Evaluation of the Immune Effect of a Trivalent Fowl Adenovirus Inactivated Vaccine Against FAdV-4/8a/8b
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Animal Research
2.2. Virus Strains, Cells and Vaccines
2.3. SPF Chicken Embryos, SPF Chickens and LMH Cell
2.4. Routine Project Inspection of Inactivated Vaccines
2.5. Correlative Analysis of Antigens of Epidemic Virus Strains
2.6. Safety Inspection of Trivalent Inactivated Vaccine
2.7. Evaluation of the Immune Effect of Trivalent Inactivated Vaccine
2.8. Antibody Durability Surveillance in Trivalent Inactivated Vaccines
2.9. Statistical Analysis
3. Results
3.1. Virus Quality Control
3.2. Cross Neutralization Test
3.3. Safety Controls of Trivalent Inactivated Vaccine
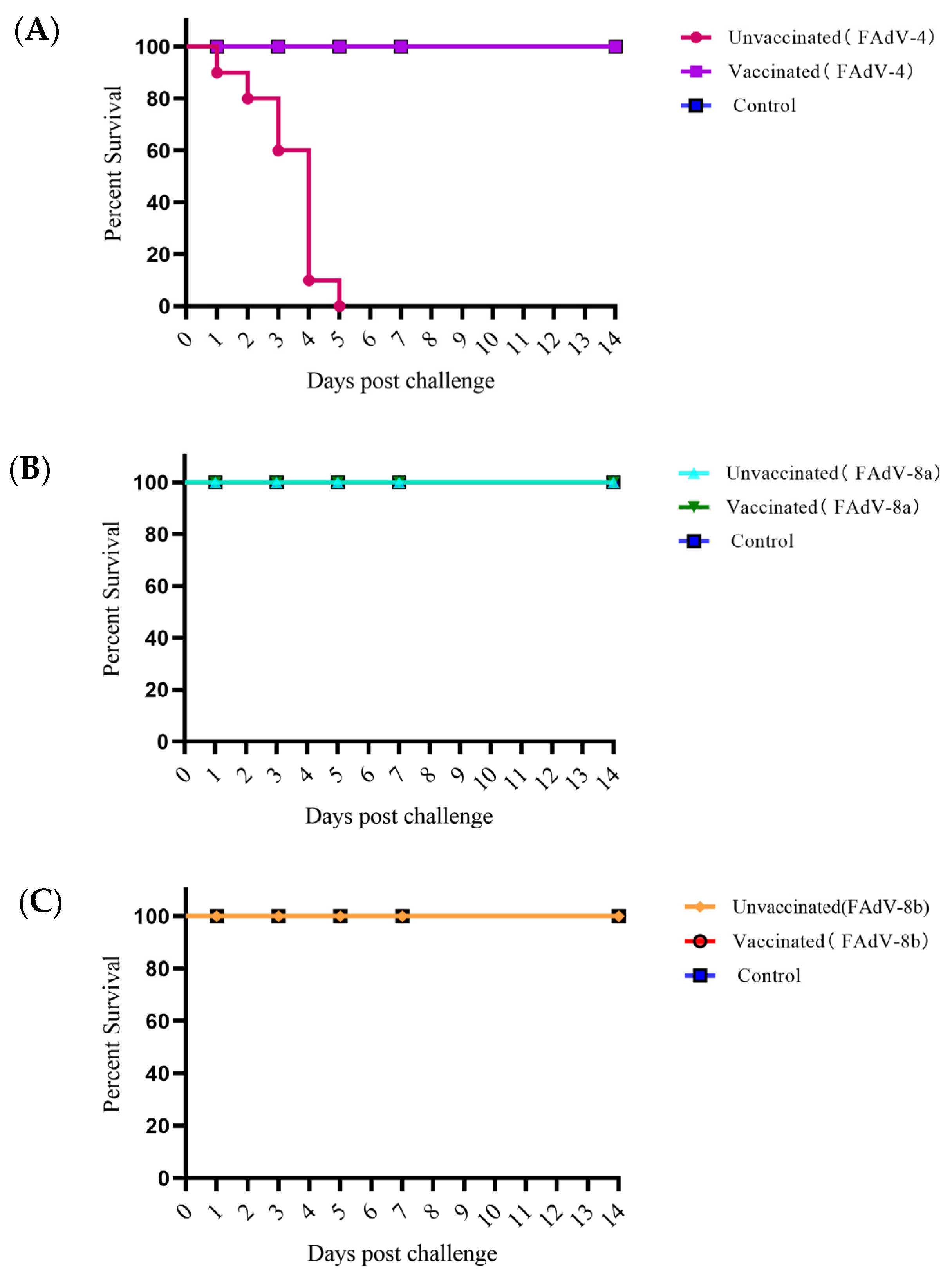
3.4. Immune Effect of the Trivalent Inactivated Vaccine
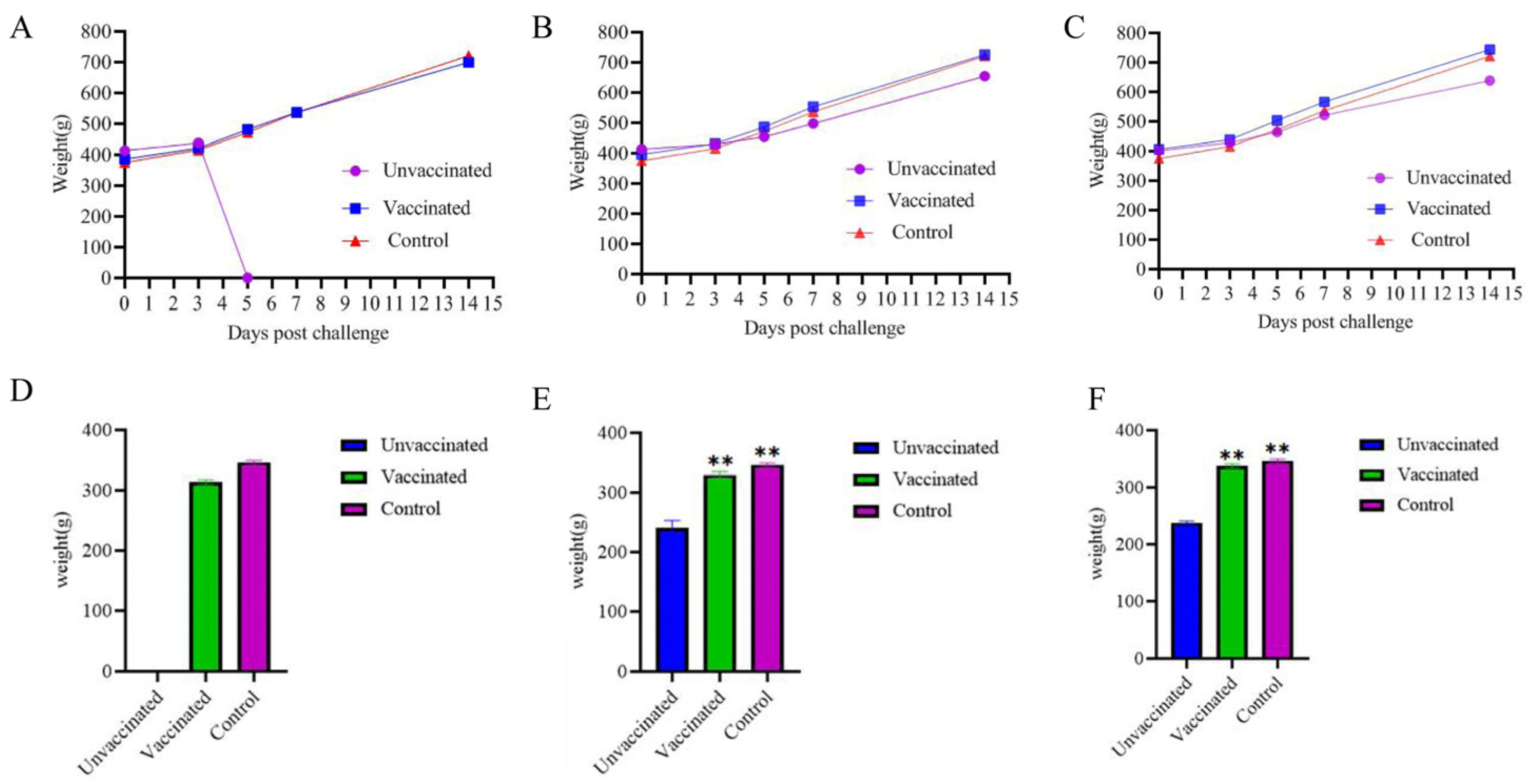
3.4.1. The Body Weight Evolution
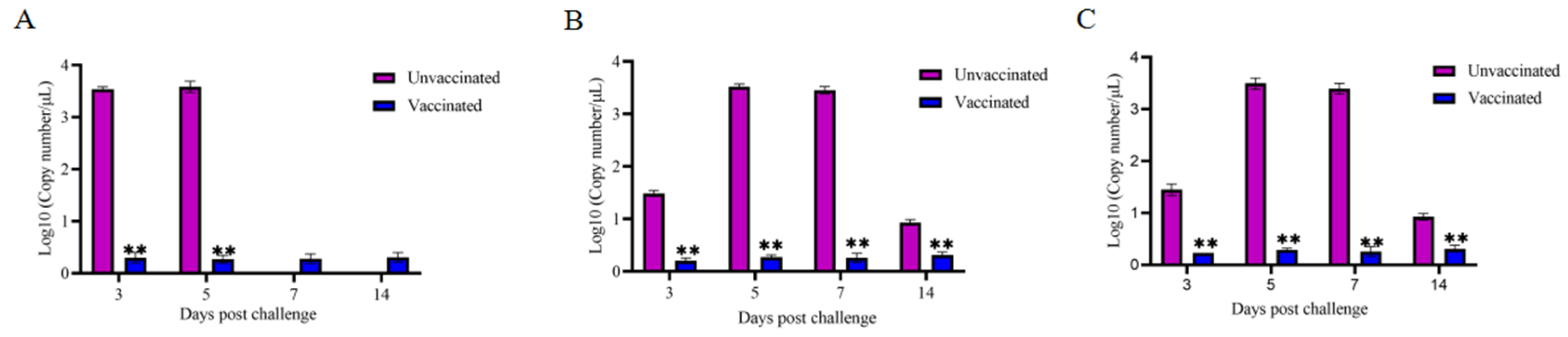
3.4.2. Virus Shedding in Cloacal of Each Group of Chickens
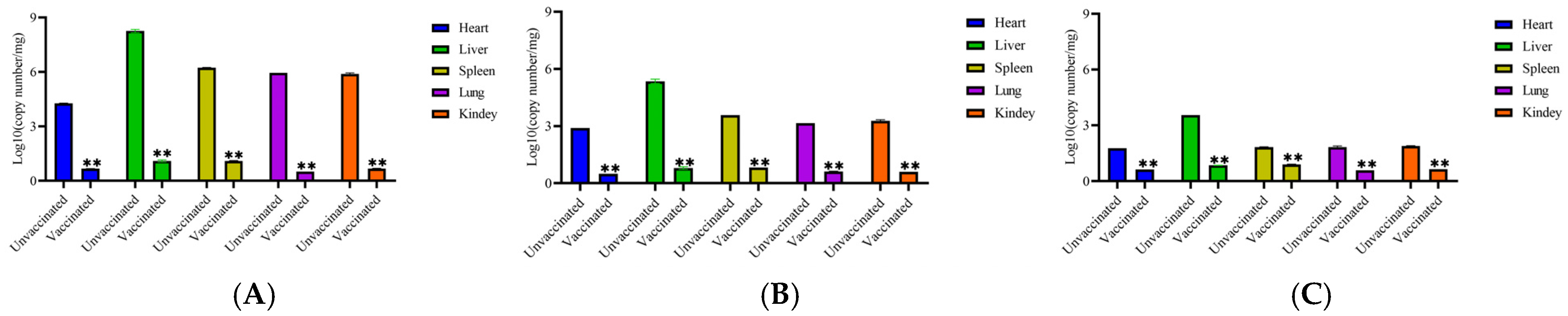
3.4.3. Virus Load in Organs of Each Group of Chickens
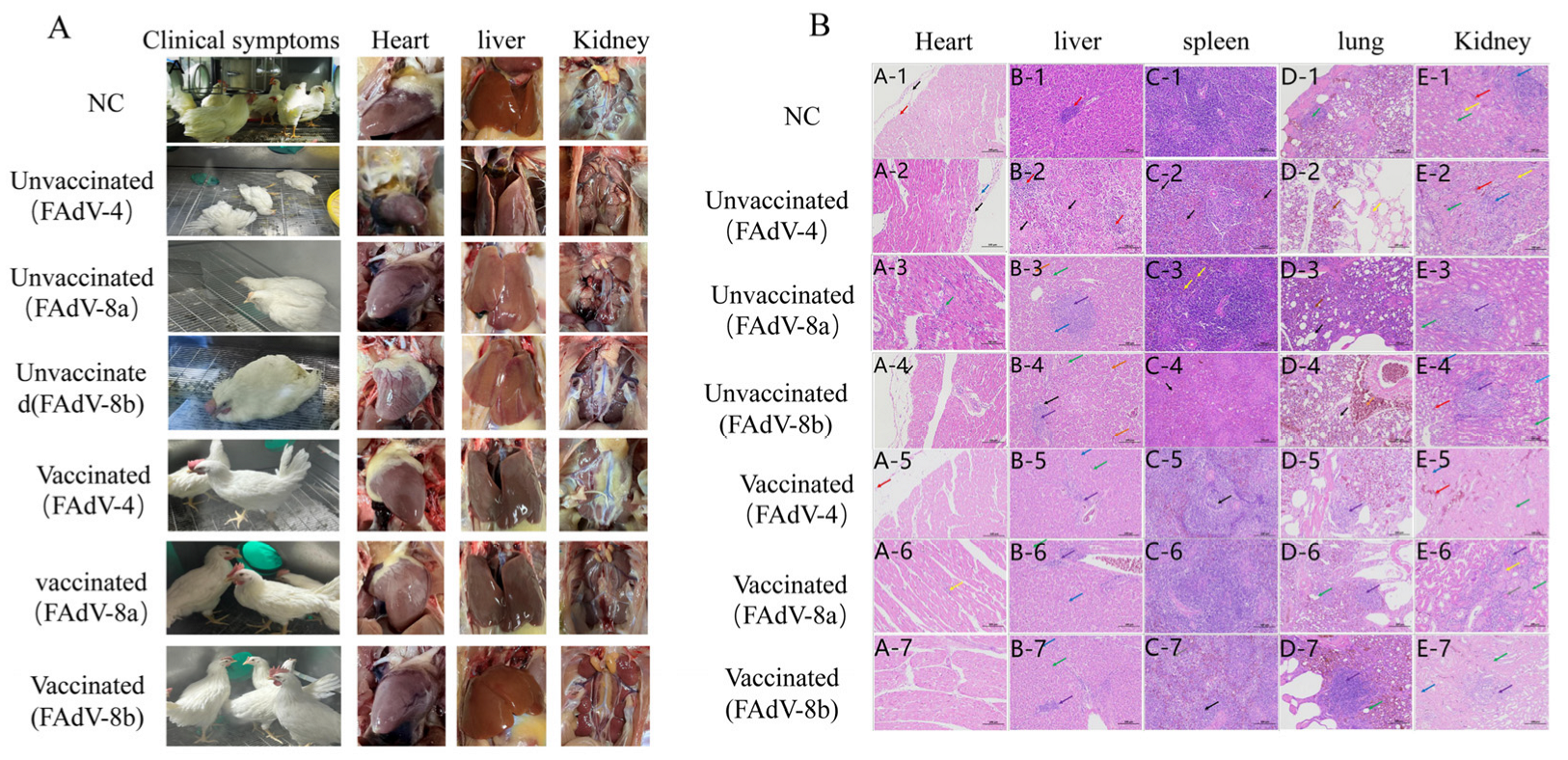
3.4.4. Clinical Symptoms and Gross Lesions
3.4.5. Histopathological Analysis
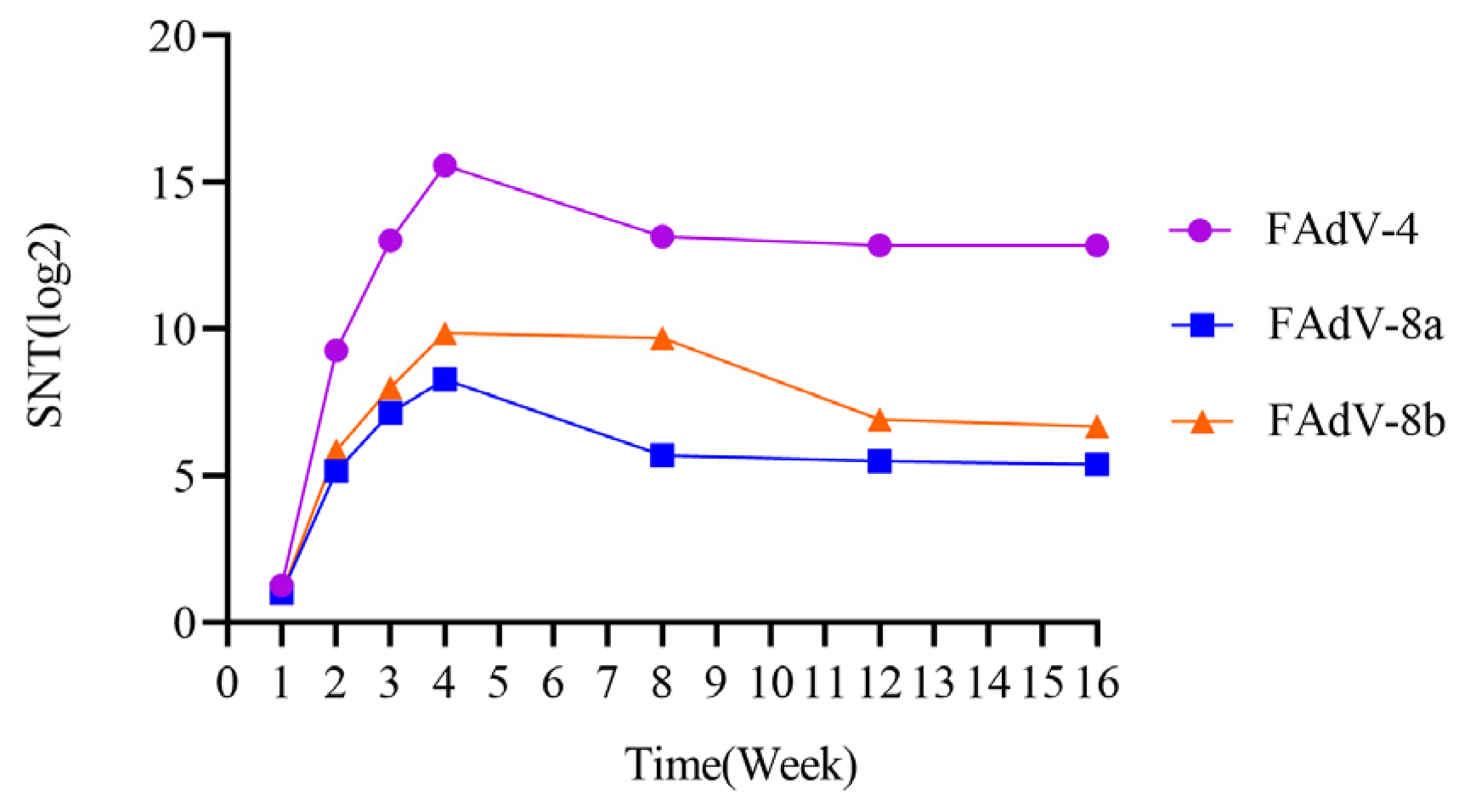
3.5. Antibody Levels in Chicken Immunized with Trivalent Inactivated Vaccine
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adair, B.; Fitzgerald, S.D. Group 1 adenovirus infections. In Diseases of Poultry; John Wiley & Sons: Hoboken, NJ, USA, 2008; Volume 12, pp. 251–266. [Google Scholar]
- Adel, A.; Mohamed, A.A.E.; Samir, M.; Hagag, N.M.; Erfan, A.; Said, M.; Arafa, A.E.S.; Hassan, W.M.M.; El Zowalaty, M.E.; Shahien, M.A. Epidemiological and molecular analysis of circulating fowl adenoviruses and emerging of serotypes 1, 3, and 8b in Egypt. Heliyon 2021, 7, e08366. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.; Ahmad, I. Efficacy of an inactivated vaccine against hydropericardium syndrome in broilers. Vet. Rec. 1990, 126, 59–60. [Google Scholar] [PubMed]
- Alvarado, I.R.; Villegas, P.; El-Attrache, J.; Jensen, E.; Rosales, G.; Perozo, F.; Purvis, L.B. Genetic characterization, pathogenicity, and protection studies with an avian adenovirus isolate associated with inclusion body hepatitis. Avian Dis. 2007, 51, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Changjing, L.; Haiying, L.; Dongdong, W.; Jingjing, W.; Youming, W.; Shouchun, W.; Jida, L.; Ping, L.; Jianlin, W.; Shouzhen, X.; et al. Characterization of fowl adenoviruses isolated between 2007 and 2014 in China. Vet. Microbiol. 2016, 197, 62–67. [Google Scholar] [CrossRef]
- Chen, L.; Yin, L.; Zhou, Q.; Li, Q.; Luo, Y.; Xu, Z.; Zhang, Y.; Xue, C.; Cao, Y. Immunogenicity and protective efficacy of recombinant fiber-2 protein in protecting SPF chickens against fowl adenovirus 4. Vaccine 2018, 36, 1203–1208. [Google Scholar] [CrossRef]
- Chen, L.; Yin, L.; Zhou, Q.; Peng, P.; Du, Y.; Liu, L.; Zhang, Y.; Xue, C.; Cao, Y. Epidemiological investigation of fowl adenovirus infections in poultry in China during 2015–2018. BMC Vet. Res. 2019, 15, 271. [Google Scholar] [CrossRef]
- Chishti, M.A.; Afzal, M.; Cheema, A.H. Preliminary studies on the development of vaccine against the “hydropericardium syndrome” of poultry. Rev. Sci. Tech. 1989, 8, 797–801. [Google Scholar] [CrossRef]
- Csontos, L. Isolation of adenoviruses from geese. Preliminary report. Acta Vet. Acad. Sci. Hung. 1967, 17, 217–219. [Google Scholar]
- De la Torre, D.; Nuñez, L.F.N.; Santander Parra, S.H.; Astolfi-Ferreira, C.S.; Piantino Ferreira, A.J. Molecular characterization of fowl adenovirus group I in commercial broiler chickens in Brazil. Virusdisease 2018, 29, 83–88. [Google Scholar] [CrossRef]
- Gomis, S.; Goodhope, A.R.; Ojkic, A.D.; Willson, P. Inclusion body hepatitis as a primary disease in broilers in Saskatchewan, Canada. Avian Dis. 2006, 50, 550–555. [Google Scholar] [CrossRef]
- Guan, R.; Tian, Y.; Han, X.; Yang, X.; Wang, H. Complete genome sequence and pathogenicity of fowl adenovirus serotype 4 involved in hydropericardium syndrome in Southwest China. Microb. Pathog. 2018, 117, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Günes, A.; Marek, A.; Grafl, B.; Berger, E.; Hess, M. Real-time PCR assay for universal detection and quantitation of all five species of fowl adenoviruses (FAdV-A to FAdV-E). J. Virol. Methods 2012, 183, 147–153. [Google Scholar] [CrossRef]
- Gupta, A.; Popowich, S.; Ojkic, D.; Kurukulasuriya, S.; Chow-Lockerbie, B.; Gunawardana, T.; Goonewardene, K.; Karunarathna, R.; Ayalew, L.E.; Ahmed, K.A.; et al. Inactivated and live bivalent fowl adenovirus (FAdV8b + FAdV11) breeder vaccines provide broad-spectrum protection in chicks against inclusion body hepatitis (IBH). Vaccine 2018, 36, 744–750. [Google Scholar] [CrossRef]
- Kiss, I.; Homonnay, Z.G.; Mató, T.; Bányai, K.; Palya, V. Research Note: An overview on distribution of fowl adenoviruses. Poult. Sci. 2021, 100, 101052. [Google Scholar] [CrossRef]
- Hegde, N.R. Cell culture-based influenza vaccines: A necessary and indispensable investment for the future. Hum. Vaccines Immunother. 2015, 11, 1223–1234. [Google Scholar] [CrossRef]
- Hess, M. Detection and differentiation of avian adenoviruses: A review. Avian Pathol. 2000, 29, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Jackwood, D.J. Multivalent virus-like-particle vaccine protects against classic and variant infectious bursal disease viruses. Avian Dis. 2013, 57, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Shi, W.; Li, Y.J.; Yu, H.; Zhao, Q.; Zhao, Y.; Liu, Y. Prevalence and pathogenicity of Group I avian adenovirus in yellow chickens from 2017 to 2022 in China. Heilongjiang Anim. Husb. Vet. Med. 2024, 22, 75–84+133–134. [Google Scholar]
- Kataria, J.M.; Dhama, K.; Nagarajan, S.; Chakraborty, S.; Kaushal, A.; Deb, R. Fowl adenoviruses causing hydropericardium syndrome in poultry. Adv. Anim. Vet. Sci. 2013, 1, 5–13. [Google Scholar]
- Kim, M.S.; Lim, T.H.; Lee, D.H.; Youn, H.N.; Yuk, S.S.; Kim, B.Y.; Choi, S.W.; Jung, C.H.; Han, J.H.; Song, C.S. An inactivated oil-emulsion fowl Adenovirus serotype 4 vaccine provides broad cross-protection against various serotypes of fowl Adenovirus. Vaccine 2014, 32, 3564–3568. [Google Scholar] [CrossRef]
- Li, P.H.; Zheng, P.P.; Zhang, T.F.; Wen, G.Y.; Shao, H.B.; Luo, Q.P. Fowl adenovirus serotype 4: Epidemiology, pathogenesis, diagnostic detection, and vaccine strategies. Poult. Sci. 2017, 96, 2630–2640. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Lu, H.; Wang, K.; Shao, H.; Mei, N.; Ye, J.Q.; Chen, H.J. Emerging of a novel natural recombinant fowl adenovirus in China. Transbound. Emerg. Dis. 2021, 68, 283–288. [Google Scholar] [CrossRef]
- Maartens, L.H.; Joubert, H.W.; Aitchison, H.; Venter, E.H. Inclusion body hepatitis associated with an outbreak of fowl adenovirus type 2 and type 8b in broiler flocks in South Africa. J. S. Afr. Vet. Assoc. 2014, 85, e1–e5. [Google Scholar] [CrossRef] [PubMed]
- Mase, M.; Mitake, H.; Inoue, T.; Imada, T. Identification of group I–III avian adenovirus by PCR coupled with direct sequencing of the hexon gene. J. Vet. Med. Sci. 2009, 71, 1239–1242. [Google Scholar] [CrossRef]
- Mazaheri, A.; Prusas, C.; Voss, M.; Hess, M. Some strains of serotype 4 fowl adenoviruses cause inclusion body hepatitis and hydropericardium syndrome in chickens. Avian Pathol. 1998, 27, 269–276. [Google Scholar] [CrossRef] [PubMed]
- McFerran, J.B.; Connor, T.J. Further studies on the classification of fowl adenoviruses. Avian Dis. 1977, 21, 585–595. [Google Scholar] [CrossRef]
- Chinese Veterinary Pharmacopoeia Commission. Veterinary Pharmacopoeia of the People’s Republic of China, 2020 ed.; China Agriculture Press: Beijing, China, 2020; Volume III, ISBN 978-7-109-27588-1. [Google Scholar]
- Meulemans, G.; Couvreur, B.; Decaesstecker, M.; Boschmans, M.; van den Berg, T.P. Phylogenetic analysis of fowl adenoviruses. Avian Pathol. 2004, 33, 164–170. [Google Scholar] [CrossRef]
- Niu, D.; Feng, J.; Duan, B.; Shi, Q.; Li, Y.; Chen, Z.; Ma, L.; Liu, H.; Wang, Y. Corrigendum to “Epidemiological survey of avian adenovirus in China from 2015 to 2021 and the genetic variability of highly pathogenic Fadv-4 isolates [Infection, Genetics and Evolution, volume 101 (2022), article number 105277]. Infect. Genet. Evol. 2022, 103, 105331. [Google Scholar] [CrossRef]
- Niu, Y.; Sun, Q.; Liu, X.; Liu, S. Mechanism of fowl adenovirus serotype 4-induced heart damage and formation of pericardial effusion. Poult. Sci. 2019, 98, 1134–1145. [Google Scholar] [CrossRef]
- Niu, Y.; Sun, Q.; Zhang, G.; Sun, W.; Liu, X.; Xiao, Y.; Shang, Y.; Liu, S. Epidemiological investigation of outbreaks of fowl adenovirus infections in commercial chickens in China. Transbound. Emerg. Dis. 2018, 65, e121–e126. [Google Scholar] [CrossRef]
- Niu, Y.J.; Sun, W.; Zhang, G.H.; Qu, Y.J.; Wang, P.F.; Sun, H.L.; Xiao, Y.H.; Liu, S.D. Hydropericardium syndrome outbreak caused by fowl adenovirus serotype 4 in China in 2015. J. Gen. Virol. 2016, 97, 2684–2690. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Wang, J.; Gao, Y.; Cui, H.; Liu, C.; Qi, X.; Zhang, Y.; Wang, Y.; Wang, X. The Natural Large Genomic Deletion Is Unrelated to the Increased Virulence of the Novel Genotype Fowl Adenovirus 4 Recently Emerged in China. Viruses 2018, 10, 494. [Google Scholar] [CrossRef]
- Roy, P.; Koteeswaran, A.; Manickam, R. Efficacy of an inactivated oil emulsion vaccine against hydropericardium syndrome in broilers. Vet. Rec. 1999, 145, 458–459. [Google Scholar] [CrossRef]
- Ruan, S.; Zhao, J.; Yin, X.; He, Z.; Zhang, G. A subunit vaccine based on fiber-2 protein provides full protection against fowl adenovirus serotype 4 and induces quicker and stronger immune responses than an inactivated oil-emulsion vaccine. Infect. Genet. Evol. 2018, 61, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Schachner, A.; Grafl, B.; Hess, M. Spotlight on avian pathology: Fowl adenovirus (FAdV) in chickens and beyond—An unresolved host-pathogen interplay. Avian Pathol. 2021, 50, 2–5. [Google Scholar] [CrossRef]
- Schachner, A.; Marek, A.; Grafl, B.; Hess, M. Detailed molecular analyses of the hexon loop-1 and fibers of fowl aviadenoviruses reveal new insights into the antigenic relationship and confirm that specific genotypes are involved in field outbreaks of inclusion body hepatitis. Vet. Microbiol. 2016, 186, 13–20. [Google Scholar] [CrossRef]
- Schachner, A.; Matos, M.; Grafl, B.; Hess, M. Fowl adenovirus-induced diseases and strategies for their control—A review on the current global situation. Avian Pathol. 2018, 47, 111–126. [Google Scholar] [CrossRef]
- Schachner, A.; Marek, A.; Jaskulska, B.; Bilic, I.; Hess, M. Recombinant FAdV-4 fiber-2 protein protects chickens against hepatitis-hydropericardium syndrome (HHS). Vaccine 2014, 32, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Schonewille, E.; Jaspers, R.; Paul, G.; Hess, M. Specific-pathogen-free chickens vaccinated with a live FAdV-4 vaccine are fully protected against a severe challenge even in the absence of neutralizing antibodies. Avian Dis. 2010, 54, 905–910. [Google Scholar] [CrossRef]
- Shah, M.; Ashraf, A.; Khan, M.; Rahman, M.; Habib, M.; Chughtai, M.; Qureshi, J. Fowl adenovirus: History, emergence, biology and development of a vaccine against hydropericardium syndrome. Arch. Virol. 2017, 162, 1833–1843. [Google Scholar] [CrossRef]
- Steer-Cope, P.A.; Sandy, J.R.; O’Rourke, D.; Scott, P.C.; Browning, G.F.; Noormohammadi, A.H. Vaccination with FAdV-8a induces protection against inclusion body hepatitis caused by homologous and heterologous strains. Avian Pathol. 2019, 48, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Steer, P.A.; O’Rourke, D.; Ghorashi, S.A.; Noormohammadi, A.H. Application of high-resolution melting curve analysis for typing of fowl adenoviruses in field cases of inclusion body hepatitis. Aust. Vet. J. 2011, 89, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Steer, P.A.; Sandy, J.R.; O’Rourke, D.; Scott, P.C.; Browning, G.F.; Noormohammadi, A.H. Chronological analysis of gross and histological lesions induced by field strains of fowl adenovirus serotypes 1, 8b and 11 in one-day-old chickens. Avian Pathol. 2015, 44, 106–113. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Y.; Gao, S.; Yang, J.; Tang, Y.; Diao, Y. Pathogenicity of fowl adenovirus serotype 4 (FAdV-4) in chickens. Infect. Genet. Evol. 2019, 75, 104017. [Google Scholar] [CrossRef]
- Sun, Q.; Li, Y.; Huang, Y.; Li, S.; Fu, Q.; Liu, S. FAdV-4 can cause more noticeable clinical symptoms compared to FAdV-8b after infecting specific pathogen free chickens. Poult. Sci. 2024, 103, 104006. [Google Scholar] [CrossRef]
- Swayne, D.E. Diseases of Poultry; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Vemula, S.V.; Ahi, Y.S.; Swaim, A.M.; Katz, J.M.; Donis, R.; Sambhara, S.; Mittal, S.K. Broadly protective adenovirus-based multivalent vaccines against highly pathogenic avian influenza viruses for pandemic preparedness. PLoS ONE 2013, 8, e62496. [Google Scholar] [CrossRef]
- Wang, B.; Song, M.; Song, C.; Zhao, S.; Yang, P.; Qiao, Q.; Cong, Y.; Wang, Y.; Wang, Z.; Zhao, J. An inactivated novel chimeric FAdV-4 containing fiber of FAdV-8b provides full protection against hepatitis-hydropericardium syndrome and inclusion body hepatitis. Vet. Res. 2022, 53, 75. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Yao, K.C.; Liu, Y.Y.; You, G.J.; Li, S.Y.; Liu, P.; Zhao, Q.; Wen Rui Wu, Y.P.; Huang, X.B.; Cao, S.J.; et al. Isolation and molecular characterization of prevalent Fowl adenovirus strains in southwestern China during 2015–2016 for the development of a control strategy. Emerg. Microbes Infect. 2017, 6, e103. [Google Scholar] [CrossRef]
- Xie, Q.; Cao, S.; Zhang, W.; Wang, W.; Li, L.; Kan, Q.; Fu, H.; Geng, T.; Li, T.; Wan, Z.; et al. A novel fiber-2-edited live attenuated vaccine candidate against the highly pathogenic serotype 4 fowl adenovirus. Vet. Res. 2021, 52, 35. [Google Scholar] [CrossRef]
- Zhang, T.; Jin, Q.; Ding, P.; Wang, Y.; Chai, Y.; Li, Y.; Liu, X.; Luo, J.; Zhang, G. Molecular epidemiology of hydropericardium syndrome outbreak-associated serotype 4 fowl adenovirus isolates in central China. Virol. J. 2016, 13, 188. [Google Scholar] [CrossRef]
- Zhao, J.; Zhong, Q.; Zhao, Y.; Hu, Y.X.; Zhang, G.Z. Pathogenicity and Complete Genome Characterization of Fowl Adenoviruses Isolated from Chickens Associated with Inclusion Body Hepatitis and Hydropericardium Syndrome in China. PLoS ONE 2015, 10, e0133073. [Google Scholar] [CrossRef] [PubMed]
- Grimes, T.M.; King, D.J. Effect of maternal antibody on experimental infections of chickens with a type-8 avian adenovirus. Avian Dis. 1977, 21, 97–112. [Google Scholar] [CrossRef] [PubMed]

| Groups | Virulent Strain | Virus Preparation | Vaccine Aqueous Phase Preparation | Preparation of Vaccine Oil Phase | Emulsify and Aliquot the Vaccine |
|---|---|---|---|---|---|
| trivalent fowl adenovirus inactivated vaccine against FAdV-4/8a/8b | FAdV-4 (107.0TCID50/mL) FAdV-8a (107.0TCID50/mL) FAdV-8b (107.0TCID50/mL) | The viral suspensions of FAdV-4, FAdV-8a, and FAdV-8b were separately filtered, concentrated threefold, and inactivated with 2% formaldehyde at 37 °C for 24 h, then mixed thoroughly in a 1:1:1 ratio. | Ninety-six parts by volume of inactivation-qualified viral suspension were mixed with 4 parts by volume of sterile Tween-80 in an emulsification tank, followed by agitation until complete dissolution of Tween-80. | Ninety-six parts by volume of mineral oil and 6 parts by volume of Span-80 were homogenized until transparent, sterilized by autoclaving at 121 °C for 30 min, and cooled for storage. | The inactivated vaccine was prepared by emulsifying the aqueous phase and oil phase in a 1:3 ratio, and then stored at 2–8 °C. |
| fowl adenovirus inactivated vaccine against FAdV-4 | FAdV-4 (107.0TCID50/mL) | The FAdV-4 viral suspension was filtered, inactivated with 2% formaldehyde at 37 °C for 24 h. | |||
| fowl adenovirus inactivated vaccine against FAdV8a | FAdV-8a (107.0TCID50/mL) | The FAdV-8a viral suspension was filtered, inactivated with 2% formaldehyde at 37 °C for 24 h. | |||
| fowl adenovirus inactivated vaccine against FAdV8b | FAdV-8b (107.0TCID50/mL) | The FAdV-8b viral suspension was filtered, inactivated with 2% formaldehyde at 37 °C for 24 h. |
| Group | Test Animal | Quantity | Vaccine Immunity | Criterion |
|---|---|---|---|---|
| Vaccine immunization group | 14 days SPF chickens | 10 | Trivalent inactivated vaccine prepared by subcutaneous injection into the neck, 1.0 mL/chicken | After 21 days of continuous observation, there was no redness, swelling, necrosis and vaccine residue at the injection site |
| blank control group | 10 | Sterile white oil was injected subcutaneously into the neck, 1.0 mL/chicken |
| Group | Test Animal | Quantity | Vaccine Immunity | Challenge Experiment | Weigh and Take Cloacal Swabs | Pathological Observation |
|---|---|---|---|---|---|---|
| Vaccine immunization group 1 | 21 days SPF chickens | 10 | Trivalent inactivated vaccine prepared by subcutaneous injection into the neck, 0.3 mL/chicken | FAdV-4 strain (RP-C4) | 3d, 5d, 7d, and 14d after challenge | After 14 days of challenge, all the chickens were dissected, and the gross lesions of the tissues were observed, and the histopathology and viral load were observed in the heart, liver, spleen, lung, and kidney |
| Vaccine immunization group 2 | FAdV-8a strain (RP-CA) | |||||
| Vaccine immunization group 3 | FAdV-8b strain (RP-CB) | |||||
| Challenge control group 4 | - | FAdV-4 strain (RP-C4) | ||||
| Challenge control group 5 | FAdV-8a strain (RP-CA) | |||||
| Challenge control group 6 | FAdV-8b strain (RP-CB) | |||||
| blank control group | - | - |
| Primers | Nucleotide Sequence (5′ → 3′) |
|---|---|
| FAV-52K-FW | ATGGCKCAGATGGCYAAGG |
| FAV-52K-RV | AGCGCCTGGGTCAAACCGA |
| Antigen | Serum | ||
|---|---|---|---|
| FAV-4 | FAV-8a | FAV-8b | |
| Neutralizing Antibody Titer (Log2) | |||
| FAV-4 | 5.0 | 0.0 | 0.0 |
| FAV-8a | 0.0 | 7.0 | 2.0 |
| FAV-8b | 0.0 | 2.0 | 12.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, Y.; Zhao, Q.; Zhao, Y.; Li, Y.; Pan, S.; Li, Y.; Liu, Y.; Shi, W. Evaluation of the Immune Effect of a Trivalent Fowl Adenovirus Inactivated Vaccine Against FAdV-4/8a/8b. Vet. Sci. 2025, 12, 549. https://doi.org/10.3390/vetsci12060549
Jiao Y, Zhao Q, Zhao Y, Li Y, Pan S, Li Y, Liu Y, Shi W. Evaluation of the Immune Effect of a Trivalent Fowl Adenovirus Inactivated Vaccine Against FAdV-4/8a/8b. Veterinary Sciences. 2025; 12(6):549. https://doi.org/10.3390/vetsci12060549
Chicago/Turabian StyleJiao, Yulan, Qianhui Zhao, Yulong Zhao, Yingjie Li, Sumin Pan, Yinming Li, Yuntao Liu, and Wanyu Shi. 2025. "Evaluation of the Immune Effect of a Trivalent Fowl Adenovirus Inactivated Vaccine Against FAdV-4/8a/8b" Veterinary Sciences 12, no. 6: 549. https://doi.org/10.3390/vetsci12060549
APA StyleJiao, Y., Zhao, Q., Zhao, Y., Li, Y., Pan, S., Li, Y., Liu, Y., & Shi, W. (2025). Evaluation of the Immune Effect of a Trivalent Fowl Adenovirus Inactivated Vaccine Against FAdV-4/8a/8b. Veterinary Sciences, 12(6), 549. https://doi.org/10.3390/vetsci12060549





