Evaluation of the Antileishmanial Activity of Some Benzimidazole Derivatives Using In Vitro and In Silico Techniques
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
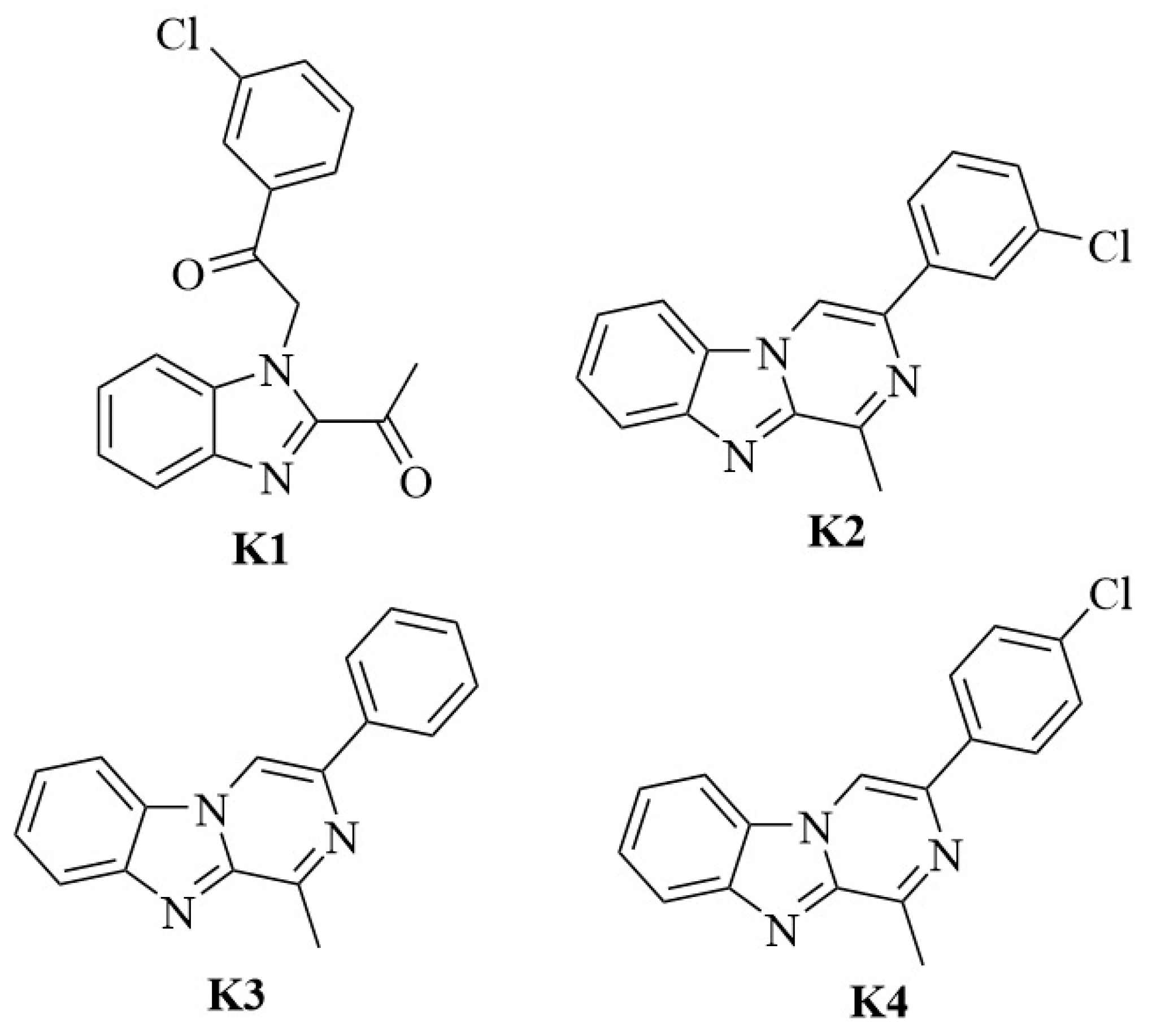
2.1. Chemistry
2.2. Cultivations of the L. major Isolates
2.3. Determination of In Vitro Antileishmanial Activity
2.4. Cytotoxicity Tests
2.5. Selectivity Index
2.6. Molecular Docking and Molecular Dynamics Simulation Studies
3. Results and Discussions
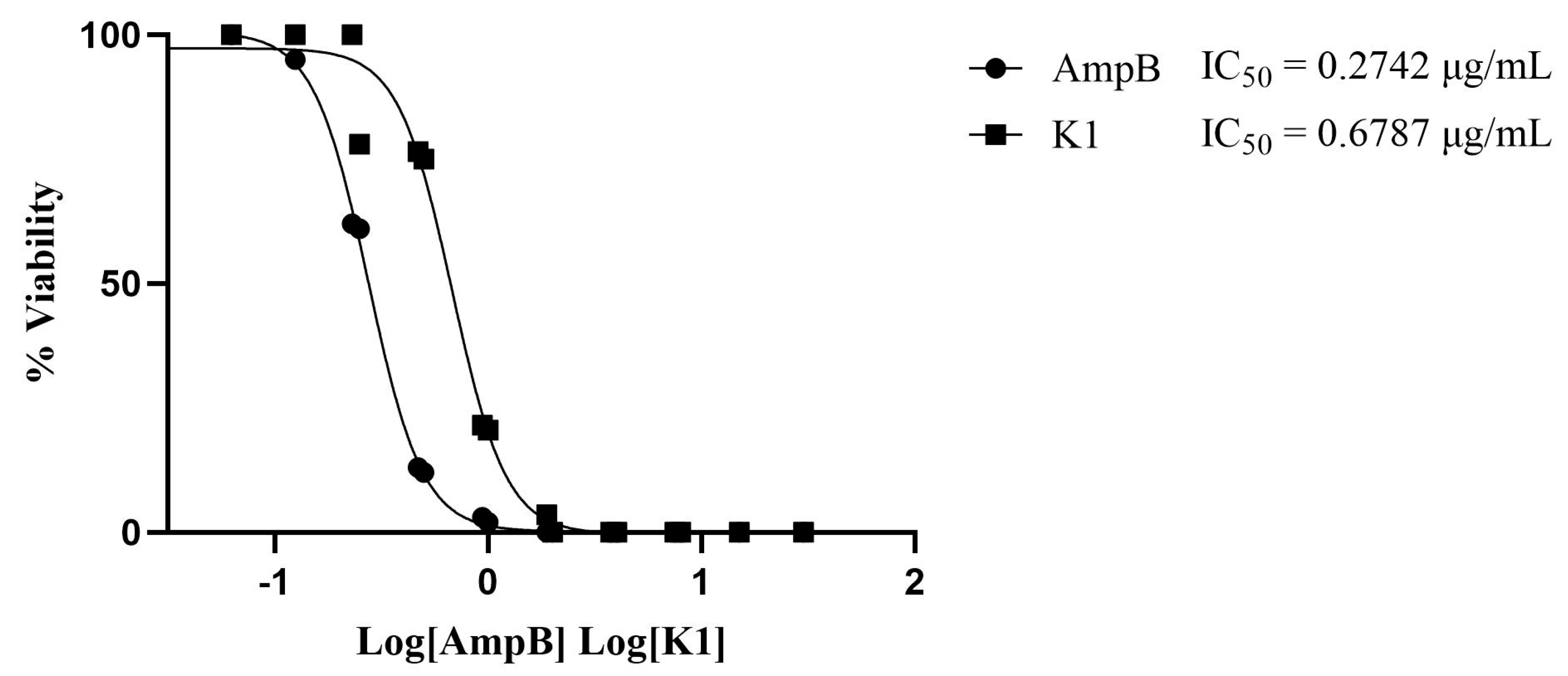
3.1. Antileishmanial Activity
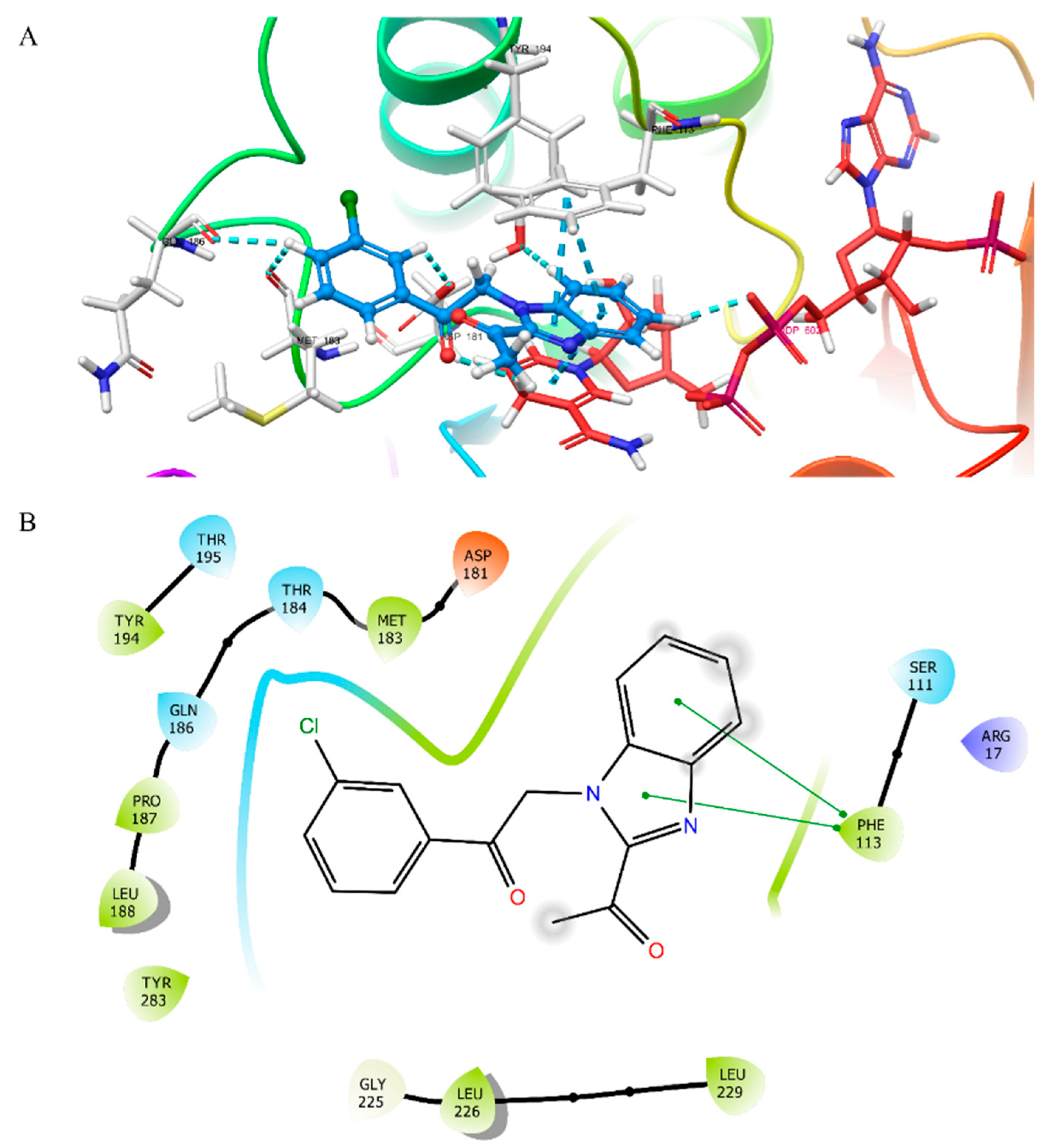
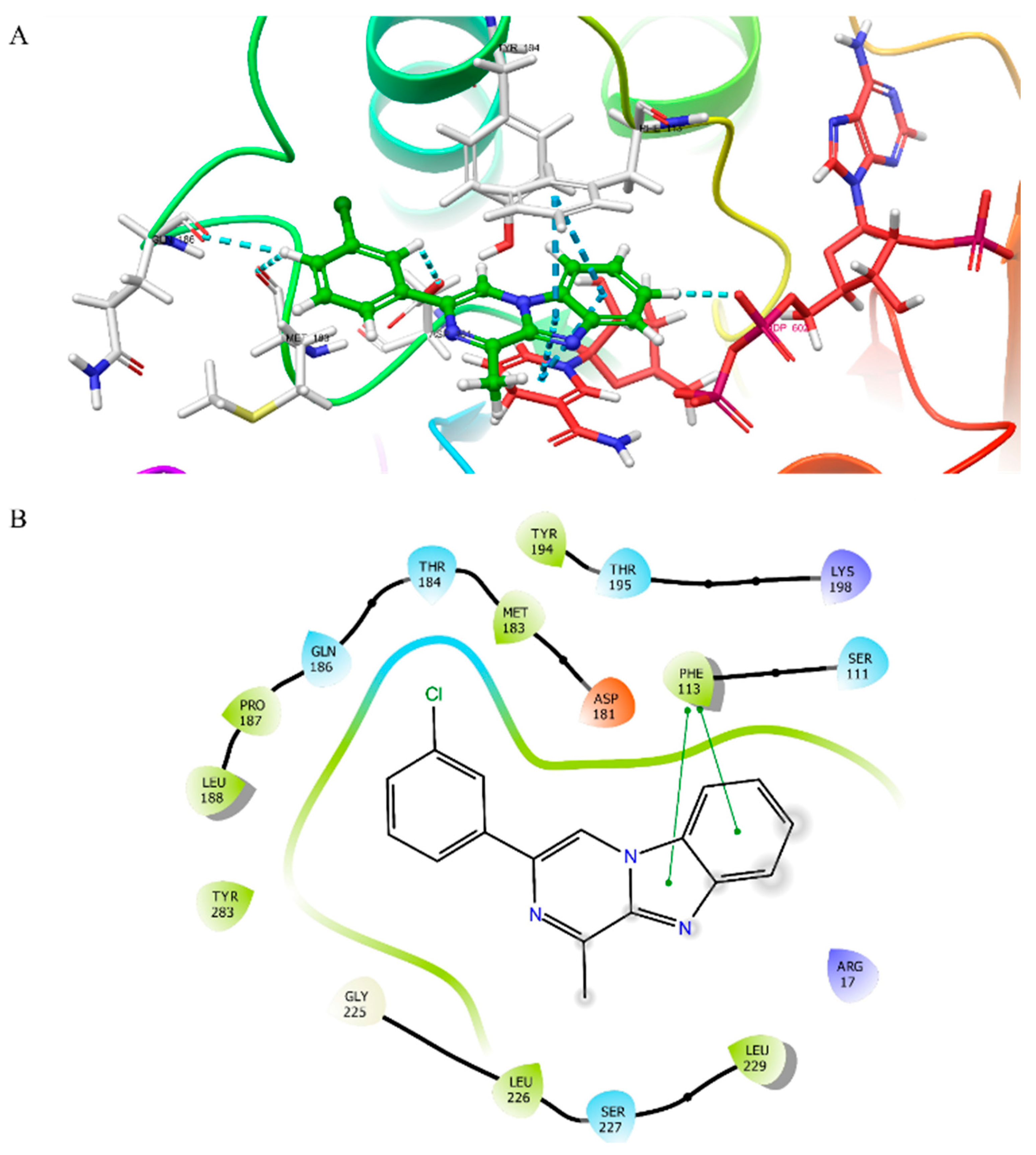
3.2. Molecular Docking Studies
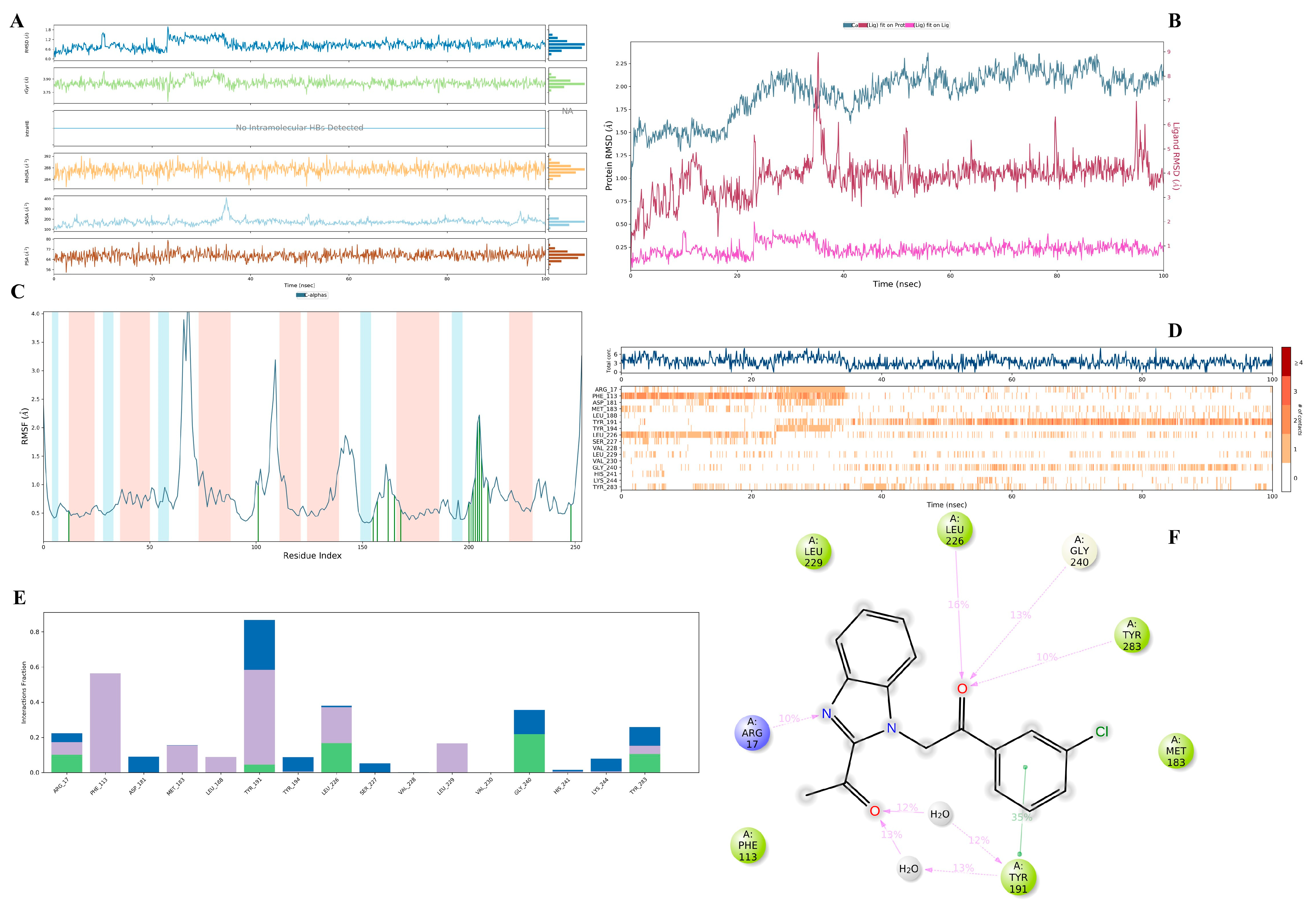
3.3. Molecular Dynamics Simulation Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Leishmaniasis. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 27 January 2025).
- World Health Organization (WHO). Fourteenth Meeting of the Strategic and Technical Advisory Group for Neglected Tropical Diseases, 22–24 June 2021; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Cosma, C.; Maia, C.; Khan, N.; Infantino, M.; Del Riccio, M. Leishmaniasis in Humans and Animals: A One Health Approach for Surveillance, Prevention and Control in a Changing World. Trop. Med. Infect. Dis. 2024, 9, 258. [Google Scholar] [CrossRef] [PubMed]
- Amela, C.; López-Gay, D.; Alberdig, J.C.; Castilla, J. Injecting drug use as risk factor for visceral leishmaniasis in AIDS patients. Eur. J. Epidemiol. 1996, 12, 91–92. [Google Scholar] [CrossRef] [PubMed]
- Meinecke, C.K.; Schottelius, J.; Oskam, L.; Fleischer, B. Congenital transmission of visceral leishmaniasis (Kala Azar) from an asymptomatic mother to her child. Pediatrics 1999, 104, e65. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Singh, S. Transfusion transmitted leishmaniasis: A case report and review of literature. Indian J. Med. Microbiol. 2006, 24, 165–170. [Google Scholar] [CrossRef]
- Akhoundi, M.; Kuhls, K.; Cannet, A.; Votýpka, J.; Marty, P.; Delaunay, P.; Sereno, D. A historical overview of the classification, evolution, and dispersion of Leishmania parasites and sandflies. PLoS Neglected Trop. Dis. 2016, 10, e0004349. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Lorusso, V.; Testini, G.; de Paiva-Cavalcanti, M.; Figueredo, L.A.; Stanneck, D.; Otranto, D. Detection of Leishmania infantum in Rhipicephalus sanguineus ticks from Brazil and Italy. Parasitol. Res. 2010, 106, 857–860. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Rossi, L.; Scroccaro, A.M.; Montarsi, F.; Caldin, M.; Furlanello, T.; Trotta, M. Detection of Leishmania infantum DNA mainly in Rhipicephalus sanguineus male ticks removed from dogs living in endemic areas of canine leishmaniosis. Parasites Vectors 2012, 5, 98. [Google Scholar] [CrossRef]
- Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. Leishmaniasis: A review. F1000Research 2017, 6, 750. [Google Scholar] [CrossRef]
- Kato, H. Epidemiology of Leishmaniasis: Risk factors for its pathology and infection. Parasitol. Int. 2025, 105, 102999. [Google Scholar] [CrossRef]
- Del Giudice, P.; Marty, P.; Lacour, J.P.; Perrin, C.; Pratlong, F.; Haas, H.; Dellamonica, P.; Le Fichoux, Y. Cutaneous leishmaniasis due to Leishmania infantum: Case reports and literature review. Arch. Dermatol. 1998, 134, 193–198. [Google Scholar] [CrossRef]
- De Silva, N.L.; De Silva, V.N.H.; Deerasinghe, A.T.H.; Rathnapala, U.L.; Itoh, M.; Takagi, H.; Weerasooriya, M.V.; Kato, H.; Yahathugoda, T.C. Development of a highly sensitive nested PCR and its application for the diagnosis of cutaneous leishmaniasis in Sri Lanka. Microorganisms 2022, 10, 990. [Google Scholar] [CrossRef] [PubMed]
- El Mazini, S.; Ejghal, R.; Bekhti, K.; Lemrani, M. The Sporadic cutaneous leishmaniasis due to Leishmania infantum in Morocco: A presumably trend towards endemicity. Acta Trop. 2022, 227, 106288. [Google Scholar] [CrossRef]
- Yadav, P.; Azam, M.; Ramesh, V.; Singh, R. Unusual observations in leishmaniasis-an overview. Pathogens 2023, 12, 297. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Available online: https://www.who.int/data/gho/data/themes/topics/gho-ntd-leishmaniasis (accessed on 28 January 2025).
- Shmueli, M.; Ben-Shimol, S. Review of Leishmaniasis Treatment: Can We See the Forest through the Trees? Pharmacy 2024, 12, 30. [Google Scholar] [CrossRef]
- Sundar, S.; Sinha, P.R.; Agrawal, N.K.; Srivastava, R.; Rainey, P.M.; Berman, J.D.; Murray, H.W.; Singh, V.P. A cluster of cases of severe cardiotoxicity among kala-azar patients treated with a high-osmolarity lot of sodium antimony gluconate. Am. J. Trop. Med. Hyg. 1998, 59, 139–143. [Google Scholar] [CrossRef]
- Gasser, R.A., Jr.; Magill, A.J.; Oster, C.N.; Franke, E.D.; Grögl, M.; Berman, J.D. Pancreatitis induced by pentavalent antimonial agents during treatment of leishmaniasis. Clin. Infect. Dis. 1994, 18, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Thakur, C.P.; Sinha, G.P.; Pandey, A.K.; Kumar, N.; Kumar, P.; Hassan, S.M.; Narain, S.; Roy, R.K. Do the diminishing efficacy and increasing toxicity of sodium stibogluconate in the treatment of visceral leishmaniasis in Bihar, India, justify its continued use as a first-line drug? An observational study of 80 cases. Ann. Trop. Med. Parasitol. 1998, 92, 561–569. [Google Scholar] [CrossRef]
- Pradhan, S.; Schwartz, R.A.; Patil, A.; Grabbe, S.; Goldust, M. Treatment options for leishmaniasis. Clin. Exp. Dermatol. 2022, 47, 516–521. [Google Scholar] [CrossRef]
- Barazorda-Ccahuana, H.L.; Goyzueta-Mamani, L.D.; Puma, M.A.C.; de Freitas, C.S.; Tavares, G.D.S.V.; Lage, D.P.; Coelho, E.A.F.; Chávez-Fumagalli, M.A. Computer-aided drug design approaches applied to screen natural product’s structural analogs targeting arginase in Leishmania spp. F1000Research 2023, 12, 93. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, R.; Liu, Y.; Yu, M.; He, Z.; Xiao, J.; Li, K.; Liu, G.; Ning, Q.; Li, Y. Progress in antileishmanial drugs: Mechanisms, challenges, and prospects. PLoS Neglected Trop. Dis. 2025, 19, e0012735. [Google Scholar] [CrossRef]
- Altamura, F.; Rajesh, R.; Catta-Preta, C.M.; Moretti, N.S.; Cestari, I. The current drug discovery landscape for trypanosomiasis and leishmaniasis: Challenges and strategies to identify drug targets. Drug Dev. Res. 2022, 83, 225–252. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Hawkins, B.A.; Du, J.J.; Groundwater, P.W.; Hibbs, D.E.; Lai, F. A Guide to In Silico Drug Design. Pharmaceutics 2022, 15, 49. [Google Scholar] [CrossRef] [PubMed]
- Prates, L.V.; Silvia, S.C.L.A.; Scotti, L.; Fechine, T.J.; Maria, B.F.J.; Keesen de Souza Lima, T.; Da Camara, R.J.; Tullius, S.M. Structure-and ligand-based approaches to evaluate aporphynic alkaloids from annonaceae as multi-target agent against Leishmania donovani. Curr. Pharm. Des. 2016, 22, 5196–5203. [Google Scholar]
- Scotti, L.; Ishiki, H.; Mendonca, F.J.B.; Da Silva, M.S.; Scotti, M.T. In-silico analyses of natural products on leishmania enzyme targets. Mini-Rev. Med. Chem. 2015, 15, 253–269. [Google Scholar] [CrossRef]
- Ansari, M.Y.; Equbal, A.; Dikhit, M.R.; Mansuri, R.; Rana, S.; Ali, V.; Sahoo, G.C.; Das, P. Establishment of correlation between in-silico and in-vitro test analysis against Leishmania HGPRT to inhibitors. Int. J. Biol. Macromol. 2016, 83, 78–96. [Google Scholar] [CrossRef]
- Herrera, A.C.; Scotti, L.; Feitosa, A.M.; Formiga, M.D.F.; Scotti, M.T. Computer-aided drug design using sesquiterpene lactones as sources of new structures with potential activity against infectious neglected diseases. Molecules 2017, 22, 79. [Google Scholar] [CrossRef]
- Kashif, M.; Hira, S.K.; Upadhyaya, A.; Gupta, U.; Singh, R.; Paladhi, A.; Khan, F.I.; Rub, A.; Manna, P.P. In silico studies and evaluation of antiparasitic role of a novel pyruvate phosphate dikinase inhibitor in Leishmania donovani infected macrophages. Int. J. Antimicrob. Agents. 2019, 53, 508–514. [Google Scholar] [CrossRef]
- Saki, J.; Shadnoush, F.; Arjmand, R.; Rahim, F. In-silico identification of the best compound against Leishmania infantum: High throughput screening of all FDA approved drugs. Turk. Parazitol. Derg. 2019, 43, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Carter, N.S.; Stamper, B.D.; Elbarbry, F.; Nguyen, V.; Lopez, S.; Kawasaki, Y.; Poormohamadian, R.; Roberts, S.C. Natural products that target the arginase in Leishmania parasites hold therapeutic promise. Microorganisms 2021, 9, 267. [Google Scholar] [CrossRef]
- Eser, M.; Çavuş, İ. In Vitro and In Silico Evaluations of the Antileishmanial Activities of New Benzimidazole-Triazole Derivatives. Vet. Sci. 2023, 10, 648. [Google Scholar] [CrossRef]
- Carter, N.S.; Kawasaki, Y.; Nahata, S.S.; Elikaee, S.; Rajab, S.; Salam, L.; Alabdulal, M.Y.; Broessel, K.K.; Foroghi, F.; Abbas, A.; et al. Polyamine metabolism in leishmania parasites: A promising therapeutic target. Med. Sci. 2022, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Barros, A.M.C.; Simplicio, F.G.; da Silva Pinto, A.C.; de Oliveira, M.D.L.; Figueiredo, V.N.; de Sousa, L.Y.L.; Franco, A.M.R.; Lima, E.S. Antileishmanial activity in silico and in vitro of semi-synthetic derivatives obtained from natural products. Sci. Plena. 2024, 20. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Kumar, L.L.; Borkotoky, S.; Murali, A. The Application of MD Simulation to Lead Identification, Vaccine Design, and Structural Studies in Combat against Leishmaniasis—A Review. Mini-Rev. Med. Chem. 2024, 24, 1089–1111. [Google Scholar] [CrossRef]
- Roberto, C.H.A.; de Sousa, D.S.; da Silva Mendes, F.R.; de Oliveira, L.M.B.; Teixeira, A.M.R.; dos Santos, H.S.; Marinho, E.S. Investigation of the antileishmanial potential of triazole-linked carvacrol–coumarin derivatives: A docking, molecular dynamics, MM/GBSA toxicity approach. J. Iran. Chem. Soc. 2025, 22, 459–473. [Google Scholar] [CrossRef]
- Javid, N.; Asadipour, A.; Salarkia, E.; Langarizadeh, M.A.; Sharifi, F.; Mahdavi, M.; Amirheidari, B.; Iraji, A.; Rezaiezadeh, H.; Hassapour, G.; et al. Synthesis and evaluation of nitrochromene derivatives as potential antileishmanial therapeutics through biological and computational studies. Sci. Rep. 2025, 15, 2571. [Google Scholar] [CrossRef] [PubMed]
- Nare, B.; Hardy, L.W.; Beverley, S.M. The roles of pteridine reductase 1 and dihydrofolate reductase-thymidylate synthase in pteridine metabolism in the protozoan parasite Leishmania major. J. Biol. Chem. 1997, 272, 13883–13891. [Google Scholar] [CrossRef] [PubMed]
- Francesconi, V.; Rizzo, M.; Pozzi, C.; Tagliazucchi, L.; Konchie Simo, C.U.; Saporito, G.; Landi, G.; Mangani, S.; Carbone, A.; Schenone, S.; et al. Identification of innovative folate inhibitors leveraging the amino dihydrotriazine motif from cycloguanil for their potential as anti-Trypanosoma brucei agents. ACS Infect. Dis. 2024, 10, 2755–2774. [Google Scholar] [CrossRef]
- Kapil, S.; Singh, P.K.; Kashyap, A.; Silakari, O. Structure based designing of benzimidazole/benzoxazole derivatives as anti-leishmanial agents. SAR QSAR Environ. Res. 2019, 30, 919–933. [Google Scholar] [CrossRef]
- Ferrari, S.; Losasso, V.; Saxena, P.; Costi, M.P. Targeting the trypanosomatidic enzymes pteridine reductase and dihydrofolate reductase. In Trypanosomatid Diseases: Molecular Routes to Drug Discovery; Wiley-Blackwell: Hoboken, NJ, USA, 2013; Volume 24, pp. 445–472. [Google Scholar]
- Adomako, A.K.; Gasu, E.N.; Mensah, J.O.; Borquaye, L.S. Antileishmanial natural products as potential inhibitors of the Leishmania pteridine reductase: Insights from molecular docking and molecular dynamics simulations. In Silico Pharmacol. 2024, 12, 70. [Google Scholar] [CrossRef]
- Hafez Ghoran, S.; Yousuf, M.; Zafar, H.; Abdjan, M.I.; Ayatollahi, S.A.; Atia-Tul-Wahab; Aminah, N.S.; Kristanti, A.N.; Aziz-Ur-Rehman; Choudhary, M.I. In vitro, in silico, and STD-NMR studies of flavonoids from Hypericum helianthemoides (Spach) Boiss. against Leishmania major pteridine reductase 1 (LmPTR1). J. Biomol. Struct. Dyn. 2025, 1–15. [Google Scholar] [CrossRef]
- Batran, R.Z.; Ebaid, M.S.; Nasralla, S.N.; Son, N.T.; Ha, N.X.; Abdelsattar Ibrahim, H.A.; Alkabbani, M.A.; Kasai, Y.; Imagawa, H.; Al-Sanea, M.M.; et al. Synthesis and mechanistic insights of Coumarinyl-Indolinone hybrids as potent inhibitors of Leishmania major. Eur. J. Med. Chem. 2025, 288, 117392. [Google Scholar] [CrossRef] [PubMed]
- Panecka-Hofman, J.; Poehner, I. Structure and dynamics of pteridine reductase 1: The key phenomena relevant to enzyme function and drug design. Eur. Biophys. J. 2023, 52, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, A.; Mirza, H.M.; Ansari, A.H.; Yasinzai, M.; Zaidi, S.Z.; Dilshad, S.; Ansari, F.L. Benzimidazole derivatives: Synthesis, leishmanicidal effectiveness, and molecular docking studies. Med. Chem. Res. 2013, 22, 3606–3620. [Google Scholar] [CrossRef]
- Tonelli, M.; Gabriele, E.; Piazza, F.; Basilico, N.; Parapini, S.; Tasso, B.; Loddo, R.; Sparatore, F.; Sparatore, A. Benzimidazole derivatives endowed with potent antileishmanial activity. J. Enzym. Inhib. Med. Chem. 2018, 33, 210–226. [Google Scholar] [CrossRef] [PubMed]
- Kaya, A.Z.; Osmaniye, D.; Evren, A.E.; Yurttaş, L.; Demirayak, Ş. Synthesis, cytotoxic activity evaluation and molecular docking studies of some benzimidazole derivatives. Cumhur. Sci. J. 2024, 45, 80–87. [Google Scholar] [CrossRef]
- Özbilgin, A.; Çavuş, İ.; Yıldırım, A.; Kaya, T.; Ertabaklar, H. Evaluation of In vitro and In vivo Drug Efficacy Over Leishmania tropica: A Pilot Study. Turk. Parazitol. Derg. 2018, 42, 11–19. [Google Scholar] [CrossRef]
- Duran, N.; Muz, M.; Culha, G.; Ozer, B. GC-MS analysis and antileishmanial activities of two Turkish propolis types. Parasitol Res. 2011, 108, 95–105. [Google Scholar] [CrossRef]
- Ozgun, G.; Kayalar, H.; Alaylı, F.; Cavus, İ.; Dönmez, F.; Ozbilgin, A. Antileishmanial Efficacy of Heracleum Persicum Desf. Ex. Fisch. Extracts Against Leishmania tropica Promastigotes. Univers. J. Pharm. Res. 2024, 9, 1–4. [Google Scholar] [CrossRef]
- Yıldırım, A.; Aksoy, T.; Kayalar, H.; Balcıoğlu, İ.C. Semen Cannabis and Oleum Hyperici: Antileishmanial activity against Leishmania tropica promastigotes and intracellular amastigotes. Parasitol. Int. 2024, 103, 102950. [Google Scholar] [CrossRef]
- Özel, Y.; Çavuş, İ.; Ünlü, M.; Özbilgin, A. Investigation of the Efficacy of Cinnamaldehyde, Cannabidiol and Eravacycline in a Malaria Model. Mikrobiyol Bul. 2023, 57, 608–624. [Google Scholar] [CrossRef]
- Ioset, J.R.; Brun, R.; Wenzler, T.; Kaiser, M.; Yardley, V. Drug Screening for Kinetoplastids Diseases: A Training Manual for Screening in Neglected Diseases. Available online: https://dndi.org/scientific-articles/2009/drug-screening-for-kinetoplastid-diseases-a-training-manual-for-screening-in-neglected-diseases/2009 (accessed on 9 February 2025).
- Evren, A.E.; Nuha, D.; Yurttaş, L. Focusing on the moderately active compound (MAC) in the design and development of strategies to optimize the apoptotic effect by molecular mechanics techniques. Eur. J. Life Sci. 2023, 1, 118–126. [Google Scholar] [CrossRef]
- Schrödinger Release. 2020-3, LigPrep 2020; Schrödinger, LLC: New York, NY, USA, 2020. [Google Scholar]
- Evren, A.E.; Dawbaa, S.; Nuha, D.; Yavuz, Ş.A.; Gül, Ü.D.; Yurttaş, L. Design and synthesis of new 4-methylthiazole derivatives: In vitro and in silico studies of antimicrobial activity. J. Mol. Struct. 2021, 1241, 130692. [Google Scholar] [CrossRef]
- Schrödinger Release. 2020-3, Glide; Schrödinger, LLC: New York, NY, USA, 2020. [Google Scholar]
- Al-Sharabi, A.A.; Evren, A.E.; Sağlık, B.N.; Yurttaş, L. Synthesis, characterization, molecular docking and molecular dynamics simulations of novel 2, 5-disubstituted-1, 3, 4-thiadiazole derivatives as potential cholinesterase/monoamine oxidase dual inhibitors for Alzheimer’s disease. J. Biomol. Struct. Dyn. 2024, 42, 13023–13041. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger Release. 2020-3, Desmond; Schrödinger, LLC: New York, NY, USA, 2020. [Google Scholar]
- Staffen, I.V.; Banhuk, F.W.; Tomiotto-Pellissier, F.; da Silva Bortoleti, B.T.; Pavanelli, W.R.; Ayala, T.S.; Menolli, R.A. Chalcone-rich extracts from Lonchocarpus cultratus roots present in vitro leishmanicidal and immunomodulatory activity. J. Pharm. Pharmacol. 2022, 74, 77–87. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eser, M.; Çavuş, İ.; Kaya, A.Z.; Evren, A.E.; Yurttaş, L. Evaluation of the Antileishmanial Activity of Some Benzimidazole Derivatives Using In Vitro and In Silico Techniques. Vet. Sci. 2025, 12, 550. https://doi.org/10.3390/vetsci12060550
Eser M, Çavuş İ, Kaya AZ, Evren AE, Yurttaş L. Evaluation of the Antileishmanial Activity of Some Benzimidazole Derivatives Using In Vitro and In Silico Techniques. Veterinary Sciences. 2025; 12(6):550. https://doi.org/10.3390/vetsci12060550
Chicago/Turabian StyleEser, Mustafa, İbrahim Çavuş, Aybüke Züleyha Kaya, Asaf Evrim Evren, and Leyla Yurttaş. 2025. "Evaluation of the Antileishmanial Activity of Some Benzimidazole Derivatives Using In Vitro and In Silico Techniques" Veterinary Sciences 12, no. 6: 550. https://doi.org/10.3390/vetsci12060550
APA StyleEser, M., Çavuş, İ., Kaya, A. Z., Evren, A. E., & Yurttaş, L. (2025). Evaluation of the Antileishmanial Activity of Some Benzimidazole Derivatives Using In Vitro and In Silico Techniques. Veterinary Sciences, 12(6), 550. https://doi.org/10.3390/vetsci12060550






