Assessment of Oxidative Stress and Biometric Data in a Captive Colony of Hamadryas Baboons (Papio hamadryas Linnaeus, 1758) at the Ravenna Zoo Safari (Italy)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Housing, and Management
2.2. Data Collection
2.3. Biometric Data in Males
2.4. Oxidative Stress Data in Males and Females
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gippoliti, S. IUCN Red List of Threatened Species: Papio hamadryas. In IUCN Red List of Threatened Species; IUCN: Gland, Switzerland, 2016. [Google Scholar]
- Dunbar, R.I.M. Primate Social Systems; Springer: Boston, MA, USA, 1988; ISBN 978-1-4684-6696-6. [Google Scholar]
- Grueter, C.; Zinner, D. Nested Societies. Convergent Adaptations of Baboons and Snub-Nosed Monkeys? Primate Rep. 2004, 70, 1–98. [Google Scholar]
- Schreier, A.L.; Swedell, L. The Fourth Level of Social Structure in a Multi-Level Society: Ecological and Social Functions of Clans in Hamadryas Baboons. Am. J. Primatol. 2009, 71, 948–955. [Google Scholar] [CrossRef]
- Phillips-Conroy, J.E.; Jolly, C.J. Sexual Dimorphism in Two Subspecies of Ethiopian Baboons (Papio hamadryas) and Their Hybrids. Am. J. Phys. Anthropol. 1981, 56, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Schreier, A.L. Feeding Ecology, Food Availability and Ranging Patterns of Wild Hamadryas Baboons at Filoha. Folia Primatol. 2010, 81, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.A.; Comuzzie, A.G.; Havill, L.M.; Karere, G.M.; Spradling, K.D.; Mahaney, M.C.; Nathanielsz, P.W.; Nicolella, D.P.; Shade, R.E.; Voruganti, S.; et al. Baboons as a Model to Study Genetics and Epigenetics of Human Disease. ILAR J. 2013, 54, 106–121. [Google Scholar] [CrossRef]
- Martin, L.J.; Mahaney, M.C.; Bronikowski, A.M.; Carey, K.D.; Dyke, B.; Comuzzie, A.G. Lifespan in Captive Baboons Is Heritable. Mech. Ageing Dev. 2002, 123, 1461–1467. [Google Scholar] [CrossRef]
- Lizarraga, S.; Daadi, E.W.; Roy-Choudhury, G.; Daadi, M.M. Age-Related Cognitive Decline in Baboons: Modeling the Prodromal Phase of Alzheimer’s Disease and Related Dementias. Aging 2020, 12, 10099. [Google Scholar] [CrossRef]
- Rainwater, D.L.; Mahaney, M.C.; VandeBerg, J.L.; Wang, X.L. Vitamin E Dietary Supplementation Significantly Affects Multiple Risk Factors for Cardiovascular Disease in Baboons. Am. J. Clin. Nutr. 2007, 86, 597–603. [Google Scholar] [CrossRef]
- Roth, G.S.; Mattison, J.A.; Ottinger, M.A.; Chachich, M.E.; Lane, M.A.; Ingram, D.K. Aging in Rhesus Monkeys: Relevance to Human Health Interventions. Science 2004, 305, 1423–1426. [Google Scholar] [CrossRef]
- Szabo, C.A.; Salinas, F.S. Neuroimaging in the Epileptic Baboon. Front. Vet. Sci. 2022, 9, 908801. [Google Scholar] [CrossRef]
- Wang, X.L.; Rainwater, D.L.; Mahaney, M.C.; Stocker, R. Cosupplementation with Vitamin E and Coenzyme Q10 Reduces Circulating Markers of Inflammation in Baboons. Am. J. Clin. Nutr. 2004, 80, 649–655. [Google Scholar] [CrossRef]
- Brent, L. The Study of Captive Baboon Behavior. In The Baboon in Biomedical Research; Springer: Berlin/Heidelberg, Germany, 2009; pp. 21–34. [Google Scholar]
- Brent, L.; Koban, T.; Ramirez, S. Abnormal, Abusive, and Stress-Related Behaviors in Baboon Mothers. Biol. Psychiatry 2002, 52, 1047–1056. [Google Scholar] [CrossRef]
- Nelson, E.E.; Winslow, J.T. Non-Human Primates: Model Animals for Developmental Psychopathology. Neuropsychopharmacology 2009, 34, 90–105. [Google Scholar] [CrossRef]
- Husain, S.; Hillmann, K.; Hengst, K.; Englert, H. Effects of a Lifestyle Intervention on the Biomarkers of Oxidative Stress in Non-Communicable Diseases: A Systematic Review. Front. Aging 2023, 4, 1085511. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, Oxidative Stress and the Biology of Ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Rainwater, D.; Vandeberg, J. Baboon Model for Dyslipidemia and Atherosclerosis. In The Baboon in Biomedical Research; Springer: Berlin/Heidelberg, Germany, 2009; pp. 225–236. ISBN 978-0-387-75990-6. [Google Scholar]
- Houldsworth, A. Role of Oxidative Stress in Neurodegenerative Disorders: A Review of Reactive Oxygen Species and Prevention by Antioxidants. Brain Commun. 2024, 6, fcad356. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Mandelker, L. Oxidative Stress, Free Radicals, and Cellular Damage. In Studies on Veterinary Medicine; Mandelker, L., Vajdovich, P., Eds.; Oxidative Stress in Applied Basic Research and Clinical Practice; Humana Press: Totowa, NJ, USA, 2011; pp. 1–17. ISBN 978-1-61779-070-6. [Google Scholar]
- Beaulieu, M. Oxidative Status: A General but Overlooked Indicator of Welfare across Animal Species? BioEssays 2024, 46, 2300205. [Google Scholar] [CrossRef]
- Strum, S.C. Weight and Age in Wild Olive Baboons. Am. J. Primatol. 1991, 25, 219–237. [Google Scholar] [CrossRef]
- Galbany, J.; Tung, J.; Altmann, J.; Alberts, S.C. Canine Length in Wild Male Baboons: Maturation, Aging and Social Dominance Rank. PLoS ONE 2015, 10, e0126415. [Google Scholar] [CrossRef]
- Costantini, D.; Casagrande, S.; De Filippis, S.; Brambilla, G.; Fanfani, A.; Tagliavini, J.; Dell’Omo, G. Correlates of Oxidative Stress in Wild Kestrel Nestlings (Falco tinnunculus). J. Comp. Physiol. B 2006, 176, 329–337. [Google Scholar] [CrossRef]
- Sohal, R.S.; Weindruch, R. Oxidative Stress, Caloric Restriction, and Aging. Science 1996, 273, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Oppler, S.H.; Palmer, S.D.; Phu, S.N.; Graham, M.L. The Role of Behavioral Management in Enhancing Clinical Care and Efficiency, Minimizing Social Disruption, and Promoting Welfare in Captive Primates. Vet. Sci. 2024, 11, 401. [Google Scholar] [CrossRef]
- Reiners, J.K.; Gregersen, H.A. How to Plan and Provide General Anesthesia for a Troop of 98 Hamadryas Baboons (Papio hamadryas) for Contraceptive and Preventative Health Interventions. Am. J. Vet. Res. 2024, 85, ajvr.23.12.0274. [Google Scholar] [CrossRef]
- Amari, M.; Brioschi, F.; Elia, L.; Rabbogliatti, V.; Capasso, M.; Venturelli, E.; Biancani, B.; Spadari, A.; Giuliano, R. Comparison of Three Anaesthetic Protocols in Captive Baboons (Papio hamadryas): Preliminary Results. Vet. Anaesth. Analg. 2024, 51, 760.e8. [Google Scholar] [CrossRef]
- Scardia, A.; Laricchiuta, P.; Stabile, M.; Acquafredda, C.; Lacitignola, L.; Uva, A.; Crovace, A.; Staffieri, F. Use of Laryngeal Mask and Anesthetic Management in Hamadryas Baboons (Papio hamadryas) Undergoing Laparoscopic Salpingectomy—A Case Series. Vet. Sci. 2023, 10, 158. [Google Scholar] [CrossRef] [PubMed]
- Leigh, S.R. Growth and Development of Baboons. In The Baboon in Biomedical Research; Springer: Berlin/Heidelberg, Germany, 2009; pp. 57–88. [Google Scholar]
- Carosi, M.; Spani, F.; Ulland, A.E.; Scalici, M.; Suomi, S.J. Clitoral Length in Immature and Mature Captive Tufted Capuchin (Sapajus spp.) Females: A Cross-Sectional Study. Am. J. Primatol. 2020, 82, e23135. [Google Scholar] [CrossRef]
- Alberti, A.; Bolognini, L.; Macciantelli, D.; Caratelli, M. The Radical Cation of N, N-Diethyl-Para-Phenylendiamine: A Possible Indicator of Oxidative Stress in Biological Samples. Res. Chem. Intermed. 2000, 26, 253–267. [Google Scholar] [CrossRef]
- Tafuri, S.; Marullo, A.; Ciani, F.; Della Morte, R.; Montagnaro, S.; Fiorito, F.; De Martino, L. Reactive Oxygen Metabolites in Alpha-Herpesvirus-Seropositive Mediterranean Buffaloes (Bubalus bubalis): A Preliminary Study. Pol. J. Vet. Sci. 2018, 21, 639–642. [Google Scholar] [CrossRef]
- Esposito, L.; Tafuri, S.; Cocchia, N.; Fasanelli, R.; Piscopo, N.; Lamagna, B.; Eguren, V.; Amici, A.; Iorio, E.L.; Ciani, F. Assessment of Living Conditions in Wild Boars by Analysis of Oxidative Stress Markers. J. Appl. Anim. Welf. Sci. 2021, 24, 64–71. [Google Scholar] [CrossRef]
- Tafuri, S.; Cocchia, N.; Carotenuto, D.; Vassetti, A.; Staropoli, A.; Mastellone, V.; Peretti, V.; Ciotola, F.; Albarella, S.; Del Prete, C.; et al. Chemical Analysis of Lepidium meyenii (Maca) and Its Effects on Redox Status and on Reproductive Biology in Stallions. Molecules 2019, 24, 1981. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Benesty, J.; Chen, J.; Huang, Y.; Cohen, I. Pearson Correlation Coefficient. In Noise Reduction in Speech Processing; Springer: Berlin/Heidelberg, Germany, 2009; pp. 37–40. [Google Scholar]
- McKnight, P.E.; Najab, J. Mann-Whitney U Test. In The Corsini Encyclopedia of Psychology; John Wiley & Sons: Hoboken, NJ, USA, 2010; p. 1. [Google Scholar]
- Hitomi, Y.; Masaki, N.; Ishinoda, Y.; Ido, Y.; Iwashita, M.; Yumita, Y.; Kagami, K.; Yasuda, R.; Ikegami, Y.; Toya, T.; et al. Effectiveness of the D-ROMs Oxidative Stress Test to Predict Long-Term Cardiovascular Mortality. Int. J. Cardiol. 2022, 354, 43–47. [Google Scholar] [CrossRef]
- Pigazzani, F.; Gorni, D.; Dyar, K.A.; Pedrelli, M.; Kennedy, G.; Costantino, G.; Bruno, A.; Mackenzie, I.; MacDonald, T.M.; Tietge, U.J.F.; et al. The Prognostic Value of Derivatives-Reactive Oxygen Metabolites (d-ROMs) for Cardiovascular Disease Events and Mortality: A Review. Antioxidants 2022, 11, 1541. [Google Scholar] [CrossRef]
- Serena, B.; Primiterra, M.; Catalani, S.; Finco, A.; Canestrari, F.; Cornelli, U. Performance Evaluation of the Innovative PAT Test, Comparison with the Common BAP Test and Influence of Interferences on the Evaluation of the Plasma Antioxidant Capacity. Clin. Lab. 2013, 59, 1091–1097. [Google Scholar]
- Rosner, B.; Glynn, R.J.; Lee, M.-L.T. The Wilcoxon Signed Rank Test for Paired Comparisons of Clustered Data. Biometrics 2006, 62, 185–192. [Google Scholar] [CrossRef]
- Ey, E.; Pfefferle, D.; Fischer, J. Do Age- and Sex-Related Variations Reliably Reflect Body Size in Non-Human Primate Vocalizations? A Review. Primates 2007, 48, 253–267. [Google Scholar] [CrossRef]
- Bercovitch, F.B. Dominance Rank and Reproductive Maturation in Male Rhesus Macaques (Macaca mulatta). J. Reprod. Fertil. 1993, 99, 113–120. [Google Scholar] [CrossRef]
- Plavcan, J.M. Sexual Dimorphism in Primate Evolution. Am. J. Phys. Anthropol. 2001, 116 (Suppl. 33), 25–53. [Google Scholar] [CrossRef]
- Smith, R.J.; Jungers, W.L. Body Mass in Comparative Primatology. J. Hum. Evol. 1997, 32, 523–559. [Google Scholar] [CrossRef]
- Higgins, P.B.; Rodriguez, P.J.; Voruganti, V.S.; Mattern, V.; Bastarrachea, R.A.; Rice, K.; Raabe, T.; Comuzzie, A.G. Body Composition and Cardiometabolic Disease Risk Factors in Captive Baboons (Papio hamadryas sp.): Sexual Dimorphism. Am. J. Phys. Anthropol. 2014, 153, 9–14. [Google Scholar] [CrossRef]
- Cabana, F.; Jayarajah, P.; Oh, P.Y.; Hsu, C.-D. Dietary Management of a Hamadryas Baboon (Papio hamadryas) Troop to Improve Body and Coat Condition and Reduce Parasite Burden: Dietary Management of Baboons. J. Zoo Aquar. Res. 2018, 6, 16–21. [Google Scholar] [CrossRef]
- Navarro-Yepes, J.; Zavala-Flores, L.; Annadurai, A.; Wang, F.; Skotak, M.; Chandra, N.; Li, M.; Pappa, A.; Martinez-Fong, D.; Razo, L.M.D.; et al. Antioxidant Gene Therapy against Neuronal Cell Death. Pharmacol. Ther. 2014, 142, 206–230. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Shibata, M.; Shimizu, T.; Shibata, S.; Toriumi, H.; Ebine, T.; Kuroi, T.; Iwashita, T.; Funakubo, M.; Kayama, Y.; et al. Differential Cellular Localization of Antioxidant Enzymes in the Trigeminal Ganglion. Neuroscience 2013, 248, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef]
- Genestra, M. Oxyl Radicals, Redox-Sensitive Signalling Cascades and Antioxidants. Cell. Signal. 2007, 19, 1807–1819. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Free Radicals: Health Implications and Their Mitigation by Herbals. J. Adv. Med. Med. Res. 2015, 7, 438–457. [Google Scholar] [CrossRef]
- Valko, M.; Izakovic, M.; Mazur, M.; Rhodes, C.J.; Telser, J. Role of Oxygen Radicals in DNA Damage and Cancer Incidence. Mol. Cell. Biochem. 2004, 266, 37–56. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The Effects of Oxidative Stress on Female Reproduction: A Review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef]
- Baskaran, S.; Finelli, R.; Agarwal, A.; Henkel, R. Reactive Oxygen Species in Male Reproduction: A Boon or a Bane? Andrologia 2021, 53, e13577. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M. Oxygen radicals-superoxide dismutase system and reproduction medicine. Nihon Sanka Fujinka Gakkai Zasshi 1993, 45, 842–848. [Google Scholar] [PubMed]
- Rajendran, P.; Nandakumar, N.; Rengarajan, T.; Palaniswami, R.; Gnanadhas, E.N.; Lakshminarasaiah, U.; Gopas, J.; Nishigaki, I. Antioxidants and Human Diseases. Clin. Chim. Acta 2014, 436, 332–347. [Google Scholar] [CrossRef]
- Khansari, N.; Shakiba, Y.; Mahmoudi, M. Chronic Inflammation and Oxidative Stress as a Major Cause of Age-Related Diseases and Cancer. Recent Pat. Inflamm. Allergy Drug Discov. 2009, 3, 73–80. [Google Scholar] [CrossRef]
- Taniyama, Y.; Griendling, K.K. Reactive Oxygen Species in the Vasculature: Molecular and Cellular Mechanisms. Hypertension 2003, 42, 1075–1081. [Google Scholar] [CrossRef]
- Wu, J.Q.; Kosten, T.R.; Zhang, X.Y. Free Radicals, Antioxidant Defense Systems, and Schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 46, 200–206. [Google Scholar] [CrossRef]
- Kongmanas, K.; Saewu, A.; Kiattiburut, W.; Baker, M.A.; Faull, K.F.; Burger, D.; Tanphaichitr, N. Accumulation of Seminolipid in Sertoli Cells Is Associated with Increased Levels of Reactive Oxygen Species and Male Subfertility: Studies in Aging Arsa Null Male Mice. Antioxidants 2021, 10, 912. [Google Scholar] [CrossRef]
- Naseer, Z.; Raza, S.; Ahmad, E.; Aksoy, M. Use of Antioxidants to Improve the Reproductive Performance in Model Laboratory Animals. In The Role of Exogenous Antioxidants in Enhancing Reproductive Function and Performance; Livre de Lyon: Lyon, France, 2022; p. 1. [Google Scholar]
- Deponte, M. Glutathione Catalysis and the Reaction Mechanisms of Glutathione-Dependent Enzymes. Biochim. Biophys. Acta 2013, 1830, 3217–3266. [Google Scholar] [CrossRef] [PubMed]
- Al-Gubory, K.H.; Garrel, C.; Faure, P.; Sugino, N. Roles of Antioxidant Enzymes in Corpus Luteum Rescue from Reactive Oxygen Species-Induced Oxidative Stress. Reprod. BioMed. Online 2012, 25, 551–560. [Google Scholar] [CrossRef]
- Glasauer, A.; Chandel, N.S. Targeting Antioxidants for Cancer Therapy. Biochem. Pharmacol. 2014, 92, 90–101. [Google Scholar] [CrossRef]
- Bardaweel, S.K.; Gul, M.; Alzweiri, M.; Ishaqat, A.; ALSalamat, H.A.; Bashatwah, R.M. Reactive Oxygen Species: The Dual Role in Physiological and Pathological Conditions of the Human Body. Eurasian J. Med. 2018, 50, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, C.; Marchi, S.; Simoes, I.C.M.; Ren, Z.; Morciano, G.; Perrone, M.; Patalas-Krawczyk, P.; Borchard, S.; Jędrak, P.; Pierzynowska, K.; et al. Mitochondria and Reactive Oxygen Species in Aging and Age-Related Diseases. Int. Rev. Cell Mol. Biol. 2018, 340, 209–344. [Google Scholar] [CrossRef] [PubMed]
- Varesi, A.; Chirumbolo, S.; Campagnoli, L.I.M.; Pierella, E.; Piccini, G.B.; Carrara, A.; Ricevuti, G.; Scassellati, C.; Bonvicini, C.; Pascale, A. The Role of Antioxidants in the Interplay between Oxidative Stress and Senescence. Antioxidants 2022, 11, 1224. [Google Scholar] [CrossRef]
- Georgiev, A.V.; Thompson, M.E.; Mandalaywala, T.M.; Maestripieri, D. Oxidative Stress as an Indicator of the Costs of Reproduction among Free-Ranging Rhesus Macaques. J. Exp. Biol. 2015, 218, 1981–1985. [Google Scholar] [CrossRef]
- Sancilio, A.; Jasienska, G.; Panter-Brick, C.; Ziomkiewicz, A.; Nenko, I.; Bribiescas, R.G. Accelerated Senescence as a Cost of Reproduction: Testing Associations between Oxidative Stress and Reproductive Effort in Rural and Urban Women. Am. J. Hum. Biol. 2021, 33, e23537. [Google Scholar] [CrossRef]
- Georgiev, A.V.; Muehlenbein, M.P.; Prall, S.P.; Emery Thompson, M.; Maestripieri, D. Male Quality, Dominance Rank, and Mating Success in Free-Ranging Rhesus Macaques. Behav. Ecol. 2015, 26, 763–772. [Google Scholar] [CrossRef]
- González, N.T.; Otali, E.; Machanda, Z.; Muller, M.N.; Wrangham, R.; Thompson, M.E. Urinary Markers of Oxidative Stress Respond to Infection and Late-Life in Wild Chimpanzees. PLoS ONE 2020, 15, e0238066. [Google Scholar] [CrossRef]
- Csiszar, A.; Podlutsky, A.; Podlutskaya, N.; Sonntag, W.E.; Merlin, S.Z.; Philipp, E.E.R.; Doyle, K.; Davila, A.; Recchia, F.A.; Ballabh, P.; et al. Testing the Oxidative Stress Hypothesis of Aging in Primate Fibroblasts: Is There a Correlation Between Species Longevity and Cellular ROS Production? J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 841–852. [Google Scholar] [CrossRef]
- Adekunbi, D.A.; Li, C.; Nathanielsz, P.W.; Salmon, A.B. Age and Sex Modify Cellular Proliferation Responses to Oxidative Stress and Glucocorticoid Challenges in Baboon Cells. Geroscience 2021, 43, 2067–2085. [Google Scholar] [CrossRef]
- Shrestha, H.K. Loss of Luteal Sensitivity to Luteinizing Hormone Underlies Luteolysis in Cattle: A Hypothesis. Reprod. Biol. 2021, 21, 100570. [Google Scholar] [CrossRef]
- Mahmoud, A.A.; Elfiky, A.M.; Abo-Zeid, F.S. The Anti-Androgenic Effect of Quercetin on Hyperandrogenism and Ovarian Dysfunction Induced in a Dehydroepiandrosterone Rat Model of Polycystic Ovary Syndrome. Steroids 2022, 177, 108936. [Google Scholar] [CrossRef]
- Ruano, C.S.M.; Miralles, F.; Méhats, C.; Vaiman, D. The Impact of Oxidative Stress of Environmental Origin on the Onset of Placental Diseases. Antioxidants 2022, 11, 106. [Google Scholar] [CrossRef] [PubMed]
- Casanueva, E.; Viteri, F.E. Iron and Oxidative Stress in Pregnancy. J. Nutr. 2003, 133, 1700S–1708S. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Oxidative Stress. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Ademuyiwa, O.; Odusoga, O.L.; Adebawo, O.O.; Ugbaja, R. Endogenous Antioxidant Defences in Plasma and Erythrocytes of Pregnant Women during Different Trimesters of Pregnancy. Acta Obstet. Gynecol. Scand. 2007, 86, 1175–1182. [Google Scholar] [CrossRef]
- Myatt, L. Review: Reactive Oxygen and Nitrogen Species and Functional Adaptation of the Placenta. Placenta 2010, 31, S66–S69. [Google Scholar] [CrossRef]
- Redman, C.W.G.; Sargent, I.L. Immunology of Pre-Eclampsia. Am. J. Reprod. Immunol. 2010, 63, 534–543. [Google Scholar] [CrossRef]
- Fogel, I.; Pinchuk, I.; Kupferminc, M.J.; Lichtenberg, D.; Fainaru, O. Oxidative Stress in the Fetal Circulation Does Not Depend on Mode of Delivery. Am. J. Obstet. Gynecol. 2005, 193, 241–246. [Google Scholar] [CrossRef]
- Sgorbini, M.; Bonelli, F.; Percacini, G.; Pasquini, A.; Rota, A. Maternal and Neonatal Evaluation of Derived Reactive Oxygen Metabolites and Biological Antioxidant Potential in Donkey Mares and Foals. Animals 2021, 11, 2885. [Google Scholar] [CrossRef] [PubMed]
- Hyatt, H.W.; Zhang, Y.; Hood, W.R.; Kavazis, A.N. Lactation Has Persistent Effects on a Mother’s Metabolism and Mitochondrial Function. Sci. Rep. 2017, 7, 17118. [Google Scholar] [CrossRef]
- Stuebe, A.M.; Rich-Edwards, J.W. The Reset Hypothesis: Lactation and Maternal Metabolism. Am. J. Perinatol. 2009, 26, 81–88. [Google Scholar] [CrossRef]
- Kirkwood, T.B.; Holliday, R. The Evolution of Ageing and Longevity. Proc. R. Soc. Lond. B Biol. Sci. 1979, 205, 531–546. [Google Scholar] [CrossRef]
- Blount, J.D.; Vitikainen, E.I.K.; Stott, I.; Cant, M.A. Oxidative Shielding and the Cost of Reproduction. Biol. Rev. Camb. Philos. Soc. 2016, 91, 483–497. [Google Scholar] [CrossRef]
- Costantini, D. Oxidative Stress in Ecology and Evolution: Lessons from Avian Studies. Ecol. Lett. 2008, 11, 1238–1251. [Google Scholar] [CrossRef]
- Dowling, D.K.; Simmons, L.W. Reactive Oxygen Species as Universal Constraints in Life-History Evolution. Proc. Biol. Sci. 2009, 276, 1737–1745. [Google Scholar] [CrossRef]
- Monaghan, P.; Metcalfe, N.B.; Torres, R. Oxidative Stress as a Mediator of Life History Trade-Offs: Mechanisms, Measurements and Interpretation. Ecol. Lett. 2009, 12, 75–92. [Google Scholar] [CrossRef]
- Speakman, J.R.; Garratt, M. Oxidative Stress as a Cost of Reproduction: Beyond the Simplistic Trade-off Model. Bioessays 2014, 36, 93–106. [Google Scholar] [CrossRef]
- Melvin, Z.E.; Dhirani, H.; Mitchell, C.; Davenport, T.R.B.; Blount, J.D.; Georgiev, A.V. Methodological Confounds of Measuring Urinary Oxidative Stress in Wild Animals. Ecol. Evol. 2022, 12, e9115. [Google Scholar] [CrossRef]
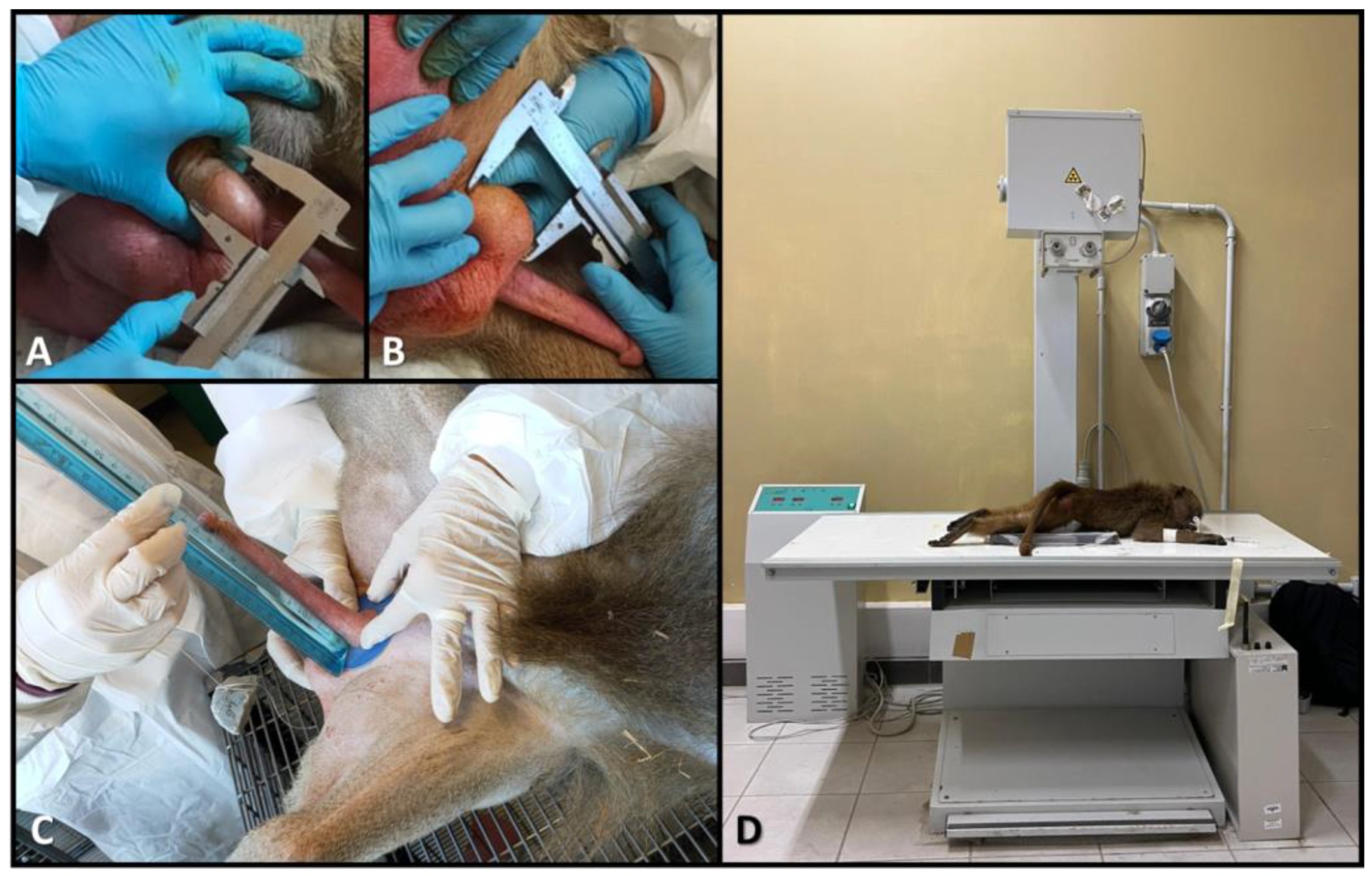
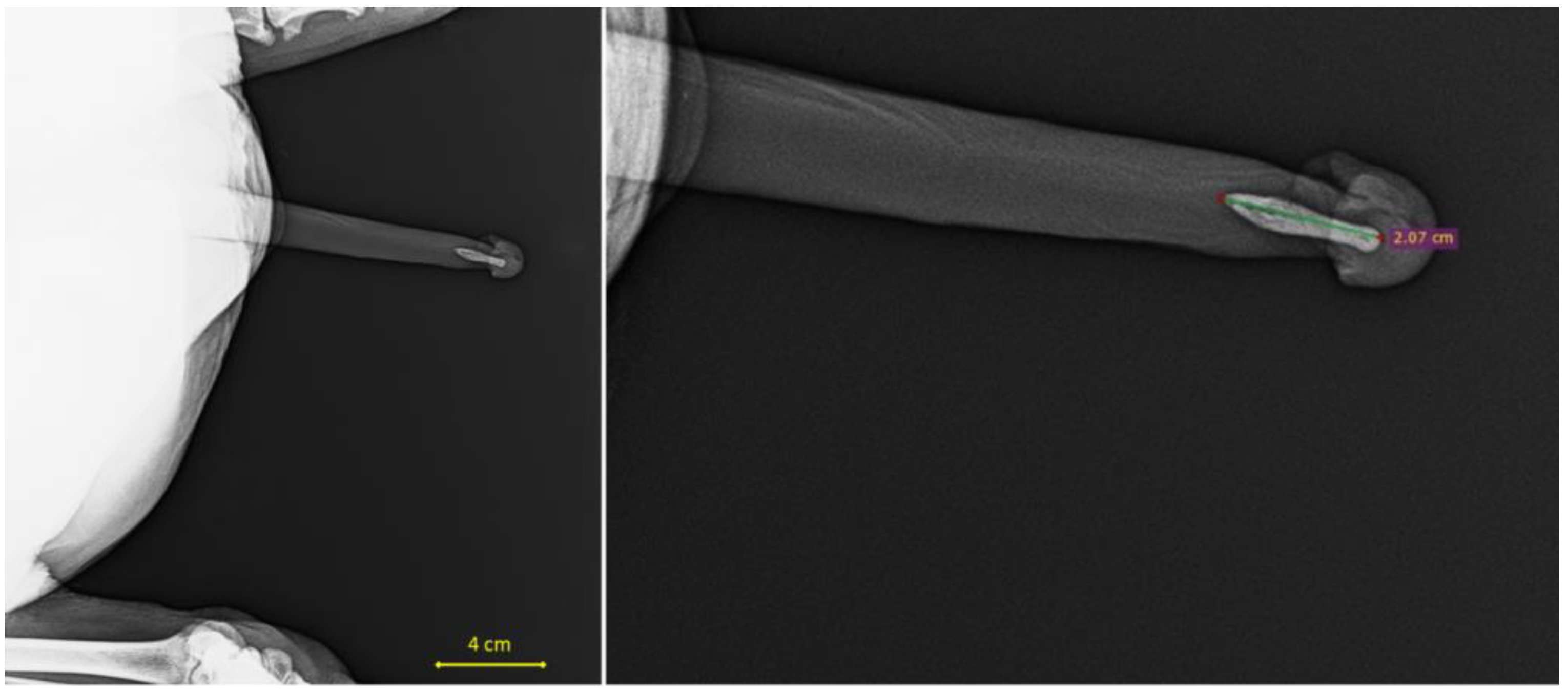
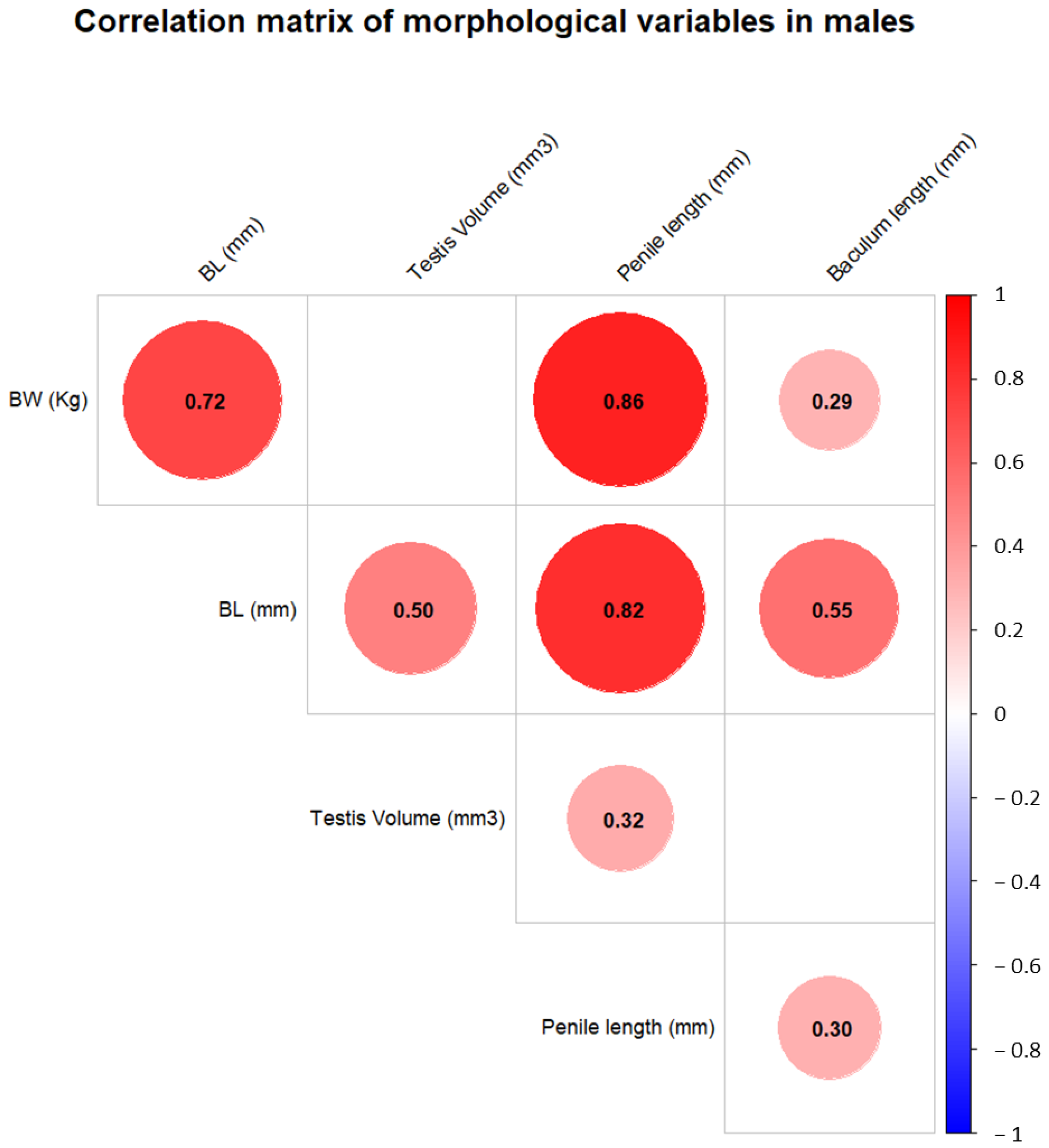
| Males | Females | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Mean | SD | Median | Min | Max | Mean | SD | Median | Min | Max |
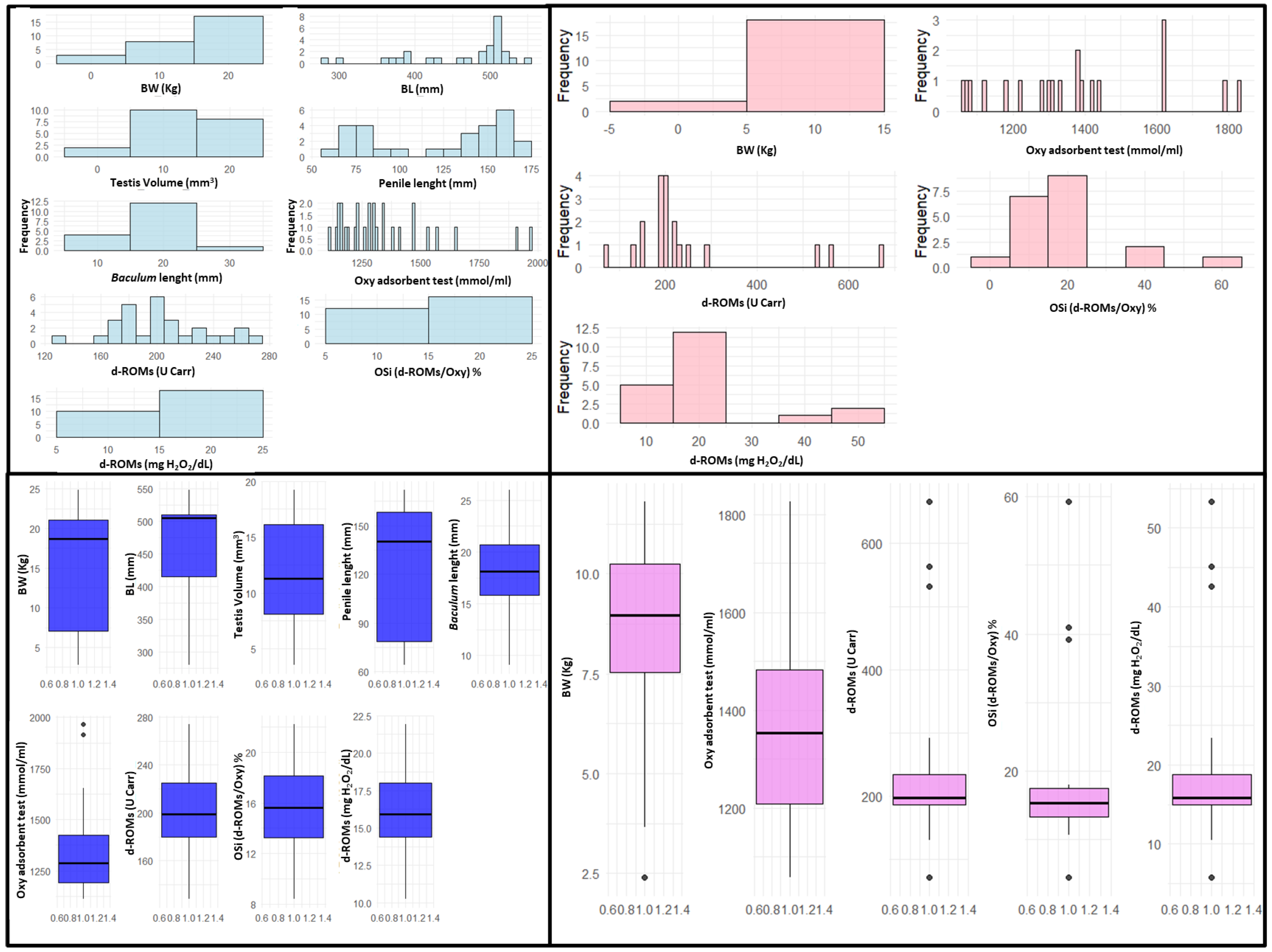
| BW (Kg) | 15.22 | 7.36 | 18.7 | 2.75 | 24.85 | 8.49 | 2.54 | 8.98 | 2.4 | 11.8 |
| BL (mm) | 462.14 | 72.75 | 505 | 280 | 548 | NA | NA | NA | NA | NA |
| Testis volume (mm3) | 11.78 | 4.94 | 11.25 | 3.53 | 19.22 | - | - | - | - | - |
| Penile length (mm) | 123.11 | 39.11 | 140 | 64 | 172 | - | - | - | - | - |
| Baculum length (mm) | 17.81 | 4.34 | 18.1 | 9.05 | 26 | - | - | - | - | - |
| OXY Adsorbent test (μmol HClO/L) | 1346.03 | 218.11 | 1288.15 | 1111.2 | 1966.3 | 1370.92 | 227.81 | 1353.1 | 1058.6 | 1825.5 |
| d-ROMs (U Carr) | 203.71 | 33.67 | 199 | 128 | 274 | 251.25 | 153.36 | 198 | 72 | 666 |
| OSi (d-ROMs/OXY × 100) | 15.52 | 3.51 | 15.6 | 8.4 | 22.2 | 18.95 | 12.78 | 15.4 | 4.5 | 59.2 |
| d-ROMs (mg H2O2/dL) | 16.3 | 2.69 | 15.92 | 10.24 | 21.92 | 20.1 | 12.27 | 15.84 | 5.76 | 53.28 |
| Variable | Test Used | p-Value | Mean (M) | Mean (F) |
|---|---|---|---|---|
| BW (Kg) | Mann–Whitney | 0.009 | 15.22 | 8.49 |
| OXY Adsorbent test (mmol/mL) | Mann–Whitney | 0.638 | 1346.03 | 1370.92 |
| d-ROMs (U Carr) | Mann–Whitney | 0.738 | 203.71 | 251.25 |
| OSi (d-ROMs/Oxy) % | Mann–Whitney | 0.958 | 15.52 | 18.94 |
| d-ROMs (mg H2O2/dL) | Mann–Whitney | 0.738 | 16.3 | 20.1 |
| Variable | Reference Values (in Homo sapiens) | Observed Mean | p-Value | Test Type |
|---|---|---|---|---|
| OXY Adsorbent test (mmol/mL) | >350 | 1356.4 | 7.1 × 10−15 | Paired Wilcoxon Test |
| d-ROMs (U Carr) | <300 | 223.5 | 4.7 × 10−6 | Paired Wilcoxon Test |
| OSi (d-ROMs/Oxy) % | <85.71% | 16.9 | 1.7 × 10−9 | Paired Wilcoxon Test |
| d-ROMs (mg H2O2/dL) | <24 | 17.9 | 4.7 × 10−6 | Paired Wilcoxon Test |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biancani, B.; Carosi, M.; Capasso, M.; Rossi, G.; Tafuri, S.; Ciani, F.; Cotignoli, C.; Zinno, F.; Venturelli, E.; Galliani, M.; et al. Assessment of Oxidative Stress and Biometric Data in a Captive Colony of Hamadryas Baboons (Papio hamadryas Linnaeus, 1758) at the Ravenna Zoo Safari (Italy). Vet. Sci. 2025, 12, 466. https://doi.org/10.3390/vetsci12050466
Biancani B, Carosi M, Capasso M, Rossi G, Tafuri S, Ciani F, Cotignoli C, Zinno F, Venturelli E, Galliani M, et al. Assessment of Oxidative Stress and Biometric Data in a Captive Colony of Hamadryas Baboons (Papio hamadryas Linnaeus, 1758) at the Ravenna Zoo Safari (Italy). Veterinary Sciences. 2025; 12(5):466. https://doi.org/10.3390/vetsci12050466
Chicago/Turabian StyleBiancani, Barbara, Monica Carosi, Michele Capasso, Giacomo Rossi, Simona Tafuri, Francesca Ciani, Chiara Cotignoli, Francesco Zinno, Elena Venturelli, Matteo Galliani, and et al. 2025. "Assessment of Oxidative Stress and Biometric Data in a Captive Colony of Hamadryas Baboons (Papio hamadryas Linnaeus, 1758) at the Ravenna Zoo Safari (Italy)" Veterinary Sciences 12, no. 5: 466. https://doi.org/10.3390/vetsci12050466
APA StyleBiancani, B., Carosi, M., Capasso, M., Rossi, G., Tafuri, S., Ciani, F., Cotignoli, C., Zinno, F., Venturelli, E., Galliani, M., & Spani, F. (2025). Assessment of Oxidative Stress and Biometric Data in a Captive Colony of Hamadryas Baboons (Papio hamadryas Linnaeus, 1758) at the Ravenna Zoo Safari (Italy). Veterinary Sciences, 12(5), 466. https://doi.org/10.3390/vetsci12050466









