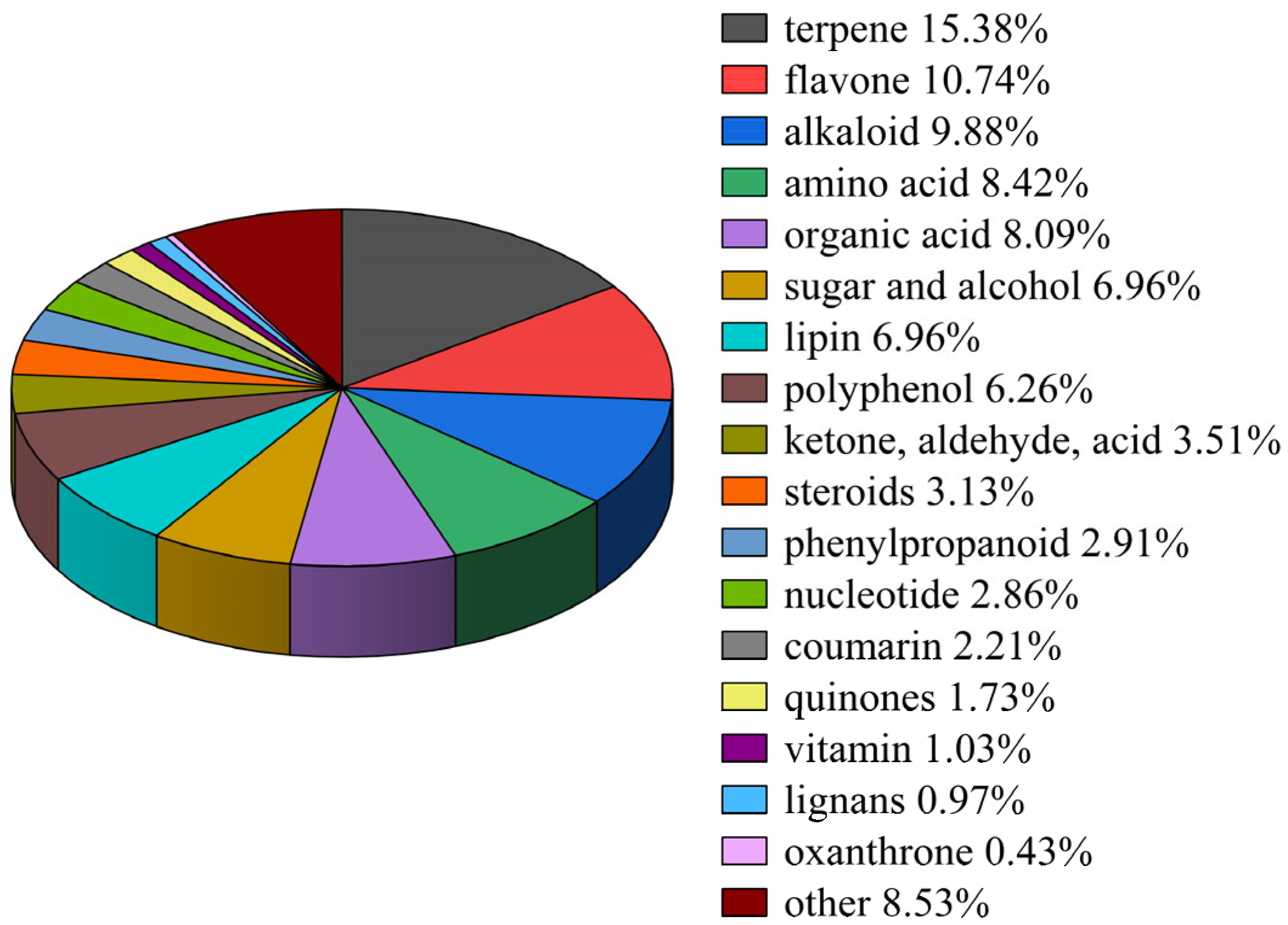
1. Introduction
The fruit, root, bark, and leaves of
Lycium barbarum have medicinal value in traditional Chinese medicine [
1]. The close relative
Lycium ruthenicum Murr. contains a variety of nutrients and is rich in polyphenols, polysaccharides, alkaloids, anthocyanins, and other biochemical components that protect the organism against inflammation, oxidation, aging, tumor growth, and fatigue [
2,
3]. Chinese herbal medicine is rich in a variety of bioactive compounds that represent a good substitute for synthetic antibiotics [
4]. In fact, previous studies indicated that—due to its variety in active substances—
Lycium barbarum has multiple biological functions including antioxidant and immune regulation [
5]. During the production process of fresh wolfberry, 5% to 10% of the residual fruit is generally discarded as waste. Considering that RBWF has a high nutritional value and contains a variety of bioactive substances—including
Lycium barbarum polysaccharide (LBP) [
6]—it is an easily available feed supplement.
Improved living standards and rapid development within animal husbandry has resulted in increased demands for high-quality livestock products. Agro-industrial by-products can be utilized in this direction. Particularly, according to the existing literature, dietary supplementation with Glycyrrhiza-derived polysaccharide extracts improved the serum biochemical characteristics and immune function of broilers [
7]. Also, dietary supplementation with LBP significantly increased IgG, IgM, IL-10, IL-2, and TNF-α levels in weaned piglets [
8]. Therefore, it is considered that RBWF might have great potential as a functional food to partially replace the conventional feed of ruminants.
Taking into consideration the nutritional characteristics of RBWF, it was incorporated into the sheep diet as a feed ingredient in the present experiment. Our previous studies [
9] were primarily concentrated on the growth performance and rumen parameters of sheep. It was determined that the inclusion of 5% RBWF could enhance ADFI and ADG in sheep and concurrently improve rumen fermentation parameters in a dose-dependent manner. We speculate that the supplementation of RBWF in the diet of fattening sheep can maintain their health status by enhancing immunity. Thus, in order to provide a theoretical basis for the application of RBWF in sheep production, the current study aimed to analyze the concentration-dependent effects of RBWF on serum biochemical, immune, and antioxidant levels as well as slaughter performance and meat quality of sheep.
4. Discussion
Apart from playing an important role in transporting nutrients to cells, blood is involved in regulation, protection, and homeostasis in mammals [
12]. Consequently, the serum biochemical index is an important indicator reflecting the health status of animals. Not only does it reflect changes within organs and tissues as well as important metabolic characteristics, but it also indicates the physiological and nutritional status according to the internal and external environment of the animals [
13,
14]. For example, serum protein content is a parameter of nitrogen metabolism as well as an index for liver function and nutritional status. Also, high levels of TP and ALB in serum can promote the healthy growth of animals [
15]. Finally, as a product of protein metabolism in the body, BUN content reflects the balance of protein catabolism and amino acids in the body and is closely related to the nitrogen content intake via the diet [
16].
Previous studies indicated that addition of polysaccharide extracts to the diet can improve the serum biochemical characteristics and immune function of animals [
17]. Our results show that increasing levels of dietary RBWF resulted in gradually increasing total protein, albumin, and urea nitrogen levels. These findings are consistent with the results of Li et al. [
18] who reported that other Chinese herbal medicine additives increased the serum TP content in Longdong black goats, further suggesting that RBWF can promote protein digestion and absorption as well as protein utilization rate. As demonstrated in earlier research [
9], the ADFI, apparent digestibility, of OM and NDF of RBWF5% was shown to be considerably elevated, indicating that RBWF may promote animal growth by synergistically regulating feeding behavior and nutrient utilization efficiency: on the one hand, it stimulates appetite to increase nutrient intake, and on the other hand, it improves feed conversion rate by improving the degradation ability of fiber substances. This could be due to the presence of active substances—such as polysaccharides—in RBWF that promote rumen fermentation, resulting in improved digestion and absorption of the nutrients in the diet, as such promoting healthy growth of sheep. Furthermore, this also indicates that RBWF can meet the growth needs of fattening sheep.
As the main source of energy, the dynamic balance of glucose absorption, transport, and metabolism is represented by the change of glucose content [
19]. Changes in the activity of LDH—a NAD-dependent kinase that exists in a variety of tissues—can reflect tissue damage or disease status [
20]. The main function of CK is promoting energy metabolism, especially during muscle contraction and ATP energy metabolism. Previous observations indicated that
Lycium barbarum leaves improve blood glucose and blood lipid levels in diabetic rats by regulating metabolic processes and reversing intestinal flora imbalances [
21]. In the current study, no significant differences in serum GLU and CK content was found. However, addition of RBWF to the diet could significantly reduce the serum LDH concentration of sheep. It is hypothesized that RBWF may reduce the damage of oxidative stress to cells by neutralizing free radicals through its antioxidant components (
Lycium barbarum polysaccharides and flavonoids), thereby inhibiting enzyme leakage caused by exercise or metabolic stress. TC and TG contents mainly reflect the fat deposition and lipid metabolism in the body. In fact, TG is the most abundant lipid in animals and functions as energy supply and energy storage. Also, whereas TC—with its important components HDL-c and LDL-c—exists in all tissues of animals, its presence in blood mainly derives from exogenous absorption as well as endogenous synthesis. Previous studies indicated that LBP limits fat formation through a dose-dependent reduction of lipid accumulation and downregulation of key transcription factors and proteins involved in the fat-formation pathway [
22]. Also, Zhuang et al. demonstrated that LBP reduced TC and LDL-c levels in fish while promoting fatty acid oxidation and hydrolysis of triglycerides [
23]. Moreover, Li et al. provided evidence that dietary supplementation with Chinese herbal medicine mixtures reduced the serum levels of cholesterol and triglycerides in laying hens [
24]. In the current study, addition of RBWF to the diet significantly reduced serum TG and LDL-c levels of sheep. This may be due to the fact that some components of RBWF, such as
Lycium barbarum polysaccharides and phytosterol, have the effect of regulating blood lipids, which can interfere with the absorption and metabolism of cholesterol and promote the excretion of cholesterol, thereby reducing the concentration of TG and LDL-c in serum [
25]. It is evident that further study is required in order to fully comprehend the regulatory mechanism of RBWF.
Immunoglobulins are proteins with non-specific immunity that function to isolate pathogens. The content of immunoglobulins in serum is an important index representing body immune function [
26]. Zhang et al. evidenced that addition of fermented wolfberry residue to the diet of sheep significantly increased the levels of IgG, IgA, IgM, and GLB while inducing expression of immune-related pathway genes resulting in improved immunity [
27]. Consistent with previous findings of Ju et al. [
28], the current study indicated that RBWF significantly increased serum levels of IgA and IgM in sheep. This suggests that RBWF could promote the function of immune cells thereby improving the immunity of livestock and poultry. The specific mechanism through which RBWF promotes immunity has not been studied. It is hypothesized that some metabolites of the RBWF-derived active ingredients could be absorbed by the rumen and intestinal flora and participate in the immune process and enhancing the immunity of the body. During growth, animals encounter a variety of stimuli that affect the body’s redox balance, resulting in increased MDA levels in the body and a decline in animal production performance [
29]. Supplementation with exogenous antioxidants can improve the body’s antioxidant status [
30]. For example, polysaccharides regulate the expression of downstream antioxidant enzymes through the endogenous antioxidant stress Nrf2/ARE pathway, resulting in significantly improved antioxidant capacity and reduced oxidative stress-related damage [
31]. In fact, LBP promotes the repair and regeneration of cavernous nerve injury by increasing serum SOD and GSH-Px activity and decreasing MDA activity [
32]. Also, previous studies evidenced that LBP resulted in upregulated expression of genes involved in fatty acid oxidation in the liver, resulting in reduced serum TC and TG levels in serum and reduced TG levels in the liver [
33]. Long et al. reported that dietary supplementation with 2000 mg/kg LBP improved the growth performance, digestive enzyme activity, antioxidant capacity, and immune function of broilers [
34]. Consistent with previous studies, the current study showed that dietary supplementation with RBWF resulted in significantly increased levels of T-AOC, SOD, and GSH-Px and reduced levels of MDA in sheep serum. It is hypothesized that active substances such as LBP and betaine contained in RBWF might enhance the body’s antioxidant capacity by mediating the humoral immunity and regulating the body’s physiological function. This should be addressed in future studies. Guo et al. previously reported that
Lycium barbarum leaves had no significant effect on the main antioxidant enzymes in rats [
35]. This inconsistent observation could be due to differences in animal species and/or feeding concentrations. As demonstrated in previous studies [
9], the simultaneous optimization of nutrient intake and utilization efficiency is directly responsible for enhancing growth performance. However, this effect is not isolated, as evidenced by serum immune and antioxidant index analysis, which revealed a significant increase in the concentrations of IgA, IgM, T-AOC, SOD and GSH-Px in RBWF5%. This suggests that it may indirectly promote growth through the ’antioxidant–immune–digestive’ interaction mechanism. In the future, it is necessary to analyze the dynamic regulation of the ’digestion–immunity–growth’ ternary relationship under nutritional intervention through joint research, so as to provide a theoretical foundation for the design of precise nutritional strategies.
Yu et al. previously reported a significant correlation between serum biochemical indexes, slaughter traits, and meat quality [
36]. Also, Tao et al. reported improved slaughter performance of finishing pigs following dietary supplementation with LBP due to significant increases in final weight, slaughter rate, and carcass length as well as reduced feed-to-weight ratio, backfat thickness, shear force, and cooking loss rate [
37]. In addition, increased final weight and eye muscle area as well as improved meat quality indicators were reported in Hu sheep following dietary supplementation with
Lycium barbarum branches and leaves [
38]. In the current experiment, RBWF had no significant effect on the slaughter traits of sheep. However, a secondary effect on the carcass weight and eye muscle area of sheep was observed, showing a trend of concentration-dependent increase with RBWF concentrations up to 5% and subsequent decrease with a RBWF concentration of 8%. Previous reports indicated that IMP can enhance the umami of meat resulting in a more delicious taste [
39]. In the current study, no significant effect on moisture, protein, and pH of longissimus dorsi muscle was found. However, a significantly increased VB1 and IMP contents were observed, suggesting that RBWF could increase the umami taste of mutton.
Amino acids are the basic unit of muscle protein, and their content directly affects the nutritional value of meat [
40]. Previous studies evidenced that the closer the proportion of essential amino acids in meat to human needs, the higher its nutritional value [
41]. For example, goat meat is a natural green, nutritious, and healthy food with a complete variety and rich content of amino acids [
42]. Amino acids also play a key role in the formation of meat flavor. Glu is one of the most important umami amino acids in meat with a high impact on meat flavor [
43]. In addition, together with other amino acids, Glu also affects the tenderness and juiciness of meat [
44]. The muscular amino acid content and composition are affected by feeding conditions and feed ingredients. For example, dietary supplementation with specific amino acids improves the intramuscular fat content and fatty acid composition of the meat with a high impact on overall meat quality [
45]. In the current experiment, dietary supplementation with RBWF significantly increased the Glu content in meat but not that of other amino acids. Next to being an important source of energy supply, fatty acids also participate in cell signal transduction and gene-expression regulation with regulatory functions in multiple cell types [
46]. Differences in fatty acid composition have a significant effect on meat quality [
47]. For example, pork and chicken contain a higher proportion of PUFA, especially n-6 fatty acid linoleic acid (LA) [
48], while beef and mutton have a high ratio of SFA to PUFA [
49,
50]. These differences affect the texture, flavor, and oxidative stability of meat [
51]. In particular, n-3 PUFA—such as α-linolenic acid (ALA)—has a beneficial effect on cardiovascular health and is high in beef and mutton [
52]. In addition, the ω-6 PUFA C18:2 contributes to prevention of cardiovascular disease by regulating lipoprotein cholesterol levels in the blood [
53]. Previous studies evidenced improved nutritional and health values of meat through changing the feed structure and nutrients of ruminants [
54]. However, this could also lead to oxidation problems during meat processing, which could affect its flavor and quality [
55]. Saturated fatty acids (SFAs) have a negative impact on musculoskeletal tissue, mainly because palmitic acid (PA, 16:0) can reduce collagen content [
56]. Unsaturated fatty acids have many positive effects on human health and are indispensable nutrients for maintaining physiological functions and promoting health [
57]. The oxidation of unsaturated fatty acids will lead to a decline in meat quality and affect the shelf life of meat. At the same time, the oxidation products of fatty acids, such as volatile substances produced during cooking, have an important impact on the flavor of meat [
58]. The current study showed that dietary supplementation with RBWF increased the muscular C20:4 contents, with no significant effect on other fatty acids. Overall, the current data evidences that RBWF resulted in improved meat flavor with no adverse effect on meat quality. Combined with serum data analysis indicating RBWF’s capacity to increase the body’s immune level, these observations further suggest that RBWF might be beneficial in cardiovascular disease prevention.
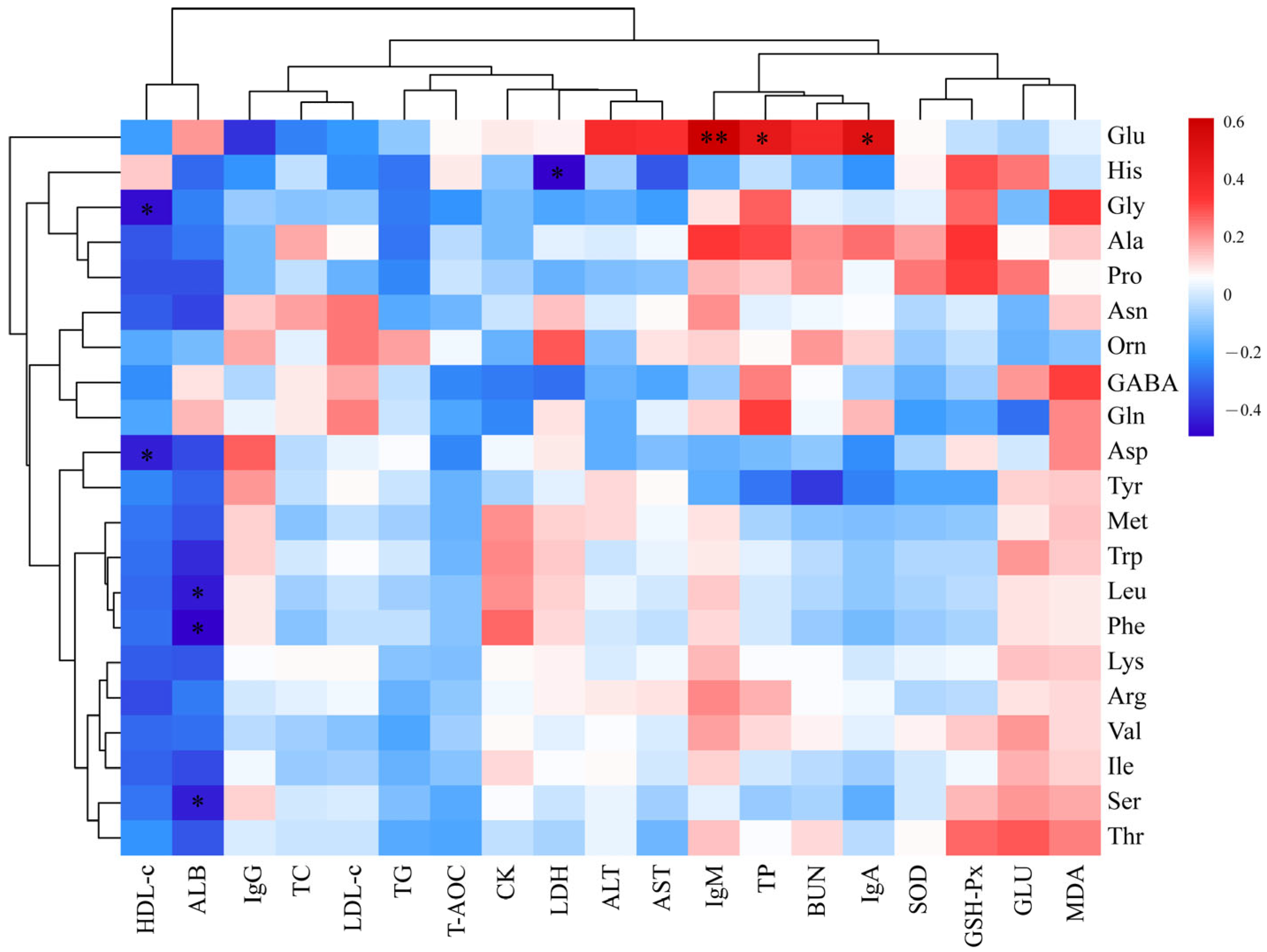
Finally, our study revealed correlations between multiple serum indicators and the muscular amino acid and fatty acid content. For example, Glu s correlated positively and significantly with serum TP, IgA, and IgM levels whereas TG and HDL were correlated negatively with most amino acids. These observations suggest that dietary RBWF could result in increased serum TP content and immunity in sheep, promote lipid metabolism, increase Glu content in muscle, and improve muscle flavor. Previous studies reported that C20:4 is a precursor of a variety of bioactive substances, such as prostaglandins, leukotrienes, and thromboxane. These substances play an important role in regulating inflammation, vasoconstriction, and platelet aggregation [
59]. The current study reported a positive correlation of C20:4 with T-AOC, SOD, and GSH-Px. Thus, dietary supplementation of RBWF could significantly increase the serum antioxidant capacity of sheep and increase the content of C20:4. However, the specific mechanism of RBWF on serum indicators and meat quality still needs further study.