Rhodotorula Yeast Culture Improved the Antioxidant Capacity, Lipid Metabolism, and Immunity of Sheep Livers
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Diet Composition
2.3. Sampling
2.4. Liver-Antioxidant Capacity
2.5. Serum Lipid Metabolism
2.6. Liver Fatty Acid Content
2.7. Liver Immune Cytokines
2.8. Liver RNA Extraction and Quantitative Real-Time PCR
2.9. Data Analysis
3. Results
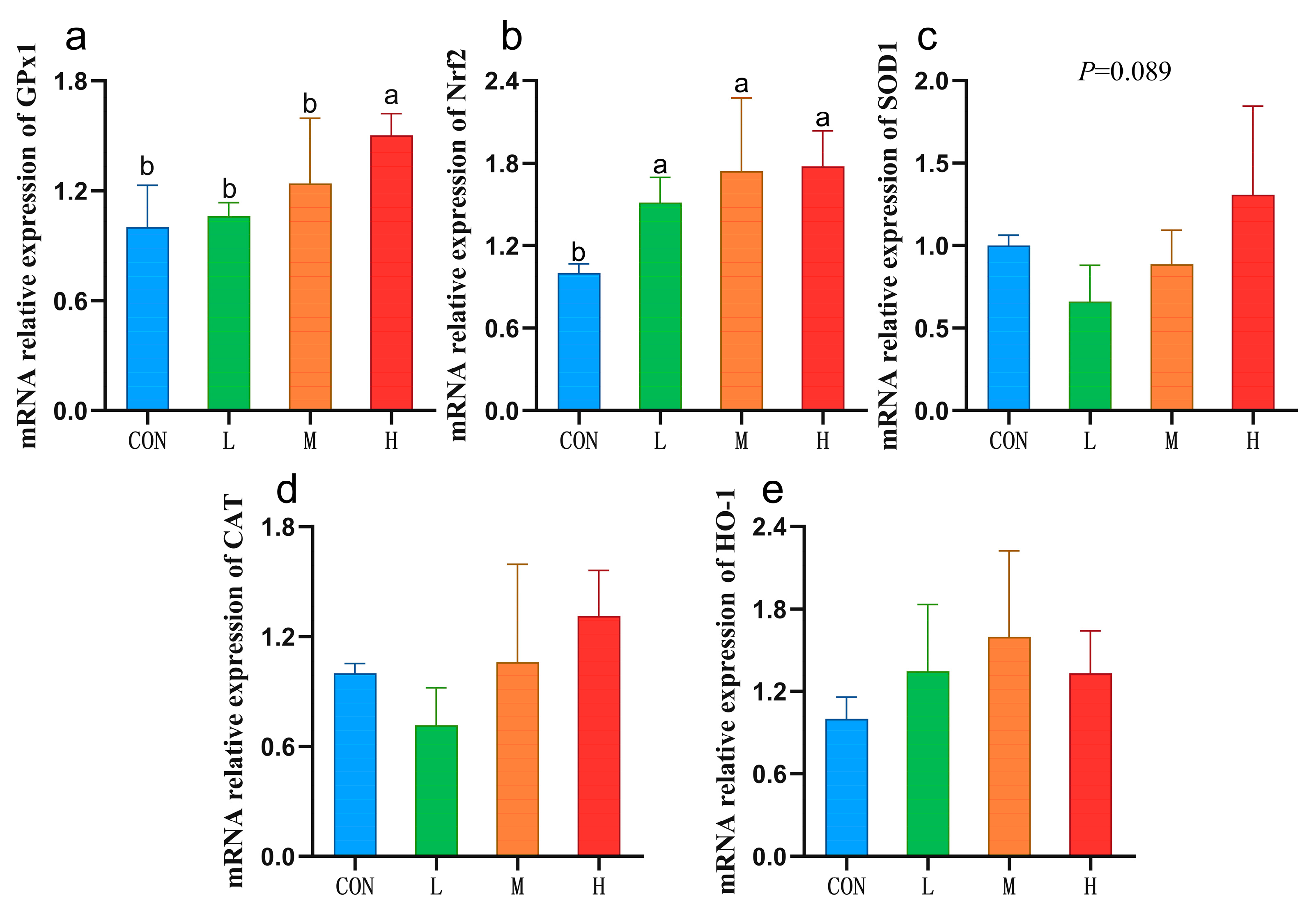
3.1. Liver Antioxidant Capacity
3.2. Expression of Liver Antioxidant-Related Genes
3.3. Serum Lipid Metabolism
3.4. Liver Fatty Acid Profile
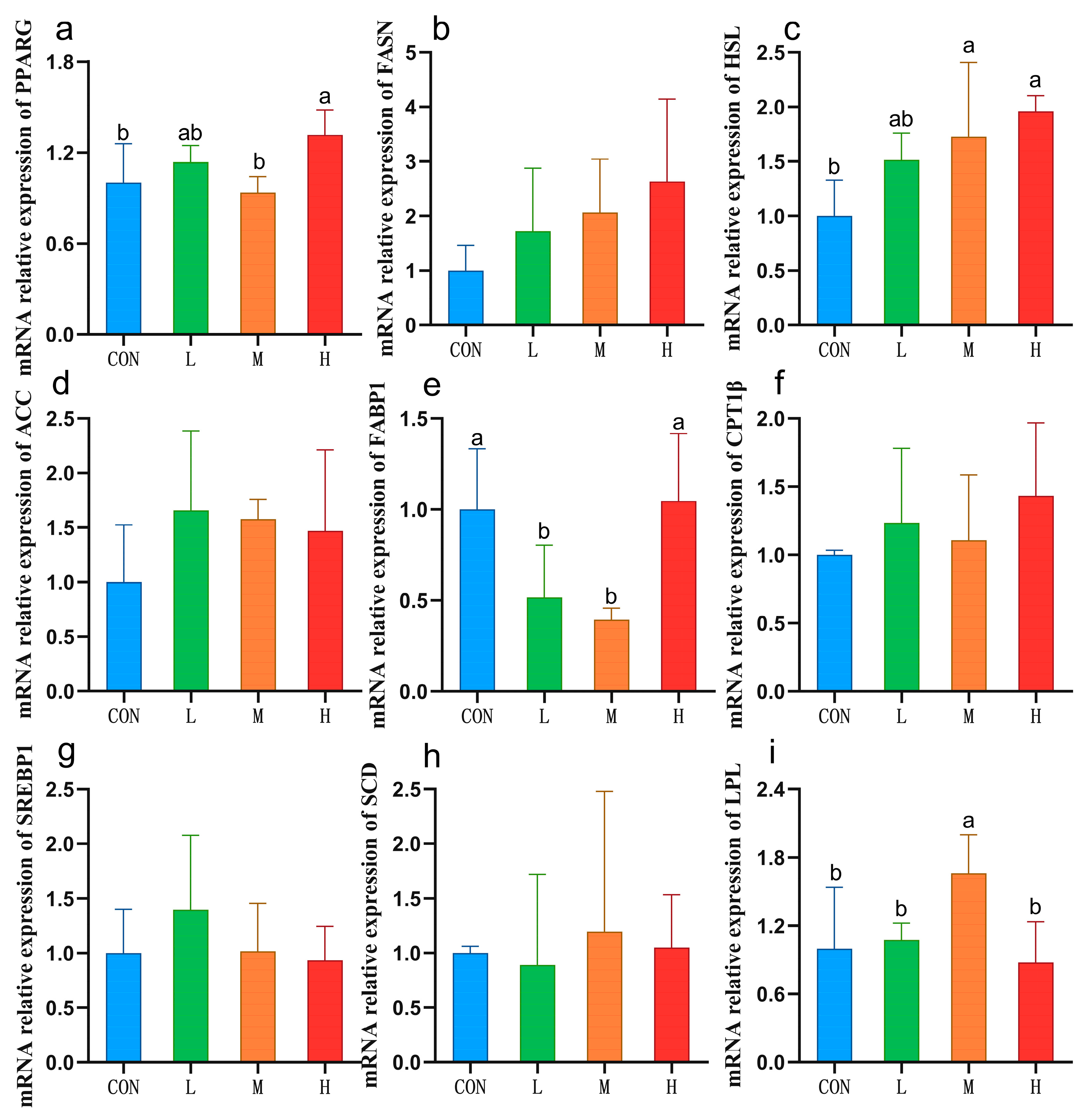
3.5. Expression of Liver Lipid Metabolism-Related Genes
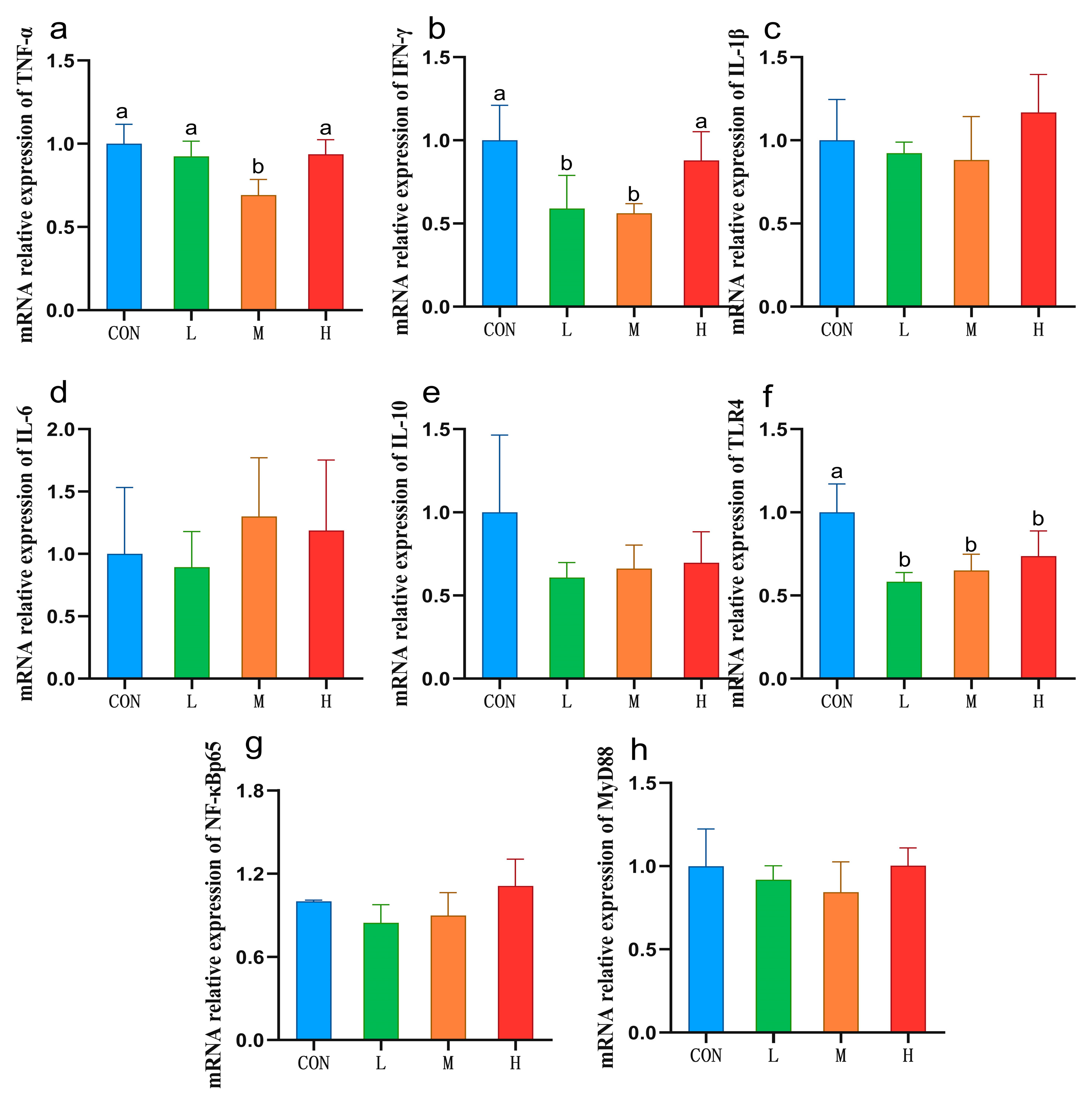
3.6. Expression of Liver Immune Cytokines and Related Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meng, M.; Li, X.; Huo, R.; Chang, G.; Shen, X. Effects of dietary disodium fumarate supplementation on muscle quality, chemical composition, oxidative stress and lipid metabolism of Hu sheep induced by high concentrate diet. Meat Sci. 2023, 201, 109176. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.X.; Cao, Q.Q.; Zhang, C.D.; Xu, T.-T.; Yue, K.; Li, Q.; Liu, F.; Wang, X.; Dong, H.-J.; Huang, S.-C.; et al. Aflatoxin B1 causes oxidative stress and apoptosis in sheep testes associated with disrupting rumen microbiota. Ecotoxicol. Environ. Saf. 2022, 232, 113225. [Google Scholar] [CrossRef]
- Van Wettere, W.H.E.J.; Kind, K.L.; Gatford, K.L.; Swinbourne, A.M.; Leu, S.T.; Hayman, P.T.; Kelly, J.M.; Weaver, A.C.; Kleemann, D.O.; Walker, S.K. Review of the impact of heat stress on reproductive performance of sheep. J. Anim. Sci. Biotechnol. 2021, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ying, G.G.; Deng, W.J. Antibiotic Residues in Food: Extraction, Analysis, and Human Health Concerns. J. Agric. Food Chem. 2019, 67, 7569–7586. [Google Scholar] [CrossRef]
- Bacanlı, M.G. The two faces of antibiotics: An overview of the effects of antibiotic residues in foodstuffs. Arch. Toxicol. 2024, 98, 1717–1725. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Agriculture and Rural Affairs of the People’s Republic of China. Available online: http://www.xmsyj.moa.gov.cn/zcjd/201907/t20190710_6320678.htm (accessed on 5 November 2024).
- Shurson, G.C. Yeast and yeast derivatives in feed additives and ingredients: Sources, characteristics, animal responses, and quantification methods. Anim. Feed Sci. Technol. 2018, 235, 60–76. [Google Scholar] [CrossRef]
- He, W.; Gao, Y.; Guo, Z.; Yang, Z.; Wang, X.; Liu, H.; Sun, H.; Shi, B. Effects of fermented wheat bran and yeast culture on growth performance, immunity, and intestinal microflora in growing-finishing pigs. J. Anim. Sci. 2021, 99, skab308. [Google Scholar] [CrossRef]
- Liu, Y.; Jia, X.; Chang, J.; Pan, X.; Jiang, X.; Che, L.; Lin, Y.; Zhuo, Y.; Feng, B.; Fang, Z.; et al. Yeast culture supplementation of sow diets regulates the immune performance of their weaned piglets under lipopolysaccharide stress. J. Anim. Sci. 2023, 101, skad226. [Google Scholar] [CrossRef]
- Kim, E.; Kyoung, H.; Hyung Koh, N.; Lee, H.; Lee, S.; Kim, Y.; Park, K.I.; Heo, J.M.; Song, M. Supplementation of live yeast culture modulates intestinal health, immune responses, and microbiota diversity in broiler chickens. J. Anim. Sci. 2022, 100, skac122. [Google Scholar] [CrossRef]
- Lin, Y.; Yu, C.; Ma, Z.; Che, L.; Feng, B.; Fang, Z.; Xu, S.; Zhuo, Y.; Li, J.; Zhang, J.; et al. Effects of Yeast Culture Supplementation in Wheat-Rice-Based Diet on Growth Performance, Meat Quality, and Gut Microbiota of Growing-Finishing Pigs. Animals 2022, 12, 2177. [Google Scholar] [CrossRef]
- Chen, H.; Liu, S.; Li, S.; Li, D.; Li, X.; Xu, Z.; Liu, D. Effects of yeast culture on growth performance, immune function, antioxidant capacity and hormonal profile in Mongolian ram lambs. Front. Vet. Sci. 2024, 11, 1424073. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Lang, M.; Zhen, Y.G.; Chen, X.; Sun, Z.; Zhao, W.; Zhang, X.; Wang, T.; Qin, G. Effects of yeast culture supplementation and the ratio of non-structural carbohydrate to fat on growth performance, carcass traits and the fatty acid profile of the longissimus dorsi muscle in lambs. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1274–1282. [Google Scholar] [CrossRef]
- Wang, H.; Su, M.; Wang, C.; Li, D.; Li, Q.; Liu, Z.; Qi, X.; Wu, Y.; Zhao, Y.; Li, T.; et al. Yeast culture repairs rumen epithelial injury by regulating microbial communities and metabolites in sheep. Front. Microbiol. 2023, 14, 1305772. [Google Scholar] [CrossRef] [PubMed]
- Halfen, J.; Carpinelli, N.; Del Pino, F.A.B.; Chapman, J.; Sharman, E.; Anderson, J.; Osorio, J. Effects of yeast culture supplementation on lactation performance and rumen fermentation profile and microbial abundance in mid-lactation Holstein dairy cows. J. Dairy Sci. 2021, 104, 11580–11592. [Google Scholar] [CrossRef]
- Ye, G.; Liu, J.; Liu, Y.; Chen, X.; Liao, S.F.; Huang, D.; Huang, K. Feeding glycerol-enriched yeast culture improves lactation performance, energy status, and hepatic gluconeogenic enzyme expression of dairy cows during the transition period. J. Anim. Sci. 2016, 94, 2441–2450. [Google Scholar] [CrossRef]
- Feng, Z.; Zhong, Y.; He, G.; Sun, H.; Chen, Y.; Zhou, W.; Lin, S. Yeast culture improved the growth performance, liver function, intestinal barrier and microbiota of juvenile largemouth bass (Micropterus salmoides) fed high-starch diet. Fish Shellfish Immunol. 2022, 120, 706–715. [Google Scholar] [CrossRef]
- Mussagy, C.U.; Ribeiro, H.F.; Santos-Ebinuma, V.C.; Schuur, B.; Pereira, J.F.B. Rhodotorula sp.-based biorefinery: A source of valuable biomolecules. Appl. Microbiol. Biotechnol. 2022, 106, 7431–7447. [Google Scholar] [CrossRef]
- Hu, P.; Mao, J.; Zeng, Y.; Sun, Z.; Deng, H.; Chen, C.; Sun, W.; Tang, Z. Isolation, Identification, and Function of Rhodotorula mucilaginosa TZR2014 and Its Effects on the Growth and Health of Weaned Piglets. Front. Microbiol. 2022, 13, 922136. [Google Scholar] [CrossRef]
- Coutinho, J.O.P.A.; Peixoto, T.S.; de Menezes, G.C.A.; Carvalho, C.R.; Ogaki, M.B.; Gomes, E.C.Q.; Rosa, C.A.; Rosa, L.H.; Arantes, R.M.E.; Nicoli, J.R.; et al. In Vitro and In Vivo Evaluation of the Probiotic Potential of Antarctic Yeasts. Probiotics Antimicrob. Proteins. 2021, 13, 1338–1354. [Google Scholar] [CrossRef]
- Kang, K.; Deng, X.; Xie, W.; Chen, J.; Lin, H.; Chen, Z. Rhodotorula mucilaginosa ZTHY2 Attenuates Cyclophosphamide-Induced Immunosuppression in Mice. Animals 2023, 13, 3376. [Google Scholar] [CrossRef]
- Sun, J.; Li, M.; Tang, Z.; Zhang, X.; Chen, J.; Sun, Z. Effects of Rhodotorula mucilaginosa fermentation product on the laying performance, egg quality, jejunal mucosal morphology and intestinal microbiota of hens. J. Appl. Microbiol. 2020, 128, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Q.; Zhao, W.; Xie, S.W.; Xie, J.-J.; Zhang, Z.-H.; Tian, L.-X.; Liu, Y.-J.; Niu, J. Effects of dietary hydrolyzed yeast (Rhodotorula mucilaginosa) on growth performance, immune response, antioxidant capacity and histomorphology of juvenile Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2019, 90, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, Y.; Zhang, Y.; Zhang, Y.; Wang, W.; Han, H.; Yang, C.; Dong, X. Superoxide dismutase ameliorates oxidative stress and regulates liver transcriptomics to provide therapeutic benefits in hepatic inflammation. PeerJ 2023, 11, e15829. [Google Scholar] [CrossRef]
- Li, T.; Jin, M.; Fei, X.; Yuan, Z.; Wang, Y.; Quan, K.; Wang, T.; Yang, J.; He, M.; Wei, C. Transcriptome Comparison Reveals the Difference in Liver Fat Metabolism between Different Sheep Breeds. Animals 2022, 12, 1650. [Google Scholar] [CrossRef]
- Kubes, P.; Jenne, C. Immune Responses in the Liver. Annu. Rev. Immunol. 2018, 36, 247–277. [Google Scholar] [CrossRef]
- Bao, G.; Liu, X.; Wang, J.; Hu, J.; Shi, B.; Li, S.; Luo, Y. Effects of Slaughter Age on Myosin Heavy Chain Isoforms, Muscle Fibers, Fatty Acids, and Meat Quality in Longissimus Thoracis Muscle of Tibetan Sheep. Front. Vet. Sci. 2021, 8, 689589. [Google Scholar] [CrossRef]
- Zhao, C. Study on the Regulatory Network of Flavor Precursors in Ujimqin Mutton. Master’s Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2022. [Google Scholar] [CrossRef]
- Ferlizza, E.; Fasoli, S.; Cavallini, D.; Bolcato, M.; Andreani, G.; Isani, G. Preliminary Study on Urine Chemistry and Protein Profile in Cows and Heifers. Pak. Vet. J. 2020, 40, 413–418. [Google Scholar] [CrossRef]
- Felini, R.; Cavallini, D.; Buonaiuto, G.; Bordin, T. Assessing the impact of thermoregulatory mineral supplementation on thermal comfort in lactating Holstein cows. Vet. Anim. Sci. 2024, 24, 100363. [Google Scholar] [CrossRef] [PubMed]
- Wilachai, K.; Paengkoum, P.; Taethaisong, N.; Thitisak, P.; Poonsuk, K.; Loor, J.J.; Paengkoum, S. Effect of Isolation Ruminal Yeast from Ruminants on In Vitro Ruminal Fermentation. Vet. Sci. 2025, 12, 155. [Google Scholar] [CrossRef]
- Li, Y.; Zhen, S.; Sun, F.; Cao, L.; Wang, L. Effects of γ-Aminobutyric Acid on Growth Performance, Immunity, Antioxidant Capacity, and Intestinal Microbiota of Growing Minks. Vet. Sci. 2024, 11, 398. [Google Scholar] [CrossRef]
- Alagbe, E.O.; Schulze, H.; Adeola, O. Growth performance, nutrient digestibility, intestinal morphology, cecal mucosal cytokines and serum antioxidant responses of broiler chickens to dietary enzymatically treated yeast and coccidia challenge. J. Anim. Sci. Biotechnol. 2023, 14, 57. [Google Scholar] [CrossRef] [PubMed]
- Roy, Z.; Bansal, R.; Siddiqui, L.; Chaudhary, N. Understanding the Role of Free Radicals and Antioxidant Enzymes in Human Diseases. Curr. Pharm. Biotechnol. 2023, 24, 1265–1276. [Google Scholar] [CrossRef]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef]
- Murakami, S.; Kusano, Y.; Okazaki, K.; Akaike, T.; Motohashi, H. NRF2 signalling in cytoprotection and metabolism. Br. J. Pharmacol. 2023. [Preprint]. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Yamazaki, K.; Kamruzzaman, M.; Bi, X.; Panthee, A.; Sano, H. Effects of Chinese herbal medicine on plasma glucose, protein and energy metabolism in sheep. J. Anim. Sci. Biotechnol. 2013, 4, 51. [Google Scholar] [CrossRef]
- Kale, D.; Fatangare, A.; Phapale, P.; Sickmann, A. Blood-Derived Lipid and Metabolite Biomarkers in Cardiovascular Research from Clinical Studies: A Recent Update. Cells 2023, 12, 2796. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Brown, M.S. A century of cholesterol and coronaries: From plaques to genes to statins. Cell 2015, 161, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Joyce, S.A.; Kamil, A.; Fleige, L.; Gahan, C.G.M. The Cholesterol-Lowering Effect of Oats and Oat Beta Glucan: Modes of Action and Potential Role of Bile Acids and the Microbiome. Front. Nutr. 2019, 6, 171. [Google Scholar] [CrossRef]
- Yu, F.; Zong, B.; Ji, L.; Sun, P.; Jia, D.; Wang, R. Free Fatty Acids and Free Fatty Acid Receptors: Role in Regulating Arterial Function. Int. J. Mol. Sci. 2024, 25, 7853. [Google Scholar] [CrossRef]
- Henderson, G.C. Plasma Free Fatty Acid Concentration as a Modifiable Risk Factor for Metabolic Disease. Nutrients 2021, 13, 2590. [Google Scholar] [CrossRef]
- Carpinelli, N.A.; Halfen, J.; Trevisi, E.; Chapman, J.; Sharman, E.; Anderson, J.; Osorio, J. Effects of peripartal yeast culture supplementation on lactation performance, blood biomarkers, rumen fermentation, and rumen bacteria species in dairy cows. J. Dairy Sci. 2021, 104, 10727–10743. [Google Scholar] [CrossRef] [PubMed]
- Malekkhahi, M.; Tahmasbi, A.M.; Naserian, A.A.; Danesh Mesgaran, M.; Kleen, J.L.; Parand, A.A. Effects of essential oils, yeast culture and malate on rumen fermentation, blood metabolites, growth performance and nutrient digestibility of Baluchi lambs fed high-concentrate diets. J. Anim. Physiol. Anim. Nutr. 2015, 99, 221–229. [Google Scholar] [CrossRef]
- Bai, H.; Zhang, H.; Wang, C.; Lambo, M.T.; Li, Y.; Zhang, Y. Effects of altering the ratio of C16:0 and cis-9 C18:1 in rumen bypass fat on growth performance, lipid metabolism, intestinal barrier, cecal microbiota, and inflammation in fattening bulls. J. Anim. Sci. Biotechnol. 2024, 15, 94. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Song, W.; Zhao, J.; Yan, W. Polyunsaturated Fatty Acids (PUFAs): Sources, Digestion, Absorption, Application and Their Potential Adjunctive Effects on Visual Fatigue. Nutrients 2023, 15, 2633. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Whittington, F.M.; Hughes, S.I. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef]
- Shih, L.M.; Tang, H.Y.; Lynn, K.S.; Huang, C.Y.; Ho, H.Y.; Cheng, M.L. Stable Isotope-Labeled Lipidomics to Unravel the Heterogeneous Development Lipotoxicity. Molecules 2018, 23, 2862. [Google Scholar] [CrossRef]
- Yang, Z.H.; Emma-Okon, B.; Remaley, A.T. Dietary marine-derived long-chain monounsaturated fatty acids and cardiovascular disease risk: A mini review. Lipids Health Dis. 2016, 15, 201. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Xu, J.; Yang, Q.; Sha, Y.; Jiao, T.; Zhao, S. Effects of yeast cultures on meat quality, flavor composition and rumen microbiota in lambs. Curr. Res. Food Sci. 2024, 9, 100845. [Google Scholar] [CrossRef]
- Nava Lauson, C.B.; Tiberti, S.; Corsetto, P.A.; Conte, F.; Tyagi, P.; Machwirth, M.; Ebert, S.; Loffreda, A.; Scheller, L.; Sheta, D.; et al. Linoleic acid potentiates CD8+ T cell metabolic fitness and antitumor immunity. Cell Metab. 2023, 35, 633–650.e9. [Google Scholar] [CrossRef]
- Tang, Y.X.; Huang, W.; Wang, Y.H.; Chen, H.; Lu, X.-Y.; Tian, Y.; Ji, X.-J.; Liu, H.-H. Engineering Yarrowia lipolytica for sustainable Cis-13, 16-docosadienoic acid production. Bioresour. Technol. 2024, 406, 130978. [Google Scholar] [CrossRef]
- Henriquez-Rodriguez, E.; Pena, R.N.; Seradj, A.R.; Fraile, L.; Christou, P.; Tor, M.; Estany, J. Carotenoid intake and SCD genotype exert complementary effects over fat content and fatty acid composition in Duroc pigs. J. Anim. Sci. 2017, 95, 2547–2557. [Google Scholar] [CrossRef]
- Pajed, L.; Taschler, U.; Tilp, A.; Hofer, P.; Kotzbeck, P.; Kolleritsch, S.; Radner, F.P.W.; Pototschnig, I.; Wagner, C.; Schratter, M.; et al. Advanced lipodystrophy reverses fatty liver in mice lacking adipocyte hormone-sensitive lipase. Commun. Biol. 2021, 4, 323. [Google Scholar] [CrossRef]
- Yuan, C.; Xu, Y.; Lu, G.; Hu, Y.; Mao, W.; Ke, L.; Tong, Z.; Xia, Y.; Ma, S.; Dong, X.; et al. AAV-mediated hepatic LPL expression ameliorates severe hypertriglyceridemia and acute pancreatitis in Gpihbp1 deficient mice and rats. Mol. Ther. 2024, 32, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Fang, K.; Wu, F.; Chen, G.; Dong, H.; Li, J.; Zhao, Y.; Xu, L.; Zou, X.; Lu, F. Diosgenin ameliorates palmitic acid-induced lipid accumulation via AMPK/ACC/CPT-1A and SREBP-1c/FAS signaling pathways in LO2 cells. BMC Complement. Altern. Med. 2019, 19, 255. [Google Scholar] [CrossRef]
- Wu, Y.; Tang, J.; Wen, Z.; Zhang, B.; Cao, J.; Zhao, L.; Guo, Z.; Xie, M.; Zhou, Z.; Hou, S. Dietary methionine deficiency stunts growth and increases fat deposition via suppression of fatty acids transportation and hepatic catabolism in Pekin ducks. J. Anim. Sci. Biotechnol. 2022, 13, 61. [Google Scholar] [CrossRef]
- Xiong, X. Effect of FABP1 on Intramuscular Fat Deposition in Xiangsu Hybrid Pigs. Master’s Thesis, Guizhou University, Guiyang, China, 2023. [Google Scholar] [CrossRef]
- Habashy, W.S.; Milfort, M.C.; Fuller, A.L.; Attia, Y.A.; Rekaya, R.; Aggrey, S.E. Effect of heat stress on protein utilization and nutrient transporters in meat-type chickens. Int. J. Biometeorol. 2017, 61, 2111–2118. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Xue, C.; Yang, Y.; Li, J.; Wang, X.; Chen, Y.; Zhang, S.; Chen, Y.; Duan, Y.; Yang, X.; et al. Lack of Nogo-B expression ameliorates PPARγ deficiency-aggravated liver fibrosis by regulating TLR4-NF-κB-TNF-α axis and macrophage polarization. Biomed. Pharmacother. 2022, 153, 113444. [Google Scholar] [CrossRef] [PubMed]
- Rockwell, C.E.; Zhang, M.; Fields, P.E.; Klaassen, C.D. Th2 skewing by activation of Nrf2 in CD4(+) T cells. J. Immunol. 2012, 188, 1630–1637. [Google Scholar] [CrossRef]
- Li, X.; An, N.; Chen, H.; Liu, D. Effects of yeast culture on growth performance, antioxidant capacity, immune function, and intestinal microbiota structure in Simmental beef cattle. Front Vet. Sci. 2025, 11, 1533081. [Google Scholar] [CrossRef]
- Chen, S.N.; Tan, Y.; Xiao, X.C.; Li, Q.; Wu, Q.; Peng, Y.-Y.; Ren, J.; Dong, M.-L. Deletion of TLR4 attenuates lipopolysaccharide-induced acute liver injury by inhibiting inflammation and apoptosis. Acta Pharmacol. Sin. 2021, 42, 1610–1619. [Google Scholar] [CrossRef]
- Li, Z.; Qi, X.; Zhang, X.; Yu, L.; Gao, L.; Kong, W.; Chen, W.; Dong, W.; Luo, L.; Lu, D.; et al. TRDMT1 exhibited protective effects against LPS-induced inflammation in rats through TLR4-NF-κB/MAPK-TNF-α pathway. Anim. Model Exp. Med. 2022, 5, 172–182. [Google Scholar] [CrossRef] [PubMed]
| Items | Content (%) |
|---|---|
| Ingredients | |
| Sheep grass | 9.21 |
| Cornstalks | 32.9 |
| Concentrate supplements | 31.57 |
| Whole plant corn silage | 26.32 |
| Total | 100 |
| Nutrient levels | |
| Metabolic energy 1 (MJ/kg) | 8.09 |
| Crude protein (%) | 11.95 |
| Ether extract (%) | 2.13 |
| Neutral detergent fibers (%) | 54.86 |
| Acidic detergent fibers (%) | 21.6 |
| Calcium (%) | 0.69 |
| Phosphorus (%) | 0.25 |
| Genes | Sequence(5′-3′) | Product Length (bp) | Accession Number |
|---|---|---|---|
| CAT | F:CCGCGCAGAAACCTGATGT R:AAGTAGCCAAAAGCCCCTGC | 196 | XM_060400054.1 |
| SOD1 | F:GGAGACCTGGGCAATGTGAA R:CCTCCAGCGTTTCCAGTCTT | 182 | NM_001145185.2 |
| GPx1 | F:CGGGACTACACCCAGATGAAT R:GTTCTTGGCGTTTTCCTGATGC | 108 | XM_004018462.5 |
| Nrf2 | F:TGTGGAGGAGTTCAACGAGC R:CGCCGCCATCTTGTTCTTG | 103 | XM_042246639.2 |
| HO-1 | F:AGGGACCAGACCTTCACAGG R:GCATAAAGCCCCACAGCAAC | 166 | XM_027967703.3 |
| PPARG | F:CTTGTGAAGGATGCAAGGGTT R:CATGCGCCCAAACCTGATG | 176 | NM_001100921.1 |
| FASN | F:AGTGGTCATTCAGGTGCGTG R:ATGACGTAGCTCTTGTGGGC | 114 | XM_027974304.3 |
| HSL | F:TCGCCTTTGAAATGCCTCTGACC R:GCTCCTTGCTGTTCTGTCCTTCC | 138 | NM_001128154.1 |
| LPL | F:CCCGGCTTTGATATTGGGAAG R:CTTTGCCAAGTTTCAGCCAGA | 171 | NM_001009394.1 |
| ACC | F:GTGGTGTGAGATCCTGTGCT R:TTAACGAGTCGCAGTTCGGT | 93 | NM_001009256.1 |
| CPT1β | F:AGCAAACCTTAGCTGTGCCA R:GCGAATCAGGCGTTTCTTCC | 168 | NM_001009259.1 |
| SREBP1 | F:GACTGCACGTTCGAAGACAT R:CTCATCGTGGAAGGAGGTGG | 164 | XM_027974786.2 |
| SCD | F:ATGGCGTTCCAGAATGACG R:AAAAGCCACGTCGGGAATTG | 103 | NM_001009254.1 |
| ACOX1 | F:CTTGCTGAATCAGGGCACCA R:TCGAAGATGAGTTCCGTGGC | 115 | XM_060395846.1 |
| IL-1β | F:TCCTCCGATGAGCTTCTGTG R:GGAGAGCCTTCAGCACACAT | 112 | NM_001009465.2 |
| IL-6 | F:ATCGCAGGTCTAATAACCACTCCAG R:GCAGGAAATTCTCAAGGCTTCTCAG | 124 | NM_001009392.1 |
| IL-10 | F:GGGTGTCTACAAAGCCATGAGTGAG R:AGGTTTATGTCGGGGAGTCTAGTCG | 143 | XM_060395938.1 |
| TNF-α | F:ACCTGGACTATGCCGAGTCT R:GAAGGGGATGAGGAGGGTCT | 127 | NM_001024860.1 |
| IFN-γ | F:AAGTTCTTGAACGGCAGCTCTGAG R:TGAGGTTAGATTTTGGCGACAGGTC | 142 | NM_001009803.1 |
| TLR4 | F:TGGGTGCGGAATGAACTGGTAAAG R:CTGGATGATATTGGCGGCGATGG | 114 | NM_001135930.1 |
| MyD88 | F:ATGGTGGTGGTTGTCTCTGAC R:GGAACTCTTTCTTCATTGGCTTGT | 139 | NM_001166183.1 |
| NF-κBp65 | F:TCTGGCCCCTATGTGGAGAT R:CCCGTGTAGCCATTGATCTTG | 155 | XM_027959295.2 |
| β-actin | F:CCCTGGAGAAGAGCTACGAG R:GGTAGTTTCGTGAATGCCGC | 131 | NM_001009784.3 |
| Items | CON | L | M | H | SEM | p-Value |
|---|---|---|---|---|---|---|
| T-SOD (U/mgprot) | 196.97 b | 205.09 b | 196.26 b | 223.31 a | 3.745 | 0.007 |
| CAT (U/mgprot) | 36.47 | 41.79 | 40.27 | 37.94 | 1.086 | 0.361 |
| GPx (U/mgprot) | 10.45 b | 14.62 a | 13.23 a | 13.74 a | 0.573 | 0.024 |
| T-AOC (μmol/gprot) | 72.48 | 71.50 | 74.26 | 73.79 | 1.311 | 0.911 |
| MDA (nmol/mgprot) | 1.26 a | 0.74 b | 0.78 b | 0.88 b | 0.062 | 0.022 |
| Items | CON | L | M | H | SEM | p-Value |
|---|---|---|---|---|---|---|
| GLU (mmol/L) | 3.41 | 3.20 | 3.34 | 3.34 | 0.076 | 0.841 |
| TC (mmol/L) | 3.06 a | 2.46 b | 2.47 b | 2.66 ab | 0.095 | 0.040 |
| TG (mmol/L) | 0.34 a | 0.31 ab | 0.22 c | 0.25 bc | 0.011 | 0.014 |
| HDL-C (mmol/L) | 1.21 a | 1.03 b | 0.98 b | 1.01 b | 0.026 | 0.001 |
| LDL-C (mmol/L) | 0.37 a | 0.22 b | 0.25 b | 0.26 b | 0.017 | 0.013 |
| FFA (mmol/L) | 0.57 | 0.56 | 0.56 | 0.53 | 0.005 | 0.056 |
| Items | CON | L | M | H | SEM | p-Value |
|---|---|---|---|---|---|---|
| C8:0 | 0.04 | 0.04 | 0.04 | 0.04 | 0.001 | 0.870 |
| C10:0 | 0.09 | 0.09 | 0.09 | 0.09 | 0.002 | 0.752 |
| C12:0 | 0.23 | 0.24 | 0.23 | 0.25 | 0.012 | 0.963 |
| C14:0 | 1.31 | 1.34 | 1.09 | 1.39 | 0.073 | 0.523 |
| C14:1 | 0.46 | 0.44 | 0.43 | 0.41 | 0.014 | 0.680 |
| C15:0 | 0.34 | 0.34 | 0.32 | 0.33 | 0.006 | 0.889 |
| C16:0 | 23.43 a | 23.28 a | 21.60 b | 23.82 a | 0.310 | 0.038 |
| C16:1 | 1.43 | 1.44 | 1.22 | 1.61 | 0.064 | 0.209 |
| C17:0 | 0.76 | 0.81 | 0.81 | 0.80 | 0.012 | 0.441 |
| C17:1 | 0.56 | 0.65 | 0.59 | 0.61 | 0.014 | 0.157 |
| C18:0 | 24.53 | 25.61 | 26.57 | 23.94 | 0.405 | 0.089 |
| C18:1C | 27.82 | 25.52 | 23.28 | 26.56 | 0.623 | 0.052 |
| C18:2n6C | 12.49 b | 13.02 b | 16.02 a | 13.27 b | 0.408 | 0.002 |
| C18:3n3 | 0.26 | 0.27 | 0.28 | 0.30 | 0.008 | 0.336 |
| C18:3n6 | 0.35 | 0.36 | 0.34 | 0.41 | 0.010 | 0.050 |
| C20:1 | 0.26 b | 0.27 b | 0.32 a | 0.25 b | 0.009 | 0.018 |
| C20:3n3 | 4.11 | 4.50 | 4.73 | 4.34 | 0.128 | 0.409 |
| C20:3n6 | 0.40 | 0.43 | 0.44 | 0.41 | 0.020 | 0.888 |
| C22:2n6 | 0.21 b | 0.23 b | 0.24 ab | 0.26 a | 0.006 | 0.013 |
| C22:6n3 | 0.91 | 1.11 | 1.08 | 1.05 | 0.050 | 0.545 |
| ΣSFAs | 50.72 | 51.75 | 50.76 | 50.67 | 0.264 | 0.435 |
| ΣMUFAs | 30.52 | 28.31 | 25.84 | 29.44 | 0.663 | 0.061 |
| ΣPUFAs | 18.73 b | 19.92 b | 23.14 a | 20.04 b | 0.521 | 0.008 |
| Items | CON | L | M | H | SEM | p-Value |
|---|---|---|---|---|---|---|
| IL-1β (pg/mg) | 6.91 | 6.01 | 6.48 | 6.74 | 0.230 | 0.568 |
| IL-6 (pg/mg) | 17.61 | 15.21 | 15.70 | 16.55 | 0.487 | 0.343 |
| IL-10 (pg/mg) | 2.02 | 2.06 | 1.83 | 2.06 | 0.052 | 0.377 |
| TNF-α (pg/mg) | 11.01 a | 9.68 ab | 8.81 b | 10.25 ab | 0.294 | 0.041 |
| IFN-γ (pg/mg) | 60.68 a | 53.13 ab | 45.65 b | 56.42 a | 1.777 | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Ma, H.; Liu, Y.; Chen, M.; Dang, J.; Su, X.; Zhao, Y.; Wang, K.; Yang, G.; Zhang, G.; et al. Rhodotorula Yeast Culture Improved the Antioxidant Capacity, Lipid Metabolism, and Immunity of Sheep Livers. Vet. Sci. 2025, 12, 314. https://doi.org/10.3390/vetsci12040314
Lu X, Ma H, Liu Y, Chen M, Dang J, Su X, Zhao Y, Wang K, Yang G, Zhang G, et al. Rhodotorula Yeast Culture Improved the Antioxidant Capacity, Lipid Metabolism, and Immunity of Sheep Livers. Veterinary Sciences. 2025; 12(4):314. https://doi.org/10.3390/vetsci12040314
Chicago/Turabian StyleLu, Xinyu, Huiru Ma, Yeqing Liu, Meiru Chen, Jianlong Dang, Xiangtan Su, Yahui Zhao, Ke Wang, Guang Yang, Gaowei Zhang, and et al. 2025. "Rhodotorula Yeast Culture Improved the Antioxidant Capacity, Lipid Metabolism, and Immunity of Sheep Livers" Veterinary Sciences 12, no. 4: 314. https://doi.org/10.3390/vetsci12040314
APA StyleLu, X., Ma, H., Liu, Y., Chen, M., Dang, J., Su, X., Zhao, Y., Wang, K., Yang, G., Zhang, G., Li, X., Gao, A., & Wang, Y. (2025). Rhodotorula Yeast Culture Improved the Antioxidant Capacity, Lipid Metabolism, and Immunity of Sheep Livers. Veterinary Sciences, 12(4), 314. https://doi.org/10.3390/vetsci12040314






