84.0) | 94.4 (87.0–100) 80.5 (67.6–93.4) 78.0 (64.2–91.3) 61.0 (45.2–77.0) 50.0 (33.6–66.3) | |
| SCC from the last monthly test: | |||
| Lipkens et al. [53] | ≥50,000 cells/mL ≥100,000 cells/mL ≥150,000 cells/mL ≥200,000 cells/mL ≥250,000 cells/mL ≥500,000 cells/mL | 86.0 (82.8–89.3) 68.6 (64.3–72.9) 58.1 (53.5–62.7) 41.9 (37.3–46.5) 36.0 (31.6–40.5) 20.9 (17.1–24.7) | 28.7 (24.5–33.0) 52.4 (47.7–57.1) 64.2 (59.8–68.7) 74.4 (70.3–78.4) 79.2 (75.4–82.9) 93.8 (91.6–96.1) |
| Geometric mean of the last 3 monthly SCC tests: | |||
| ≥50,000 cells/mL ≥100,000 cells/mL ≥150,000 cells/mL ≥200,000 cells/mL ≥250,000 cells/mL ≥500,000 cells/mL | 82.4 (78.8–85.9) 67.1 (62.6–71.5) 49.4 (44.7–54.1) 37.6 (33.1–42.4) 32.9 (28.5–37.4) 12.9 (9.8–16.1) | 32.5 (28.1–36.9) 59.5 (54.9–64.1) 71.6 (67.3–75.8) 79.3 (75.5–83.1) 85.3 (82.0–88.7) 95.4 (93.4–97.4) | |
| McDougall et al. [34] | Last SCC result (>108,000 cells/mL) Peak SCC value (>152,000 cells/mL) Mean SCC level (>105,000 cells/mL) | 86 82 76 | 71 74 80 |
| Rowe et al. [31] | >200,000 cells/mL or >2 cases of CM during lactation | 72 (57–84) | 44 (42–47) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ut, I.D.; Berean, D.I.; Bogdan, L.M.; Ciupe, S.; Gog Bogdan, S. Selective Dry Cow Therapy in Modern Dairy Management: Balancing Udder Health and Antimicrobial Stewardship. Vet. Sci. 2025, 12, 580. https://doi.org/10.3390/vetsci12060580
Ut ID, Berean DI, Bogdan LM, Ciupe S, Gog Bogdan S. Selective Dry Cow Therapy in Modern Dairy Management: Balancing Udder Health and Antimicrobial Stewardship. Veterinary Sciences. 2025; 12(6):580. https://doi.org/10.3390/vetsci12060580
Chicago/Turabian StyleUt, Ionela Delia, Daniel Ionut Berean, Liviu Marian Bogdan, Simona Ciupe, and Sidonia Gog Bogdan. 2025. "Selective Dry Cow Therapy in Modern Dairy Management: Balancing Udder Health and Antimicrobial Stewardship" Veterinary Sciences 12, no. 6: 580. https://doi.org/10.3390/vetsci12060580
APA StyleUt, I. D., Berean, D. I., Bogdan, L. M., Ciupe, S., & Gog Bogdan, S. (2025). Selective Dry Cow Therapy in Modern Dairy Management: Balancing Udder Health and Antimicrobial Stewardship. Veterinary Sciences, 12(6), 580. https://doi.org/10.3390/vetsci12060580





