1. Introduction
Copper (Cu) is an integral component of cellular physiology due to its unique chemical properties. Unlike most physiologically relevant metals, Cu accepts and donates electrons under physiological conditions and cycles between two oxidation states. Cu is an essential element in the synthesis of ceruloplasmin, which plays a key role in the metabolism of iron by helping to convert stored forms of iron into forms available for use by the body, thereby promoting iron absorption and utilization [
1]. Cu is also a component of a variety of antioxidant enzymes, including Cu/Zn-superoxide dismutase (Cu/Zn-SOD, SOD1), which can remove superoxide radicals from the body and protect cells from oxidative stress damage [
2].
Cu plays a very important role in the body of ruminants. It is an essential trace element for maintaining the normal physiological function, biochemical metabolism and the growth and development of ruminants. As early as the 1940s, hypocopper in cattle had been reported [
3]. Today, copper deficiency in cattle is still common. In addition to the direct primary copper deficiency caused by insufficient copper intake, the main cause of copper deficiency in cattle is secondary copper deficiency caused by interference with copper absorption. Due to the interaction between Cu, molybdenum and sulfur (Cu-Mo-S) in the rumen of ruminants, the level of Cu absorption by ruminants is much lower than that of non-ruminants [
4]. In western China, yaks develop a disease locally known as “sway disease” characterized by loss of appetite, diarrhea, pica, poor growth, emaciation, dyskinesia (walking slowly with slight signs of rock and shivering) and sway, which is caused by secondary copper deficiency [
5]. Yaks in the northern Qinghai–Tibet Plateau of China have symptoms of secondary copper deficiency induced by molybdenum. Yaks with copper deficiency are emaciated and unstable, and the symptoms of copper deficiency are most serious during pregnancy and lactation [
6]. From 2014 to 2019, copper deficiency occurred in almost all cattle farms in eastern and western Canada, with 20–40% of cows in each farm having lower serum copper content than normal, and the majority of such copper deficient cows showed symptoms of copper deficiency [
7]. Copper deficiency can also cause morphological changes in bovine heart, resulting in significant damage to myocardium, high lipid oxidation level, and significantly reduced Cu-Zn superoxide dismutase activity [
8].
It has been shown that adequate copper concentrations improve intracellular GSH content in oocytes and cumulus cells, the DNA integrity of cumulus cells during in vitro maturation of bovine oocytes, and the development of preimplantation embryos [
9]. Additionally, Cu may promote the secretion of luteinizing hormone-releasing hormone from isolated hypothalamic granules [
10,
11]. Copper deficiency can indirectly affect reproductive processes by impairing bone development and keratinization, reducing antioxidant capacity in cows, and impacting angiogenesis [
12,
13]. In 1936, researchers found that ovulation could be induced in model animals by injection of copper salts [
11]. Cu can maintain the erratic activity of pituitary hormones in the blood and also promote the release of gonadotropin-releasing hormone and luteinizing hormone. Studies have shown that Cu
2+ levels in follicular fluid affect steroid hormone production by follicular granulosa cells [
14]. Cu can also affect the normal development and ovulation of follicles. Studies have shown that the number of follicles in the ovary is significantly reduced by short-term high-dose Cu treatment, and long-term high-dose Cu treatment can affect the whole ovary [
15]. In the reproductive system of female mammals, oocytes, as female reproductive cells, have become important experimental subjects in reproductive and developmental biology research due to their unique biological characteristics. Granulocytes (GCs) play multiple key roles in the follicular microenvironment: on the one hand, they support follicular growth by providing nutrients and bioactive factors, and on the other hand, they regulate the maturation process of oocytes by synthesizing reproductive hormones such as estradiol (E2) and progesterone (P4) [
16]. From the perspective of cellular interaction, GCs coordinate signal communication between oocytes and follicular membrane cells through their proliferative activity and endocrine function, thereby participating in the regulation of various stages of follicular development [
16]. Jiang et al. further confirmed that GCs are not only an ideal cell model for studying the regulation of follicle development by steroid hormones, but also a core regulator for maintaining the stability of the follicular environment and promoting oocyte development [
17]. Our previous studies have shown that Cu
2+ can induce steroid hormone secretion in BGCs (Bos grunniens granlosa cells), but the specific mechanism remains unclear.
The aim of this study is to elucidate the mechanism by which Cu2+ affects the secretion of granulosa cell hormones. Quantitative real-time PCR, Western blotting and transcriptome analysis were used to elucidate the effect of Cu2+ on steroid hormone synthesis in BGCs and the underlying molecular mechanisms. The results of this study may provide new insights and theoretical support for addressing reproductive disorders associated with copper deficiency in yaks.
2. Materials and Methods
2.1. Materials
Cu sulfate (CuSO4; CAS No. 7758-98-7; AR 99%) was sourced from Chengdu Kelong Chemical Co., Ltd. (Chengdu, China). Ammonium tetrathiomolybdate (TTM; CAS No. 15060-55-6) was obtained from Sigma Aldrich (St. Louis, MO, USA). Phenylmethanesulfonyl fluoride (PMSF; CAS No. ST506), Nonidet P-40 (NP40) lysis buffer (Cat No. P0013F), BeyoECL Star (Cat No. P0018AM), and bicinchoninic acid (BCA) protein assay kit (Cat No. P0012) were purchased from Beyotime Biotechnology (Shanghai, China). Estradiol (E2) Enzyme-linked immunosorbent assay (ELISA) kits (Cat No. MM-0023O1) and progesterone (P4) ELISA kits (Cat No. MM-50918O1) were sourced from Mmbio (Jiangsu, China). Follicle-stimulating hormone (FSH; Cat No. F8470) was obtained from Solarbio (Beijing, China), and testosterone (Cat No. T102169) was obtained from Aladdin (Shanghai, China). Superoxide Dismutase (SOD) typed assay kit (Cat No. A001-2-2) was obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
The antibodies used in this study included: anti-rabbit StAR (bs-3570R, Bioss, Beijing, China, 1:500), anti-rabbit CYP19A1 (ET1703-77, HuaBio, Zhejiang, China, 1:1000), anti-rabbit CYP11A1 (13363-1-AP, HuaBio, 1:1000), anti-rabbit HSD3B1 (HA500082, HuaBio, 1:1000), anti-rabbit HSD17B1 (ER1910-88, HuaBio, 1:1000), anti-rabbit LOX (ET1706-31, HuaBio, 1:1000).
2.2. Cell Culture and Treatments
Ovarian tissues from yaks (
n = 18; aged 3 to 4 years) were procured from a slaughterhouse located in Chengdu. BGCs were extracted with reference to previous studies [
18]. The cells were seeded into T25 culture flasks after adjusting the concentration to 5 × 10
6 and then incubated in a cell incubator at 37 °C and 5% CO
2. In subsequent experiments aimed at investigating alterations in steroidogenesis within BGC cultures, the BGC were cultured in a basal medium (DMEM/F-12 containing 1% penicillin-streptomycin-amphotericin B and 10% FBS) supplemented with 20 ng/mL testosterone and 30 mIU of follicle-stimulating hormone (FSH) [
19,
20]. TTM is a high-affinity copper complex with excellent safety qualities. It forms a stable three-way complex with serum albumin and Cu
2+, so that the complex Cu
2+ cannot be used by cells, and is often used to chelate intracellular Cu
2+ [
21]. In this trial, low Cu
2+ status was determined by TTM and SOD1 activity was used as a marker of Cu
2+ status [
22]. A higher Cu
2+ state was established by additional supplementation of CuSO
4. BGCs were divided into five groups: control group, TTM group (TTM 10, 20 μmol), CuSO
4 group (CuSO
4 150, 300 μmol). They were treated for 12 and 24 h, respectively.
2.3. Cellular Cu Assay
Cell samples and media were collected and quantified utilizing a cell counting instrument (IC1000; Countstar, Shanghai, China), followed by analysis through inductively coupled plasma mass spectrometry (ICP-MS). The samples underwent digestion with 69–70% nitric acid at a temperature of 95 °C for a duration of 2 h. Subsequent to digestion, the samples were filtered using polytetrafluoroethylene membrane filters and subsequently analyzed with an Agilent 7800 ICP-MS system (Agilent Technologies, Inc., Santa Clara, CA, USA).
2.4. Transcriptome Sequencing and Transcriptomic Analysis
BGCs were divided into three groups: a control group, a TTM group (TTM at 20 μmol), and a Cu group (CuSO
4 at 300 μmol), with three biological replicates per group. Cell samples were collected 24 h post-treatment. Sequencing and analysis were conducted by Novogene Ltd. (Beijing, China). RNA was extracted from the cells using standard protocols, and RNA integrity was assessed using an Agilent 2100 Bioanalyzer. Complementary DNA (cDNA) libraries were constructed with the Illumina TruSeq RNA Sample Preparation Kit (Illumina, CA, USA). All samples were aligned with the bovine genome (
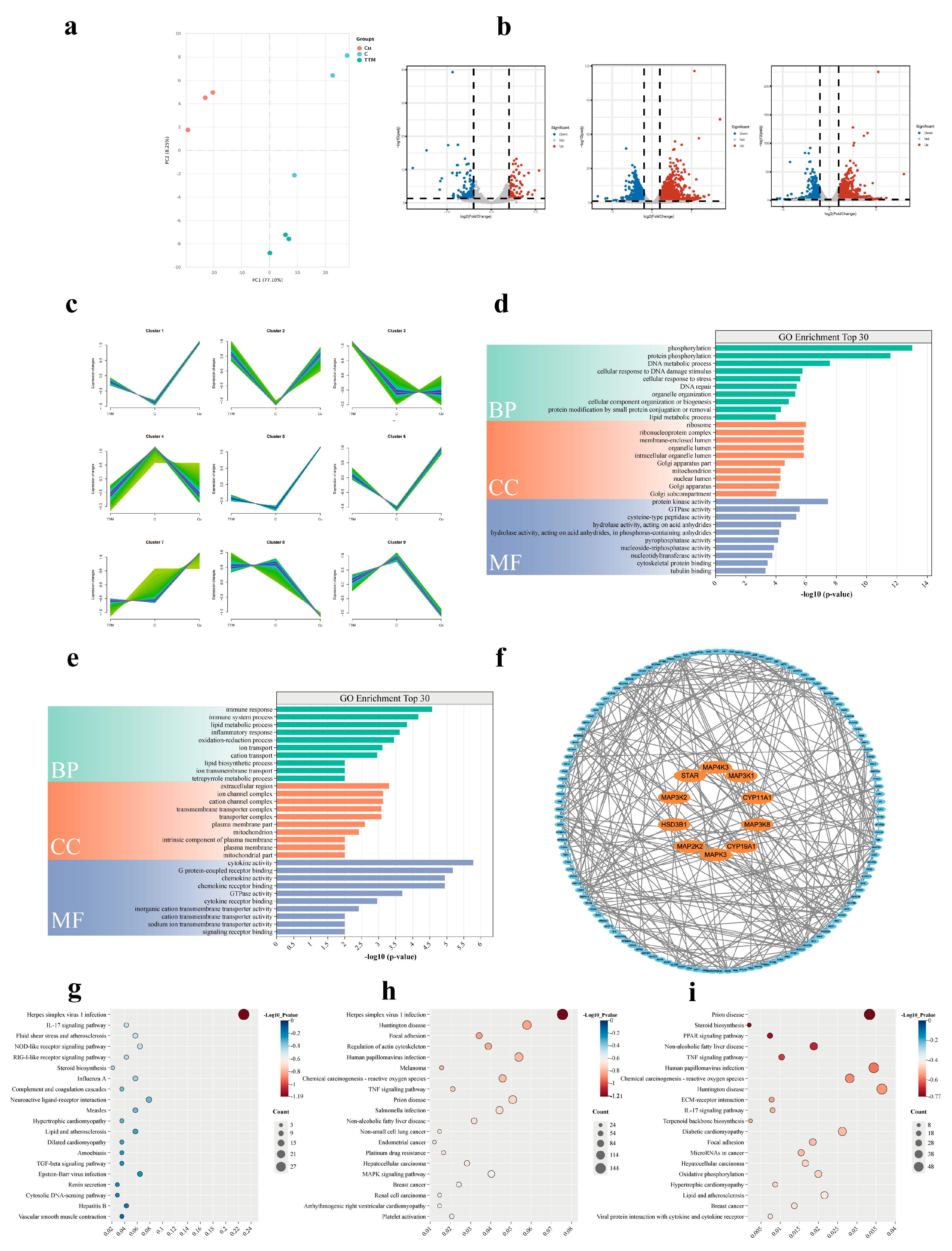
http://ftp.ensembl.org/pub/release-105/fasta/bos_taurus/) (accessed on 12 June 2024) to meet library screening requirements. Principal component analysis (PCA) was performed based on the gene expression values (FPKM) across all samples. Differentially expressed genes (DEGs) between groups were identified using a significance threshold of
p ≤ 0.05. Following differential gene analysis, Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses were performed using the ClusterProfiler R software (1.21.1.202) package. Genes associated with steroid hormones and the MAPK signaling pathway were selected based on enrichment results. Protein–protein interaction (PPI) networks were constructed using the STRING database (
https://cn.string-db.org/) (accessed on 13 September 2024) with Homo sapiens protein interaction data, considering interactions with scores above the 0.7 threshold as significant. The resulting PPI networks were visualized using Cytoscape software (3.10.3).
2.5. Cells SOD1 Activity Assay
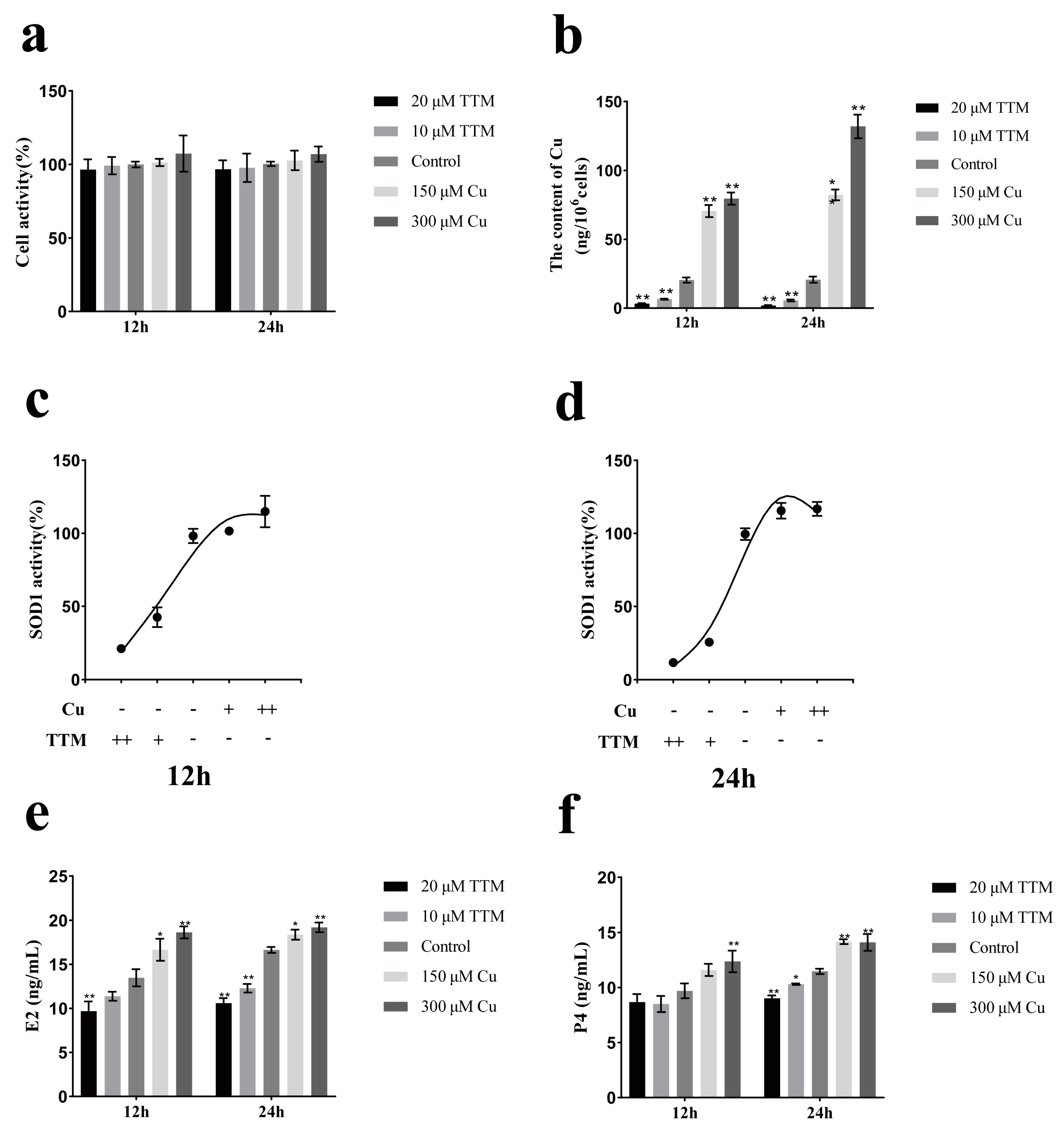
After BGCs were treated with CuSO4 or TTM for 12 and 24 h, cells were washed 1–2 times with precooled saline on ice or at 4 °C. The precipitate was homogenized in precooled saline at 4 °C and collected in a centrifuge tube. Then, the collection was centrifuged at 4 °C at a speed of 12,000 r/min, and the resulting supernatant was detected according to the SOD1 genotyping assay kit instructions.
2.6. Cell Activity Assay
BGCs were cultured in 96-well plates until they reached approximately 60% confluence. Following this, the culture medium was replaced with varying concentrations of CuSO4 and TTM, and the cells were treated for either 12 or 24 h. Upon completion of the treatment, the wells were rinsed two to three times with phosphate-buffered saline (PBS). Subsequently, 90 μL of fresh medium and 10 μL of MTT (Methylthiazolyldiphenyl-tetrazolium bromide) solution (M1020, Solarbio, Beijing, China) were introduced into each well, and the incubation continued for a duration of 4 h. At the conclusion of the incubation period, the supernatant was removed, and 110 μL of formazan solution was added to each well. The plate was then gently agitated at a low speed for 10 min to ensure complete dissolution of the resulting crystals. Finally, the absorbance of each well was quantified at a wavelength of 490 nm using an enzyme-linked immunoassay reader.
2.7. ELISA
The treated cell culture medium was collected, and the clear supernatant was isolated through centrifugation at speeds ranging from 2000 to 3000 RPM. Estradiol (E2, MM-0023O1) and progesterone (P4, MM-50918O1) were sourced from Mmbio in China and were allowed to equilibrate at room temperature prior to their application. Samples, standards, and blank controls were established, and precoated microtiter wells were prepared accordingly. The specimens, standards, and horseradish peroxidase (HRP)-labeled assay antibodies were sequentially added, followed by incubation at elevated temperatures and a thorough washing procedure. The colorimetric reaction was initiated by the substrate TMB (3, 3′,5,5′-Tetramethylbenzidine), which exhibits a blue color in the presence of peroxidase and transitions to yellow upon the addition of an acidic solution. The intensity of the resulting color is directly proportional to the concentration of E2 or P4 present in the sample. The absorbance of each well was measured at a wavelength of 450 nm utilizing an enzyme-linked immunoassay reader.
2.8. Western Blotting
BGC was cleaved with NP40 and 1% phenylmethane sulfonyl fluoride (PMSF). Lysate supernatants were collected and protein concentrations were quantified using the BCA Protein Assay kit. Subsequently, the separated protein samples were separated by SDS-PAGE and then transferred to PVDF membranes. Finally, ImageJ2x software was used to analyze significant changes in protein expression.
2.9. Quantitative Reverse Transcription PCR (RT q-PCR)
After drug treatment with BGCs, total RNA was extracted using an animal total RNA isolation kit (RE-03014, Foregene, Chengdu, China). RNA was reverse transcribed into complementary DNA (cDNA) using the PrimeScript
®RT Kit (RR047A, Takara, Tokyo, Japan). Primers were designed and synthesized by Sangyo Bioengineering (Shanghai, China) Ltd., as detailed in
Table 1. CT values were obtained using SYBR PRIME qPCR (FASTHS) (BG0014, Baoguang, Chongqing China) after predenaturation, denaturation, annealing, and extension. The amplification was monitored using the LightCycler
®480 Real-Time PCR System (Bio-Rad, Hercules, CA, USA), with the β-actin gene employed as an internal control. The quantification of mRNA expression levels was performed using the 2−ΔΔCT method.
2.10. Statistical Analysis
Data analysis was performed using GraphPad Prism 8.0 and SPSS software (version 21.0, IBM Corporation, Armonk, NY, USA). Independent samples t-tests and one-way analysis of variance (ANOVA) were employed to compare data across different groups. The results are presented as means ± standard deviation, with a p-value of less than 0.05 indicating statistical significance.
4. Discussion
Cu is an essential trace element for the growth and development of humans and animals. It is a cofactor for many proteins and enzymes. Due to their unique digestive system, ruminants have a much lower level of Cu absorption than non-ruminants [
4]. Molybdenum induced secondary Copper deficiency is present in yaks from the northern Tibetan Plateau of China, and Copper deficiency is most severe during pregnancy and lactation. Granulosa cells are important participants in follicular development and female reproduction, and the steroid hormones estradiol and progesterone secreted by granulosa cells are essential for maintaining the reproductive process of female animals [
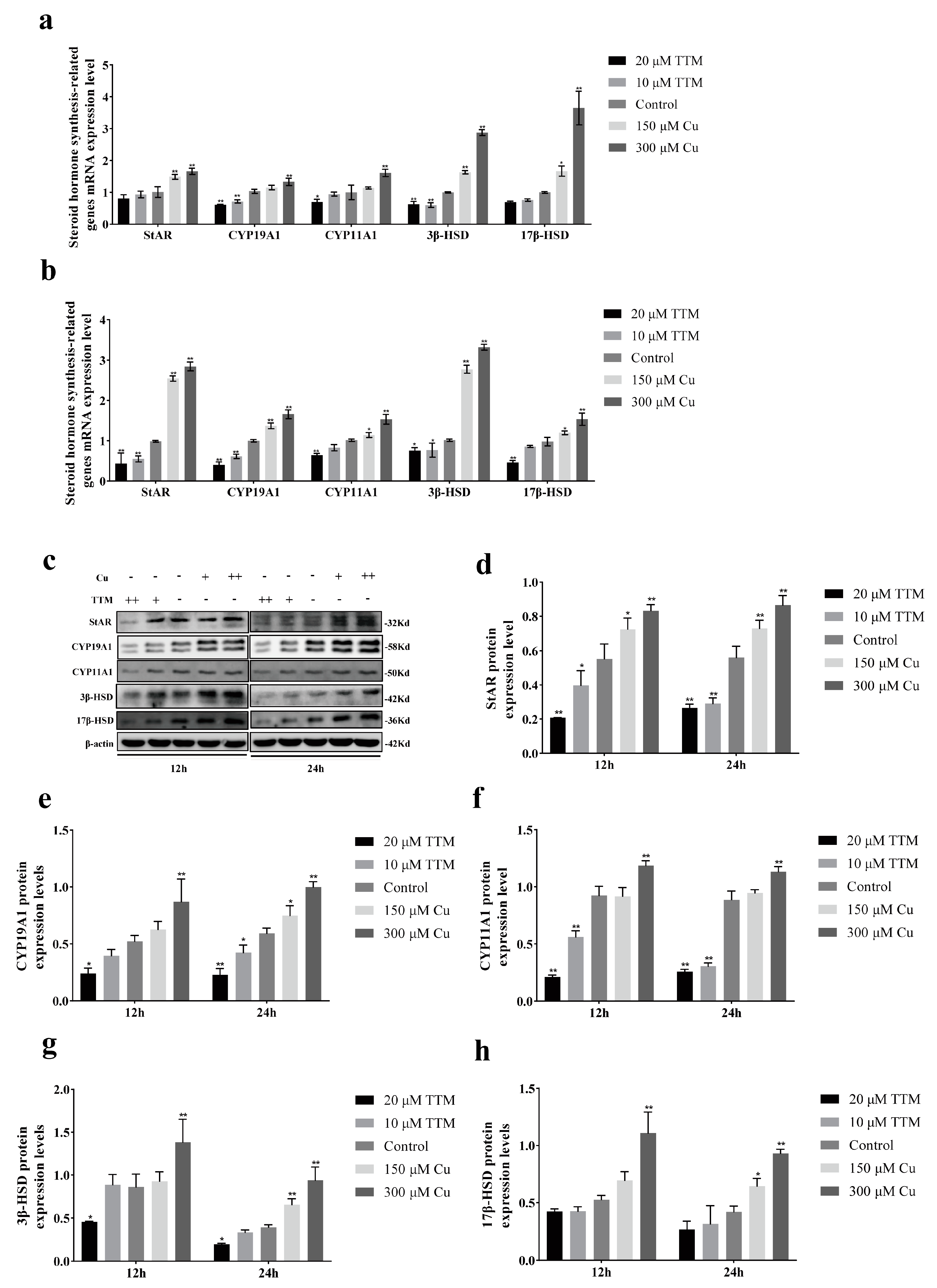
23]. This study found that Cu promoted hormone secretion by regulating the expression of steroid synthetase, such as StAR, CYP19A1, 3β-HSD, etc. Transcriptome sequencing further revealed that Cu was significantly enriched in the MAPK signaling pathway. This study demonstrates the molecular mechanism of Cu regulating BGCs hormone secretion, and provides a new idea for the treatment of reproductive disorders related to copper deficiency in yaks.
Granulosa cells can provide a large number of substances and growth factors for follicles, and can also promote oocyte maturation and follicle development by secreting steroid hormones such as E2 and progesterone P4. In this study, Cu
2+ and TTM were used to treat primary BGCs, and then the Cu
2+ status in BGCs was determined by detecting intracellular copper concentration and SOD1 activity. The rise and fall in SOD1 and intracellular copper concentrations indicate that BGCs are in a state of high or low copper utilization. The results showed that there was a positive correlation between Cu
2+ content and hormone secretion of BGCs. Alexander V Sirotkin et al. showed that Cu nanoparticles could promote the release of E2 and P4 from ovarian granulosa cells [
24]. S Roychoudhury et al. found that the addition of copper during the in vitro culture of porcine follicle granulosa cells could promote the secretion of steroid hormones [
25]. This study also showed that Cu
2+ promotes the gene and protein expression levels of steroid synthetase in BGCs (
Figure 2). This is similar to the results of J C Veltman, where transient treatment of Cu
2+ in mitochondria promotes a significant increase in cytochrome P450-dependent steroid 11β-hydroxylase activity [
26]. In conclusion, the results of the present study indicate that Cu
2+ promotes the secretion of E2 and P4 from BGCs. StAR is a transporter located in the mitochondrial membrane that facilitates the transfer of cholesterol from the outer mitochondrial membrane to the inner mitochondrial membrane. This process is crucial, as it serves as a key rate-limiting step in the biosynthesis of steroid hormones. CYP11A1 initiates the synthesis of steroid hormones, while 3β-HSD and 17β-HSD function as dehydrogenases.
Transcriptome sequencing results showed that copper level significantly affected the gene expression profile of BGCs. Compared with the control group, the expression of 2773 genes was up-regulated and 1973 genes were down-regulated in the Cu group. In contrast, 213 genes were up-regulated and 94 genes were down-regulated in the TTM group. Further analysis showed that the expression of steroid synthetase genes (such as StAR, CYP19A1, CYP11A1, 3β-HSD, 17β-HSD) was significantly up-regulated in the Cu group, which was consistent with the results of hormone detection, indicating that Cu2+ enhanced the hormone secretion ability of BGCs by promoting the expression of steroid synthetase. Functional enrichment analysis of these DEGs showed that the Cu group was significantly enriched in MAPK signaling pathway, TNF signaling pathway and other pathways related to cell proliferation and hormone synthesis, while the TTM group was enriched in herpes simplex virus type 1 infection, the IL-17 signaling pathway and the steroid biosynthesis pathway. Notably, DEGs in the TTM group were significantly enriched in steroid biosynthesis pathways, but their expression levels were generally down-regulated, suggesting that copper deficiency may lead to reduced hormone secretion by inhibiting the expression of genes related to steroid synthesis. This finding is consistent with the results of hormone assays in the TTM group and further validates the critical role of Cu2+ in the hormone synthesis of BGCs.
These results suggest that copper may affect the hormone secretion function of BGCs by regulating the MAPK signaling pathway and the expression of related genes. In addition, protein interaction network analysis showed significant interactions between MAPK pathway related genes (such as MAPK3K1, MAPK3K8, MAPK3, etc.) and steroid synthetase genes, further supporting the hypothesis that Cu
2+ regulates hormone synthesis through the MAPK signaling pathway. Studies have shown that copper ions are transported by copper transporter 1 (Ctr1) to the insides of cells, and enter the mitochondria through specific chaperone proteins (such as COX17) and transporters (such as SCO1/2) to participate in the assembly of respiratory chain enzymes. When copper is out of equilibrium, excess copper significantly increases reactive oxygen species (ROS) production by interfering with the electron transport chain and catalyzing the Fenton reaction. ROS acts as a second messenger to activate downstream ERK1/2, which can phosphorylate transcription factors such as CREB (cAMP Response Element-Binding Protein) and SF-1 (Steroidogenic Factor 1), thereby promoting the transcription of genes such as StAR and CYP19A1 [
25,
26,
27]. In addition, when copper ions are introduced into cells by Ctr1, they are reduced to Cu⁺ by intracellular reductases, and copper chaperones such as CCS, COX17, and ATOX1 transport Cu⁺ to specific target proteins and activate, such as superoxide dismutase 1 (SOD1), cytochrome C oxidase (CCO), and other enzymes in the Golgi apparatus. Copper has also been shown to regulate the phosphorylation activity of metallochaperones such as CCS by interacting with the metal-binding domains of MEK1/2 or ERK1/2 [
28]. In conclusion, copper ions can activate MAPK signaling pathways through multiple pathways, but the mechanism of their influence in BGCs hormone secretion remains unclear. The transcriptomic results of this paper suggest that the MAPK signaling pathway is involved in the production of steroid hormones in BGC by copper, so how copper affects the hormone secretion of BGCs through the MAPK signaling pathway will be the focus of our future research.
Taken together, the transcriptome data reveal the molecular mechanism by which Cu2+ affects the hormone secretion of BGCs by regulating the expression of genes involved in MAPK signaling pathway and steroid synthesis. These results provide an important molecular basis for a better understanding of the role of Cu in yak reproduction and provide potential targets for the treatment of reproductive disorders associated with Copper deficiency. Although this study revealed the promoting effect of Cu2+ on hormone synthesis in BGCs, further in vivo experiments are needed to verify its effectiveness and explore the effects of other potential signaling pathways.