Use of Fermented Black Tea (Camellia sinensis) Factory Wastes in Standard Rat Diets
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. The Preparation of Tissues for Histopathologic Analysis
2.4. Biochemical Analyses
2.5. Total Antioxidant Status, Total Oxidant Status, and Oxidative Stress Index (OSI) Assays
2.6. Statistical Analysis
3. Results
3.1. Biochemical Findings
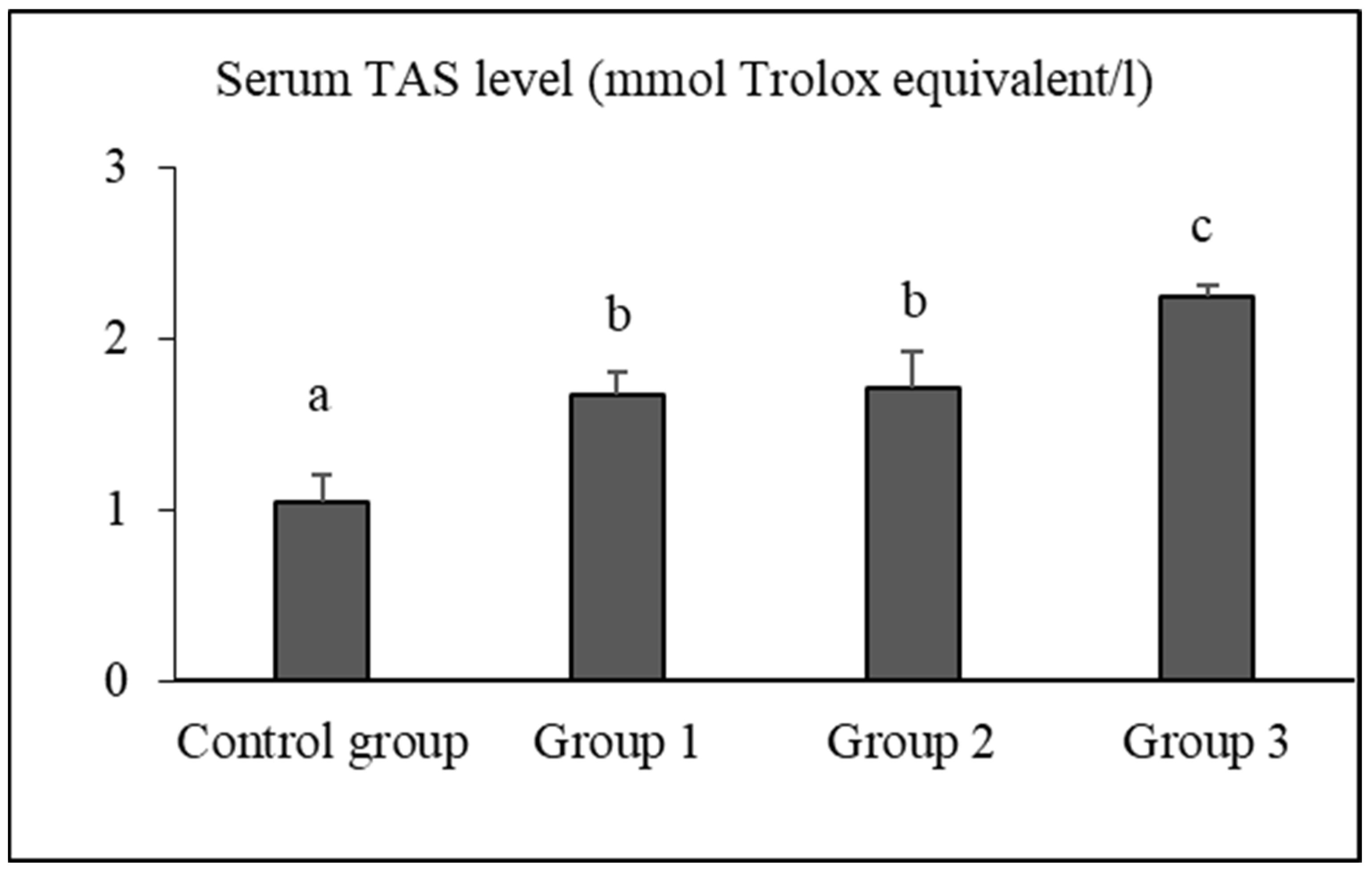
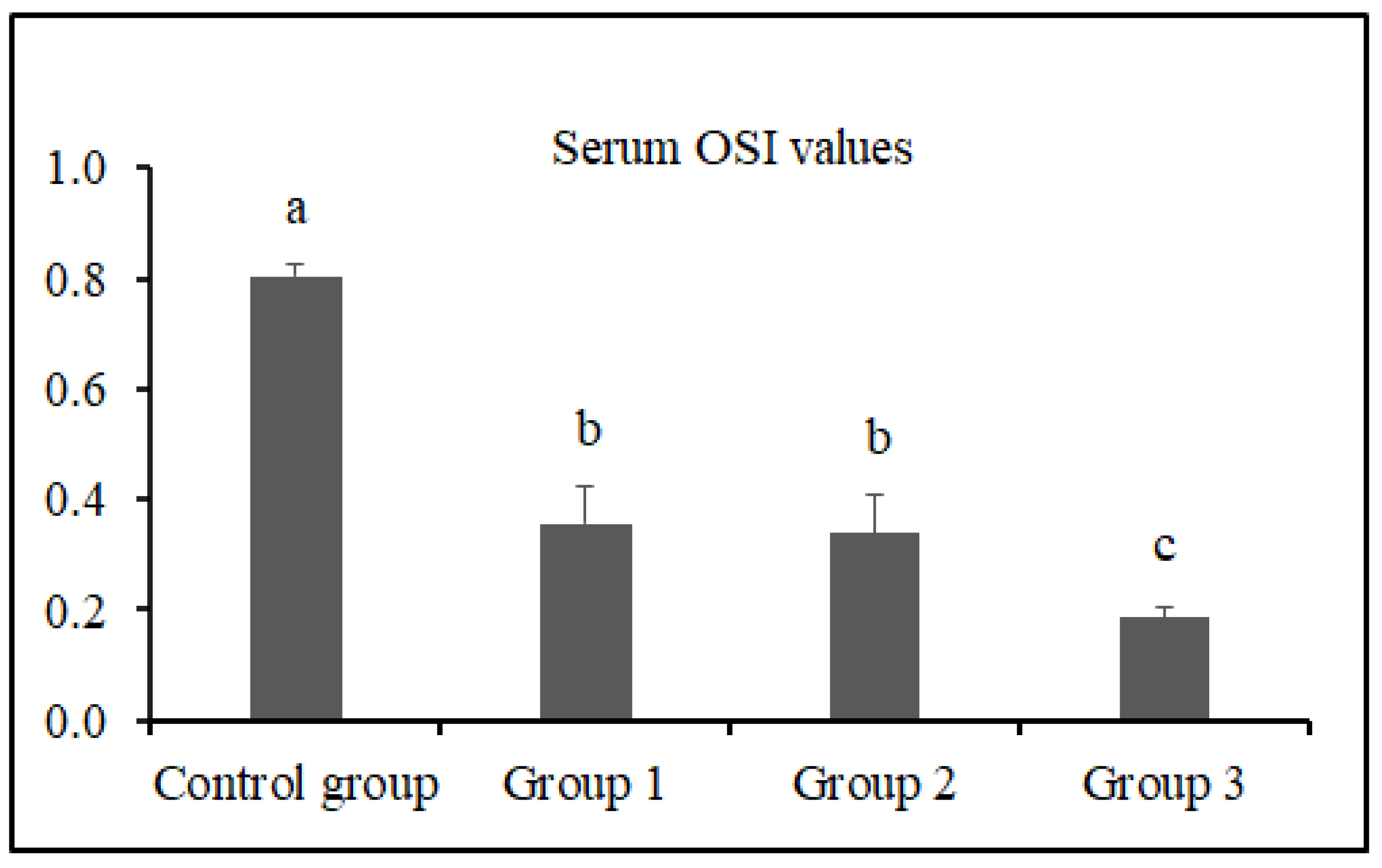
3.2. Serum TAS, TOS, and OSI Levels
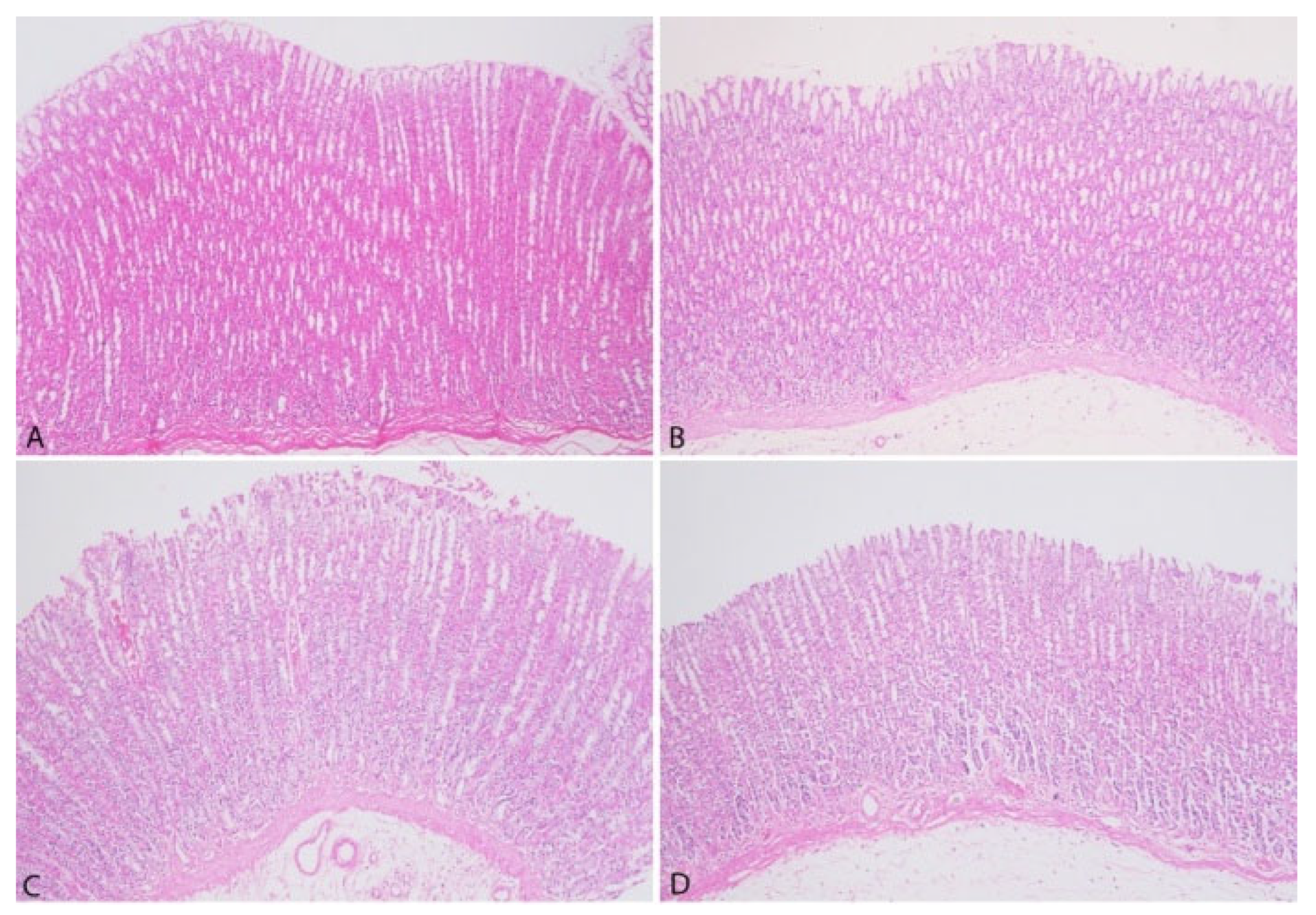
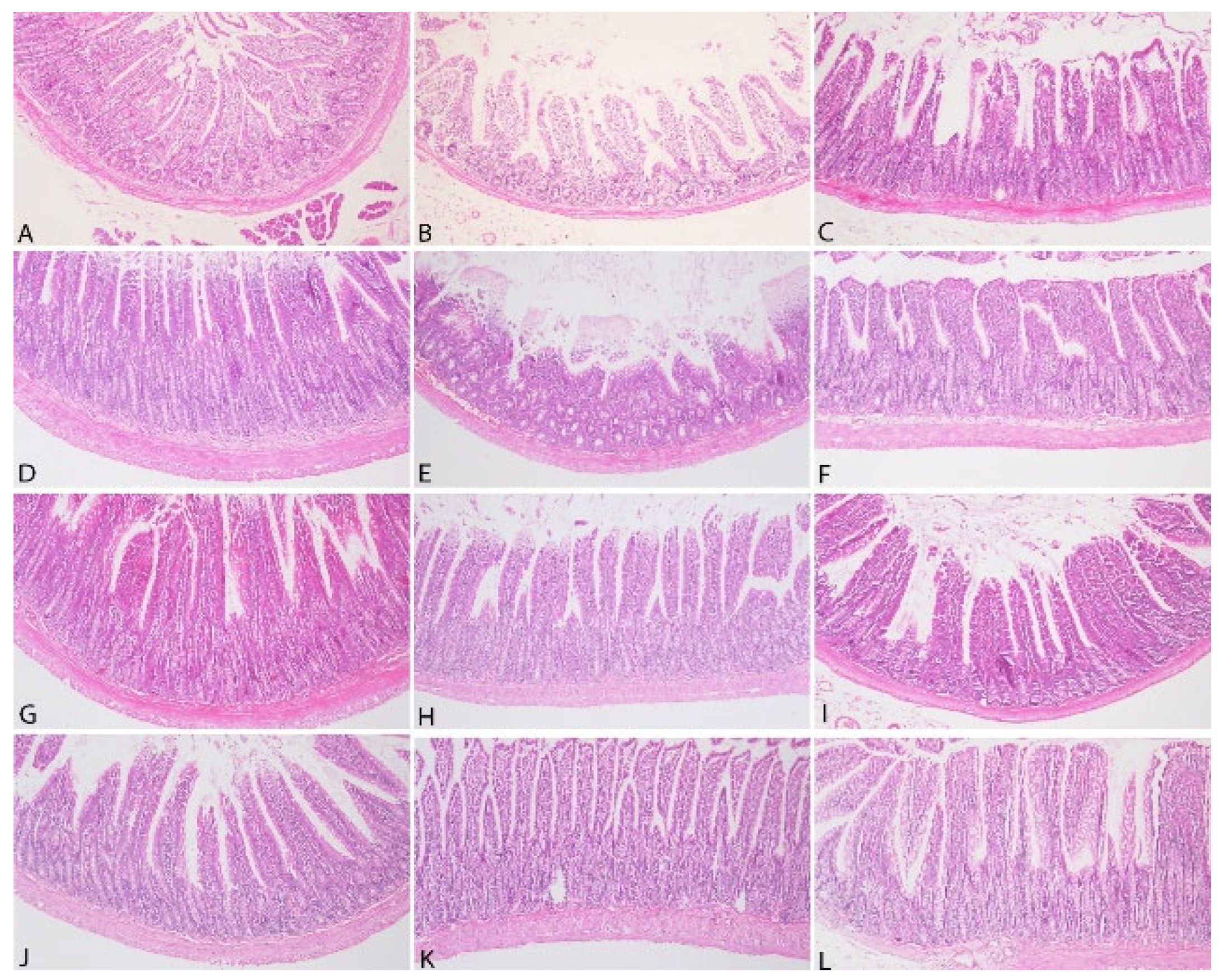
3.3. Pathological Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, S.; Awasthi, M.K.; Wang, Y.; Xu, P. Current Understanding in Conversion and Application of Tea Waste Biomass: A Review. Bioresour. Technol. 2021, 338, 125530. [Google Scholar] [CrossRef]
- Wang, H.; Provan, G.J.; Helliwell, K. Tea Flavonoids: Their Functions, Utilisation and Analysis. Trends Food Sci. Technol. 2000, 11, 152–160. [Google Scholar] [CrossRef]
- Chowdhury, A.; Sarkar, S.; Chowdhury, A.; Bardhan, S.; Mandal, P.; Chowdhury, M. Tea Waste Management: A Case Study from West Bengal, India. Indian J. Sci. Technol. 2016, 9, 42. [Google Scholar] [CrossRef]
- Altay, H.Ö. Siyah Çay Üretiminin Farkli Aşamalarinda Ortaya Çikan Atiklarin Değerlendirme Olanaklarinin Arttirilmasi Amaçli Özelliklerinin Belirlenmesi. Master’s Thesis, Akdeniz Üniversitesi Fen Bilimleri Enstitüsü Gıda Mühendisliği, Antalya, Türkiye, July 2021. [Google Scholar]
- Güngör, A.; Akgül, G.; Bakan, M.F.; Erdem, E. Chemically exfoliated refined carbon from industrial tea waste for capacitive energy storage. Phys. Scr. 2024, 99, 035959. [Google Scholar] [CrossRef]
- Priya, M.B.; Anitha, V.; Shanmugam, M.; Ashwath, B.; Sylva, S.D.; Vigneshwari, S.K. Efficacy of chlorhexidine and green tea mouthwashes in the management of dental plaque-induced gingivitis: A comparative clinical study. Contemp. Clin. Dent. 2015, 6, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Mercantepe, T.; Yılmaz, A.; Topçu, A.; Bilgin, A.; Tümkaya, L.; Mercantepe, F. Investigation of the effect of Camellia Sinensis essence cream on skin burns. Life 2025, 15, 176. [Google Scholar] [CrossRef]
- Karaçimen, E.; Değirmenci, E. Brewing contradictions: State intervention and commodity dynamics in tea agriculture in Turkey. J. Agrar. Change 2025, 25, e12619. [Google Scholar] [CrossRef]
- Huang, Y.; He, Y.; Peng, Z.; Hu, H.; Yang, M.; Pan, H.; Zhao, S.; Li, Y. Effect of Pu-erh tea pomace on the composition and diversity of cecum microflora in Chahua chicken No. 2. Front. Vet. Sci. 2023, 10, 1289546. [Google Scholar] [CrossRef]
- Ko, S.Y.; Yang, C.J. Effect of Green Tea Probiotics on the Growth Performance, Meat Quality and Immune Response in Finishing Pigs. Asian Aust. J. Anim. Sci. 2008, 21, 1339–1347. [Google Scholar] [CrossRef]
- Rahmatillah, R.; Ramdani, D.; Hernaman, I.; Jayanegara, A.; Hidayatik, N. Evaluation of the Effects of Green Tea Extract as a Dietary Supplement in Sheep on Gas Production, Volatile Fatty Acids, and Digestibility. Vet. World 2024, 17, 2204–2210. [Google Scholar] [CrossRef]
- Aşık, B.B.; Kütük, C. Çay Atığı Kompostunun Çim Alanların Oluşturulmasında Kullanım Olanağı. Uludağ Üniv. Zir. Fak. Derg. 2012, 26, 47–57. [Google Scholar]
- Seth, D.; Athparia, M.; Singh, A.; Rathore, D.; Venkatramanan, V.; Channashettar, V.; Prasad, S.; Maddirala, S.; Sevda, S.; Kataki, R. Sustainable Environmental Practices of Tea Waste—A Comprehensive Review. Environ. Sci. Pollut. Res. 2025, 32, 7449–7467. [Google Scholar] [CrossRef]
- Glendinning, L.; Genç, B.; Wallace, J.; Watson, M. Metagenomic analysis of the cow, sheep, reindeer and red deer rumen. Sci. Rep. 2021, 11, 1990. [Google Scholar] [CrossRef] [PubMed]
- Vastolo, A.; Serrapica, F.; Cavallini, D.; Fusaro, I.; Atzori, A.S.; Todaro, M. Editorial: Alternative and novel livestock feed: Reducing environmental impact. Front. Vet. Sci. 2024, 11, 1441905. [Google Scholar] [CrossRef]
- Pushpalatha, R.; Roshni, T.; Sruthy, S.; Upadhyay, G. Potential mitigation practices to reduce methane emissions from livestock in rural India and policy recommendations. Environ. Monit. Assess. 2025, 197, 289. [Google Scholar] [CrossRef] [PubMed]
- Altan, A. Özel Gıdalar Teknolojisi, 1st ed.; Çukurova Üniversitesi Ziraat Fakültesi Ofset Atölyesi: Adana, Türkiye, 1997. [Google Scholar]
- Fernando, V.; Roberts, G.R. Effect of Process Parameters on Seasonal Development of Flavour in Black Tea. J. Sci. Food Agric. 1984, 35, 71–76. [Google Scholar] [CrossRef]
- Yalınkılıç, M.K.; Altun, L.; Kalay, Z. Çay Fabrikaları Çay Yaprağı Artıklarının Kompostlaştırılarak Orman Fidanlıklarında Organik Gübre Olarak Kullanılması. Ekoloji Derg. 1996, 18, 28–32. [Google Scholar]
- Kacar, B. Çay—Çay Bitkisi, Biyokimyası, Gübrelenmesi, İşleme Teknolojisi, 1st ed.; Nobel Yayınevi: Ankara, Türkiye, 2010; ISBN 978-605-395-359-3. [Google Scholar]
- Kunjikutty, N.; Ramachandran, P.; Devasia, P.A.; Thomas, C.T.; Nadakumaran, M. Evaluation of the Feeding Value of Tea Waste (Camellia thea) as an Ingredient in the Ration for Growing Pigs. Kerala J. Vet. Sci. 1977, 8, 127–132. [Google Scholar]
- Konwar, B.K.; Medhi, A.K.; Ahmed, H.F.; Saikia, A.; Das, P.C. Effect of Feeding Factory Tea Waste in Starter Chicks. Indian J. Poult. Sci. 1985, 20, 122. [Google Scholar]
- İmik, H.; Şeker, E. Farklı Tanen Kaynaklarının Tiftik Keçilerinde Yem Tüketimi, Canlı Ağırlık Artışı, Tiftik Verimi ve Kalitesi Üzerine Etkisi. Lalahan Hay. Arş. Ens. Derg. 1999, 39, 85–100. [Google Scholar]
- Ahmed, S.T.; Lee, J.W.; Mun, H.S.; Yang, C.J. Effects of Supplementation with Green Tea By-Products on Growth Performance, Meat Quality, Blood Metabolites and Immune Cell Proliferation in Goats. J. Anim. Physiol. Anim. Nutr. 2016, 99, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Nasehi, M.; Torbatinejad, N.; Rezaie, M.; Ghoorchi, T. Effect of Polyethylene Glycol Addition on Nutritive Value of Green and Black Tea Co-Products in Ruminant Nutrition. Asian J. Anim. Vet. Adv. 2017, 12, 254–260. [Google Scholar] [CrossRef]
- Angga, W.A.; Rizal, Y.; Mahata, M.E.; Yuniza, A.; Mayerni, R. Potential of Waste Tea Leaves (Camellia sinensis) in West Sumatra to Be Processed into Poultry Feed. Pak. J. Nutr. 2018, 17, 287–293. [Google Scholar] [CrossRef]
- Özyılmaz, N.; Genç, B. Determination of Nutrient Content and In Vitro Digestibility Values of Organic and Conventional Tea (Camellia sinensis) Factory Wastes. Int. J. Vet. Anim. Res. 2019, 2, 29–33. [Google Scholar]
- Zahedifar, M.; Fazaeli, H.; Safaei, A.R.; Alavi, S.M. Chemical Composition and In Vitro and In Vivo Digestibility of Tea Waste in Sheep. Iran. J. Appl. Anim. Sci. 2019, 9, 87–93. [Google Scholar]
- Genç, B.; Bilgici, C.A. Use of Tea (Camellia sinensis) Factory Waste in Laboratory Animal Breeding. In Advanced and Contemporary Studies in Health Sciences; Demirel, S., Ed.; Duvar Press: İzmir, Türkiye, 2023. [Google Scholar]
- Balentine, D.A.; Wiseman, S.A.; Bouwens, L.C. The Chemistry of Tea Flavonoids. Crit. Rev. Food Sci. Nutr. 1997, 37, 693–704. [Google Scholar] [CrossRef]
- İmik, H.; Tuncer, Ş.D.; Aylanç, A.; Aytaç, M.; Erdoğan, İ. The Effect of Tea Wastes Added to the Rations of Akkaraman Lambs on Some Performance Parameters. Ankara Üniv. Vet. Fak. Derg. 2002, 49, 51–57. [Google Scholar]
- Ma, Y.; Han, R.; He, Q.; Wang, Y.; Chen, X.; Li, J. Adsorption of Zn(II) from Aqueous Solution by Tea Waste. J. Hazard. Mater. 2008, 154, 1025–1030. [Google Scholar]
- Zheng, Q.; Han, C.; Zhong, Y.; Wen, R.; Zhong, M. Effects of Dietary Supplementation with Green Tea Waste on Growth, Digestive Enzyme and Lipid Metabolism of Juvenile Hybrid Tilapia, Oreochromis niloticus × O. aureus. Fish Physiol. Biochem. 2017, 43, 361–371. [Google Scholar] [CrossRef]
- Wang, Z.; Ahmad, W.; Zhu, A.; Zhao, S.; Ouyang, Q.; Chen, Q. Recent Advances Review in Tea Waste: High-Value Applications, Processing Technology, and Value-Added Products. Sci. Total Environ. 2024, 946, 174225. [Google Scholar] [CrossRef]
- Tan, C.Y.; Zhong, R.Z.; Tan, Z.L.; Han, X.F.; Tang, S.X.; Xiao, W.J.; Sun, Z.H.; Wang, M. Dietary Inclusion of Tea Catechins Changes Fatty Acid Composition of Muscle of Goats. Lipids 2011, 46, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Camargo, L.E.A.; Pedroso, L.S.; Vendrame, S.C.; Mainardes, R.M.; Khalil, N.M. Antioxidant and Antifungal Activities of Camellia sinensis (L.) Kuntze Leaves Obtained by Different Forms of Production. Braz. J. Biol. 2016, 76, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Gramza-Michałowska, A.; Kobus-Cisowska, J.; Kmiecik, D.; Korczak, J.; Helak, B.; Dziedzic, K.; Górecka, D. Antioxidative Potential, Nutritional Value and Sensory Profiles of Confectionery Fortified with Green and Yellow Tea Leaves (Camellia sinensis). Food Chem. 2016, 211, 448–454. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, N.; Dhanda, A.; Yadav, A.; Aggarwal, N.K. In Vivo Assessment of Antimicrobial, Antioxidant, Antigenotoxic and Antimutagenic Effects of Different Extracts from Camellia sinensis. Syst. Microbiol. Biomanuf. 2024, 4, 282–293. [Google Scholar] [CrossRef]
- Halliwell, B. Understanding Mechanisms of Antioxidant Action in Health and Disease. Nat. Rev. Mol. Cell Biol. 2024, 25, 13–33. [Google Scholar] [CrossRef]
- British Columbia Ministry of Forests. Collection of Specimens. In Techniques and Procedures for Collecting, Preserving, Processing, and Storing Botanical Specimens; Ministry of Forests: Victoria, BC, Canada, 1996; pp. 2–5. [Google Scholar]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 19th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2012. [Google Scholar]
- Keskin, H. Gıda Kimyası; İstanbul Üniversitesi Yayınları: İstanbul, Türkiye, 1975; pp. 1046–1047. [Google Scholar]
- Yalçın, S. Yem Analizleri. In Yemler, Yem Hijyeni ve Teknolojisi; Ergün, A., Tuncer, Ş.D., Eds.; Ankara Üniversitesi Veteriner Fakültesi Ders Kitabı: Ankara, Türkiye, 2011; Chapter 8; p. 372. [Google Scholar]
- ISO 14502-1:2005; Determination of Substances Characteristic of Green and Black Tea. Part 1: Content of Total Polyphenols in Tea. Colorimetric Method Using Folin–Ciocalteu Reagent. International Organization for Standardization: Geneva, Switzerland, 2005; pp. 1–8.
- ISO 14502-2005; Content of Catechins in Green Tea—Method Using High-Performance Liquid Chromatography. International Organization for Standardization: Geneva, Switzerland, 2005.
- ISO 10720-740; Steel and Iron—Determination of Nitrogen Content—Thermal Conductimetric Method after Fusion in a Current of Inert Gas. International Organization for Standardization: Geneva, Switzerland,, 1997.
- TS ISO 10720; Conformity Assessment—Requirements for the Operation of Various Types of Bodies Performing Inspection. Turkish Standards Institution: Geneva, Switzerland, 2012.
- Luna, L.G. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology, 3rd ed.; McGraw-Hill: New York, NY, USA, 1968; pp. 111–112. [Google Scholar]
- Erel, O. A Novel Automated Direct Measurement Method for Total Antioxidant Capacity Using a New Generation, More Stable ABTS Radical Cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Erel, O. A New Automated Colorimetric Method for Measuring Total Oxidant Status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef]
- Yang, Y.; Dai, C.; Chen, X.; Zhang, B.; Li, X.; Yang, W.; Feng, J. Role of Uranium Toxicity and Uranium-Induced Oxidative Stress in Advancing Kidney Injury and Endothelial Inflammation in Rats. BMC Pharmacol. Toxicol. 2024, 25, 14. [Google Scholar] [CrossRef]
- Bostancı, Ş.; Koca, İ. Theanine Contest of Tea Waste. In Proceedings of the 5th International Agriculture Congress, Istanbul, Türkiye, 21–24 August 2019. [Google Scholar]
- Bayrak, A.; Tekin, A.; Çalıkoğlu, E.; Poyrazoğlu, E. Effects of Flushing Periods, Growing Elevation and Processing Methods on Some Volatile Organic Compounds of Black Tea. In Proceedings of the 5th International Congress on Food Technology, Thessaloniki, Greece, 9–11 March 2007; Volume 2, pp. 69–76. [Google Scholar]
- Wu, Y.; Li, Q.; Cao, J.; Fan, F.; Gan, L.; Wu, R.; Jin, J.; Chen, R.; Sun, L.; Zhang, Z.; et al. Aged Black Tea Alleviates Constipation in Mice by Modulating Intestinal Neurotransmitters and Decreasing AQP3 and AQP9 Expression. Food Nutr. Res. 2023, 67, 9513. [Google Scholar] [CrossRef]
- Matsuda, H.; Nakamura, S.; Morikawa, T.; Muraoka, O.; Yoshikawa, M. New Biofunctional Effects of the Flower Buds of Camellia sinensis and Its Bioactive Acylated Oleanane—Type Triterpene Oligoglycosides. J. Nat. Med. 2016, 70, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Sur, T.K.; Chatterjee, S.; Hazra, A.K.; Pradhan, R.; Chowdhury, S. Acute and Sub-Chronic Oral Toxicity Study of Black Tea in Rodents. Indian J. Pharmacol. 2015, 47, 167–172. [Google Scholar]
- Isbrucker, R.A.; Edwards, J.A.; Wolz, E.; Davidovich, A.; Bausch, J. Safety Studies on Epigallocatechin Gallate (EGCG) Preparations. Part 2: Dermal, Acute and Short-Term Toxicity Studies. Food Chem. Toxicol. 2006, 44, 636–650. [Google Scholar] [CrossRef]
- Wang, D.; Xu, K.; Zhong, Y.; Luo, X.; Xiao, R.; Hou, Y.; Bao, W.; Yang, W.; Yan, H.; Yao, P.; et al. Acute and Subchronic Oral Toxicities of Pu-erh Black Tea Extract in Sprague-Dawley Rats. J. Ethnopharmacol. 2011, 134, 156–164. [Google Scholar] [CrossRef]
- Al-Attar, A.M.; Zari, T.A. Influences of Crude Extract of Tea Leaves, Camellia sinensis, on Streptozotocin Diabetic Male Albino Mice. Saudi J. Biol. Sci. 2010, 17, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Salem, R.R.; Mohamed, A.M.; El-Kenawy, A.M. Protective Effect of Green Tea Against the Hematological, Biochemical, Histopathological and Ultrastructural Changes in Rat Liver Induced by Subchronic Exposure to Melamine. Curr. Top. Toxicol. 2018, 14, 95–108. [Google Scholar]
- Azeez, A.A.; Mustafa, E.M.; Shoshin, M.A.O. Effect of Camellia sinensis on Fat Peroxidation and Ox-LDL in Rats. Arch. Razi Inst. 2021, 76, 949–955. [Google Scholar]
- Ayalogu, E.O.; Snelling, J.; Lewis, D.F.; Talwar, S.; Clifford, M.N.; Ioannides, C. Induction of Hepatic CYP1A2 by the Oral Administration of Caffeine to Rats: Lack of Association with the Ah Locus. Biochim. Biophys. Acta 1995, 1272, 89–94. [Google Scholar] [CrossRef]
- Goasduff, T.; Dréano, Y.; Guillois, B.; Méndez-Ferrer, J.; Berthou, F. Induction of Liver and Kidney CYP1A1/1A2 by Caffeine in Rat. Biochem. Pharmacol. 1996, 52, 1915. [Google Scholar] [CrossRef]
- Chan, P.C.; Ramot, Y.; Malarkey, D.E.; Blackshear, P.; Kissling, G.E.; Travlos, G.; Nyska, A. Fourteen-Week Toxicity Study of Green Tea Extract in Rats and Mice. Toxicol. Pathol. 2010, 38, 1070–1084. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Y.; Zeng, L.; Dong, F.; Tu, Y.; Yang, Z. Occurrence of Functional Molecules in the Flowers of Tea (Camellia sinensis) Plants: Evidence for a Second Resource. Molecules 2018, 23, 790. [Google Scholar] [CrossRef]
- Li, B.; Jin, Y.; Xu, Y.; Wu, Y.; Xu, J.; Tu, Y. Safety Evaluation of Tea (Camellia sinensis (L.) O. Kuntze) Flower Extract: Assessment of Mutagenicity, and Acute and Subchronic Toxicity in Rats. J. Ethnopharmacol. 2011, 133, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Takami, S.; Imai, T.; Hasumura, M.; Cho, Y.M.; Onose, J.; Hirose, M. Evaluation of Toxicity of Green Tea Catechins with 90-Day Dietary Administration to F344 Rats. Food Chem. Toxicol. 2008, 46, 2224–2229. [Google Scholar] [CrossRef]
- Gür, E.S.; Gülten, T.; Serdar, Z.; Colakoğulları, M. Chronic Black Tea Administration Protects Plasma Proteins, Plasma, Liver and Kidney Lipids Against Oxidation. Med. Sci. Monit. 2006, 12, 102–105. [Google Scholar]
- Da Silva, E.L.; Piskula, M.; Terao, J. Enhancement of Antioxidative Ability of Rat Plasma by Oral Administration of (–)-Epicatechin. Free Radic. Biol. Med. 1998, 24, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Serafini, M.; Ghiselli, A.; Ferro-Luzzi, A. In Vivo Antioxidant Effect of Green and Black Tea in Man. Eur. J. Clin. Nutr. 1996, 50, 28–32. [Google Scholar]
- Forester, S.C.; Lambert, J.D. The Role of Antioxidant Versus Pro-Oxidant Effects of Green Tea Polyphenols in Cancer Prevention. Mol. Nutr. Food Res. 2011, 55, 844–854. [Google Scholar] [CrossRef]
- Yan, Z.; Zhong, Y.; Duan, Y.; Chen, Q.; Li, F. Antioxidant Mechanism of Tea Polyphenols and Its Impact on Health Benefits. Anim. Nutr. 2020, 6, 115–123. [Google Scholar] [CrossRef]
- Patel, R.; Maru, G. Polymeric Black Tea Polyphenols Induce Phase II Enzymes via Nrf2 in Mouse Liver and Lungs. Free Radic. Biol. Med. 2008, 44, 1897–1911. [Google Scholar]
- Qi, G.; Mi, Y.; Fan, R.; Li, R.; Wang, Y.; Li, X.; Huanga, S.; Liu, X. Tea Polyphenols Ameliorate Hydrogen Peroxide-And Constant Darkness-Triggered Oxidative Stress via Modulating the Keap1/Nrf2 Transcriptional Signaling Pathway in HepG2 Cells and Mice Liver. RSC Adv. 2017, 7, 32198–32208. [Google Scholar] [CrossRef]
- Bogovski, P.; Day, N. Accelerating Action of Tea on Mouse Skin Carcinogenesis. Cancer Lett. 1977, 3, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Hirose, M.; Hoshiya, T.; Akagi, K.; Takahashi, S.; Hara, Y.; Ito, N. Effects of Green Tea Catechins in a Rat Multi-Organ Carcinogenesis Model. Carcinogenesis 1993, 14, 1549–1553. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.; Morrissey, R.; Crowell, J.; McCormick, D. Subchronic Oral Toxicity of Green Tea Polyphenols in Rats and Dogs. Toxicol. Sci. 1999, 48, 57–58. [Google Scholar]
- McCormick, D.; Johnson, W.; Morrissey, R.; Crowell, J. Subchronic Oral Toxicity of Epigallocatechin Gallate (EGCG) in Rats and Dogs. Toxicol. Sci. 1999, 48, 57. [Google Scholar]
- Stratton, S.P.; Bangert, J.L.; Alberts, D.S.; Dorr, R.T. Dermal Toxicity of Topical (−)-Epigallocatechin-3-Gallate in BALB/c and SKH1 Mice. Cancer Lett. 2000, 158, 47–52. [Google Scholar] [CrossRef]
- Gomes, A.; Das, M.; Vedasiromoni, J.R.; Ganguly, D.K. Proconvulsive Effect of Tea (Camellia sinensis) in Mice. Phytother. Res. 1999, 13, 376–379. [Google Scholar] [CrossRef]
- Yamane, T.; Hagiwara, N.; Tateishi, M.; Akachi, S.; Kim, M.; Okuzumi, J.; Kitao, Y.; Inagake, M.; Kuwata, K.; Takahashi, T. Inhibition of Azoxymethane-Induced Colon Carcinogenesis in Rat by Green Tea Polyphenol Fraction. Jpn. J. Cancer Res. 1991, 82, 1336–1339. [Google Scholar] [CrossRef]

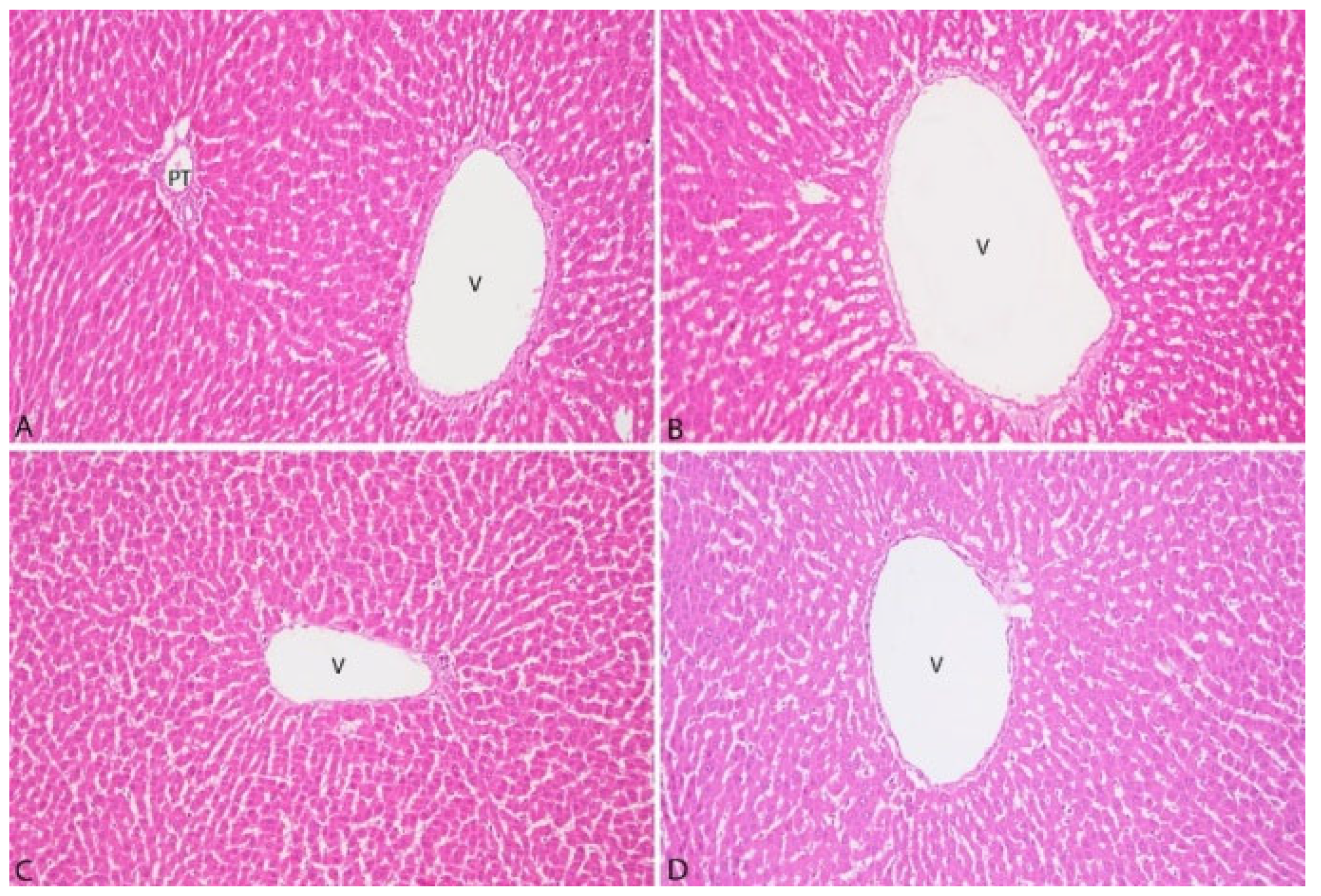
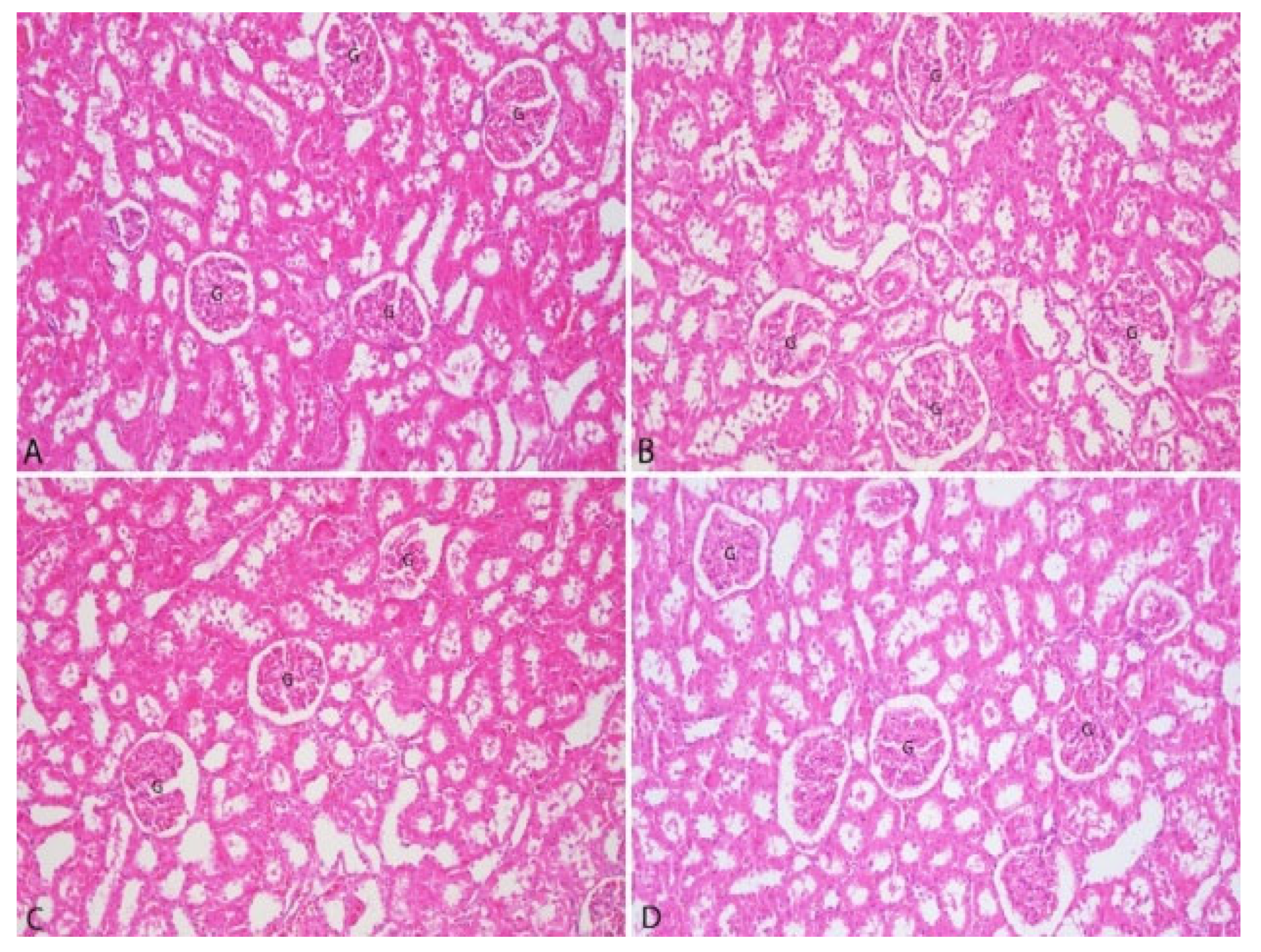
| Liver | Kidney |
|---|---|
| Bile Pigment in Hepatocytes | Proximal Tubular Epithelium Degeneration |
| Vacuolar Degeneration | Distal Tubular Epithelium Degeneration |
| Macrovesicular Steatosis | Crystalluria |
| Microvesicular Steatosis | Hypercellularity in Glomeruli |
| Inflammatory Cell Infiltration in the Portal Area | Glomerulosclerosis |
| Portal Area Fibrosis | Expansion of Bowman’s Space |
| Central Vein Congestion | Inflammatory Cell Infiltration in Interstitial Tissue |
| Congestion in the Sinusoids | |
| Stomach | Intestine |
| Lamina Epithelium Desquamation | Lamina Epithelium Desquamation |
| Inflammatory Cell Infiltration in Lamina Propria | Inflammatory Cells in Lamina Propria |
| Submucosal Edema | Lieberkühn Glands Hyperplasia |
| Submucosal Fibrosis | Lymphatic Dilatation |
| Parameters | Groups | ||||
|---|---|---|---|---|---|
| FTFW | C 0% | 1 (3%) | 2 (5%) | 3 (10%) | |
| TP (ppm) | 5.35 | 1.19 | 1.20 | 1.38 | 1.42 |
| TPDM (ppm) | 1.72 | n | n | n | 0.10 |
| GA (ppm) | 0.10 | n | n | n | n |
| Caffeine (ppm) | 1.17 | n | n | n | 0.09 |
| ECG (PPM) | 0.28 | n | n | n | n |
| DM (%) | 92.00 | 93.00 | 93.20 | 92.90 | 93.00 |
| CP (%) | 14.00 | 24.00 | 24.00 | 24.00 | 24.00 |
| EE (%) | 1.20 | 4.00 | 4.00 | 4.00 | 4.00 |
| CA | 3.50 | 8.40 | 8.38 | 8.41 | 8.40 |
| CC | 18.00 | 6.00 | 6.02 | 6.03 | 6.05 |
| ME (kcal/kg) | 2400 | 2700 | 2700 | 2700 | 2700 |
| Groups | Internal Organs | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Heart (g) | Liver (g) | Lung (g) | Stomach (g) | Spleen (g) | Kidney (g) | Brain (g) | Small Intestine (g) | Large Intestine (g) | Small Intestine Length (cm) | Large Intestine Length (cm) | |
| C (0%) | 1.22 ± 0.05 | 10.22 ± 0.38 | 2.02 ± 0.13 | 1.78 ± 0.09 | 0.66 ± 0.03 | 2.97 ± 0.09 | 1.49 ± 0.11 | 9.52 ± 0.48 | 4.17 ± 0.36 | 120.20 ± 3.06 | 21.40 ± 1.39 |
| 1 (3%) | 1.30 ± 0.03 | 11.24 ± 0.37 | 2.24 ± 0.12 | 1.83 ± 0.05 | 0.64 ± 0.02 | 3.05 ± 0.12 | 1.60 ± 0.10 | 9.79 ± 0.41 | 4.45 ± 0.30 | 128.60 ± 2.80 | 19.30 ± 1.00 |
| 2 (5%) | 1.23 ± 0.03 | 10.91 ± 0.46 | 2.18 ± 0.08 | 1.86 ± 0.08 | 0.70 ± 0.02 | 3.08 ± 0.18 | 1.45 ± 0.11 | 9.67 ± 0.36 | 4.48 ± 0.32 | 122.80 ± 2.86 | 19.70 ± 0.59 |
| 3 (10%) | 1.27 ± 0.04 | 11.23 ± 0.38 | 2.10 ± 0.10 | 1.96 ± 0.07 | 0.63 ± 0.02 | 2.98 ± 0.09 | 1.41 ± 0.10 | 9.85 ± 0.36 | 4.85 ± 0.22 | 121.40 ± 3.67 | 21.50 ± 1.20 |
| p-value | 0.505 | 0.257 | 0.537 | 0.384 | 0.345 | 0.918 | 0.652 | 0.942 | 0.500 | 0.252 | 0.365 |
| Groups | Week | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| First Day | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | |
| C (0%) | 218.70 ± 7.03 | 261.10 ± 10.00 | 298.10 ± 12.26 | 324.60 ± 13.55 | 345.10 ± 13.37 | 367.20 ± 13.89 | 384.80 ± 14.46 | 396.70 ± 14.45 | 415.60 ± 15.72 | 422.60 ± 15.83 |
| 1 (3%) | 219.10 ± 7.18 | 270.10 ± 8.28 | 309.70 ± 10.12 | 338.50 ± 11.08 | 365.30 ± 11.45 | 391.30 ± 12.28 | 409.70 ± 13.34 | 424.80 ± 12.88 | 442.30 ± 14.00 | 449.60 ± 14.07 |
| 2 (5%) | 217.70 ± 5.52 | 261.50 ± 9.35 | 300.00 ± 9.81 | 328.70 ± 10.60 | 354.60 ± 10.58 | 377.40 ± 11.15 | 394.60 ± 11.95 | 407.40 ± 12.99 | 422.50 ± 13.65 | 432.40 ± 14.21 |
| 3 (10%) | 218.80 ± 8.66 | 267.60 ± 9.95 | 299.80 ± 10.66 | 335.30 ± 11.46 | 358.80 ± 10.12 | 381.80 ± 9.94 | 400.90 ± 10.87 | 413.00 ± 10.34 | 430.10 ± 9.78 | 439.20 ± 10.67 |
| p-value | 0.999 | 0.878 | 0.868 | 0.834 | 0.654 | 0.555 | 0.571 | 0.480 | 0.546 | 0.543 |
| Parameter | Groups | |||
|---|---|---|---|---|
| C (0%) | 1 (3%) | 2 (5%) | 3 (10%) | |
| Glucose (mg/dL) | 129.4 ± 25.0 | 128.8 ± 20.1 | 130.1 ± 24.4 | 126.7 ± 20.2 |
| Total protein (g/L) | 64.2 ± 3.1 | 62.1 ± 4.1 | 61.5 ± 3.4 | 61.4 ± 2.9 |
| Albumin (g/L) | 30.5 ± 1.5 | 29.4 ± 2.3 | 29.8 ± 1.6 | 30.6 ± 1.4 |
| Triglyceride (mg/dL) | 43.8 ± 12.9 | 34.4 ± 11.5 | 40.1 ± 12.8 | 38.1 ± 11.4 |
| Total cholesterol (mg/dL) | 50.8 ± 8.4 | 49.9 ± 8.6 | 51.1 ± 8.2 | 52.0 ± 11.0 |
| HDL cholesterol (mg/dL) | 26.8 ± 4.5 | 25.2 ± 3.3 | 27.7 ± 2.7 | 29.9 ± 3.8 |
| LDL cholesterol (mg/dL) | 13.3 ± 1.2 | 13.2 ± 1.0 | 14.1 ± 0.9 | 13.4 ± 1.3 |
| Total bilirubin (mg/dL) | 0.54 ± 0.35 | 0.43 ± 0.24 | 0.57 ± 0.21 | 0.44 ± 0.31 |
| AST (U/L) | 65.8 ± 10.6 | 57.3 ± 7.4 | 59.6 ± 13.3 | 59.8 ± 9.8 |
| ALT (U/L) | 28.9 ± 5.8 | 27.1 ± 4.6 | 29.3 ± 5.2 | 28.2 ± 2.1 |
| ALP (U/L) | 127.9 ± 32.9 | 120.2 ± 23.0 | 133.1 ± 32.4 | 135.8 ± 29.5 |
| GGT (U/L) | 3.5 ± 0.5 | 3.7 ± 0.7 | 3.3 ± 0.7 | 3.9 ± 0.7 |
| LDH (U/L) | 241.1 ± 20.2 | 253.0 ± 23.9 | 261.6 ± 27.7 | 254.3 ± 32.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genç, B.; Kuruca, N.; Yarım, G.F.; Güvenç, T.; Özan, E.; Müftüoğlu, B.; İde, T.; Erdoğan, A.N.D.; Odacı, S. Use of Fermented Black Tea (Camellia sinensis) Factory Wastes in Standard Rat Diets. Vet. Sci. 2025, 12, 451. https://doi.org/10.3390/vetsci12050451
Genç B, Kuruca N, Yarım GF, Güvenç T, Özan E, Müftüoğlu B, İde T, Erdoğan AND, Odacı S. Use of Fermented Black Tea (Camellia sinensis) Factory Wastes in Standard Rat Diets. Veterinary Sciences. 2025; 12(5):451. https://doi.org/10.3390/vetsci12050451
Chicago/Turabian StyleGenç, Buğra, Nilüfer Kuruca, Gül Fatma Yarım, Tolga Güvenç, Emre Özan, Bahadır Müftüoğlu, Tayfun İde, Aşkın Nur Derinöz Erdoğan, and Serdar Odacı. 2025. "Use of Fermented Black Tea (Camellia sinensis) Factory Wastes in Standard Rat Diets" Veterinary Sciences 12, no. 5: 451. https://doi.org/10.3390/vetsci12050451
APA StyleGenç, B., Kuruca, N., Yarım, G. F., Güvenç, T., Özan, E., Müftüoğlu, B., İde, T., Erdoğan, A. N. D., & Odacı, S. (2025). Use of Fermented Black Tea (Camellia sinensis) Factory Wastes in Standard Rat Diets. Veterinary Sciences, 12(5), 451. https://doi.org/10.3390/vetsci12050451






