Effects of Ladder-Climbing Exercise on Mammary Cancer: Data from a Chemically Induced Rat Model
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
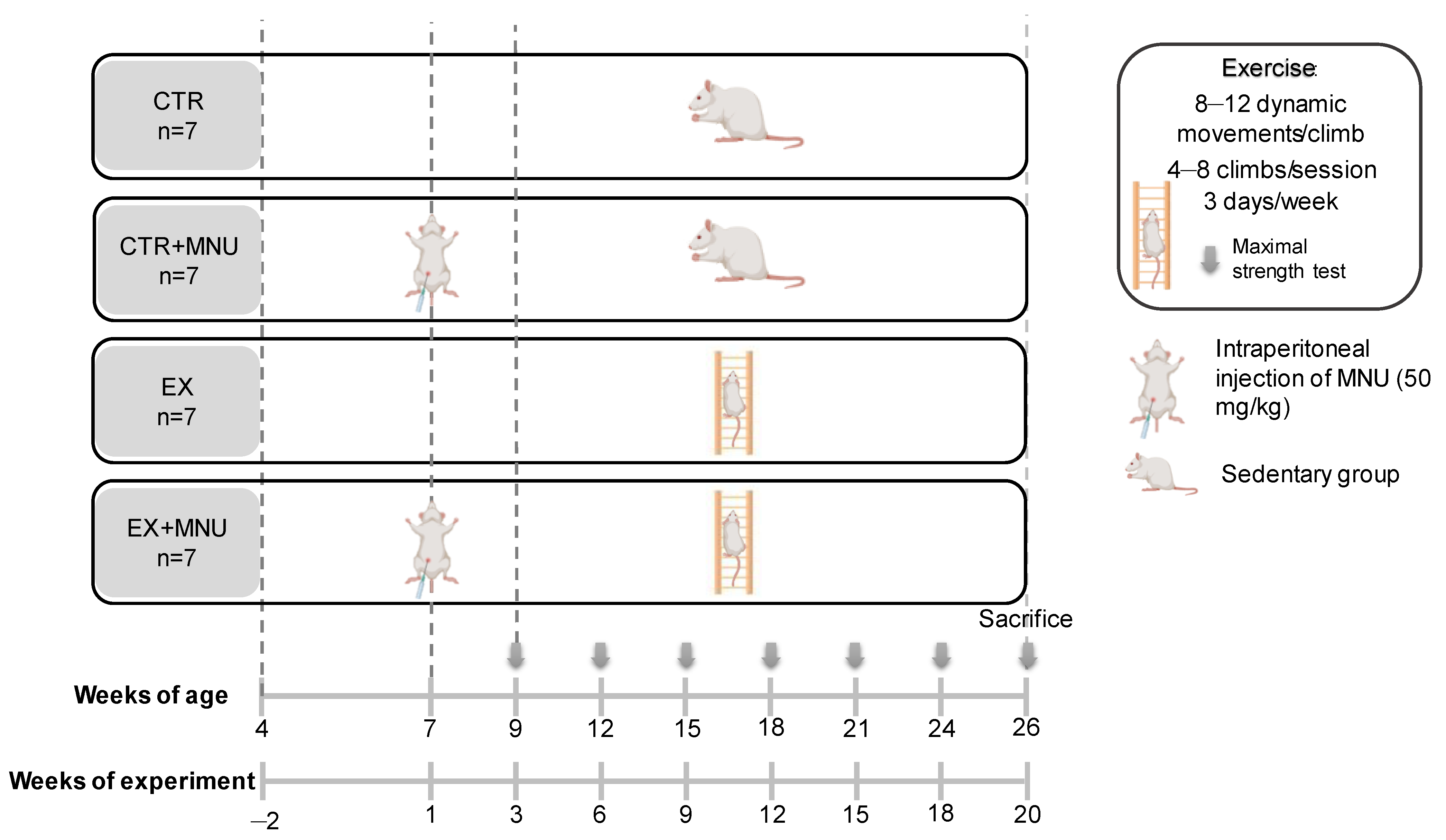
2.2. Animals and Experimental Design
2.3. Exercise Training
2.4. Maximal Strength Test
2.5. Sacrifice and Necropsy of the Animals
2.6. Blood Samples Analysis
2.7. Histological and Immunohistochemical Analysis of Mammary Tumors
2.8. Statistical Analysis
3. Results
3.1. General Findings
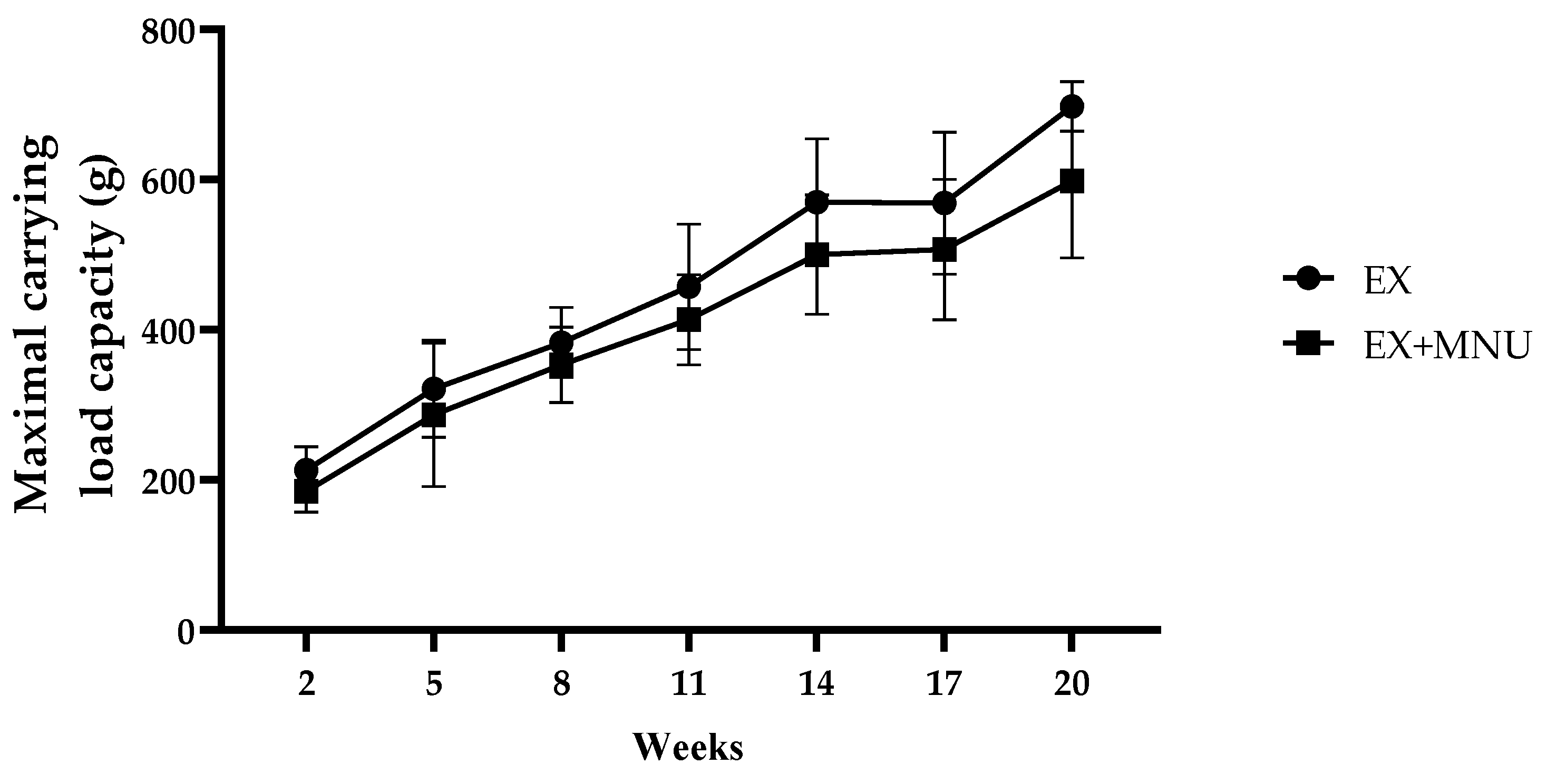
3.2. Maximal Strength Test
3.3. Blood Samples Analysis
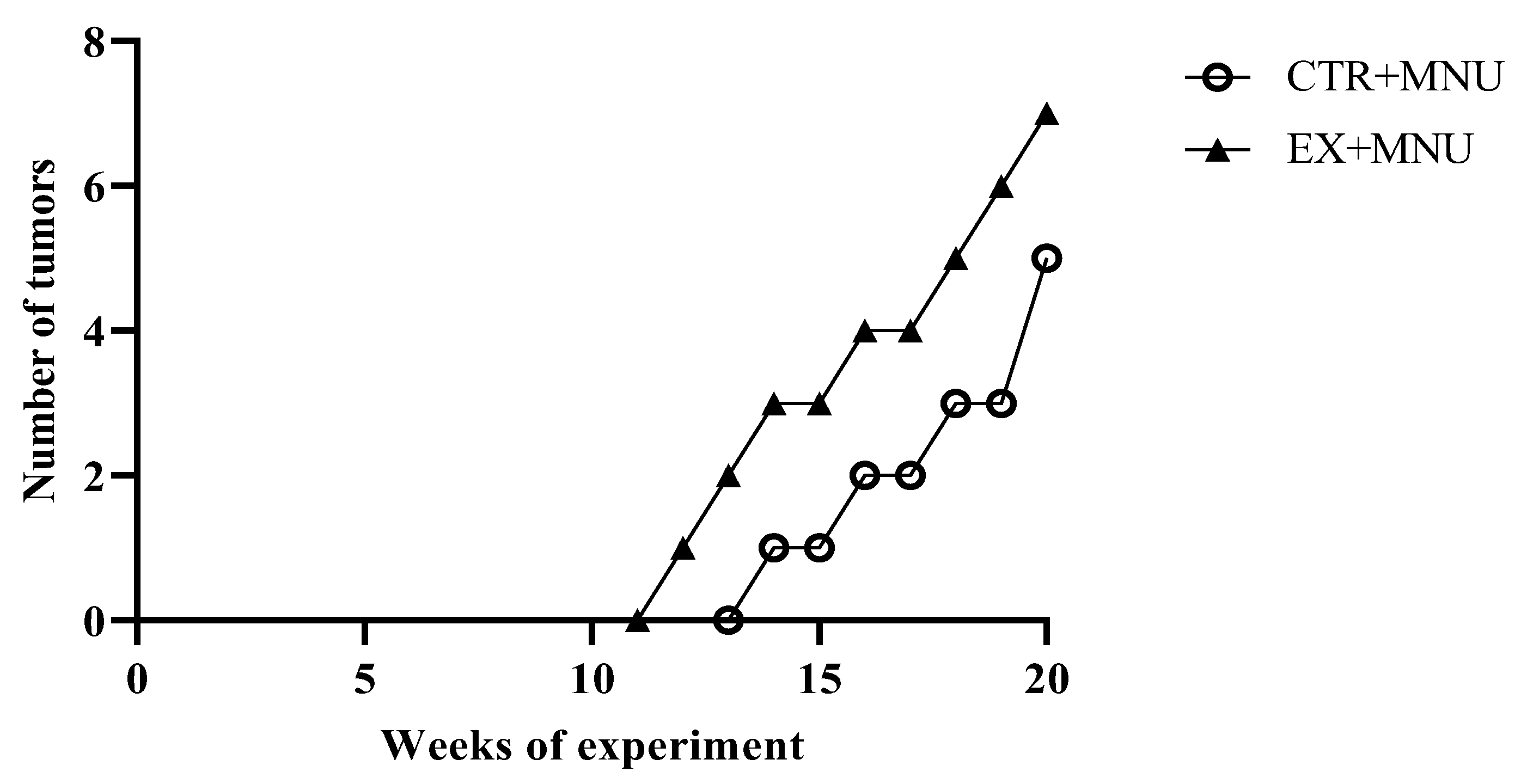
3.4. Mammary Tumours
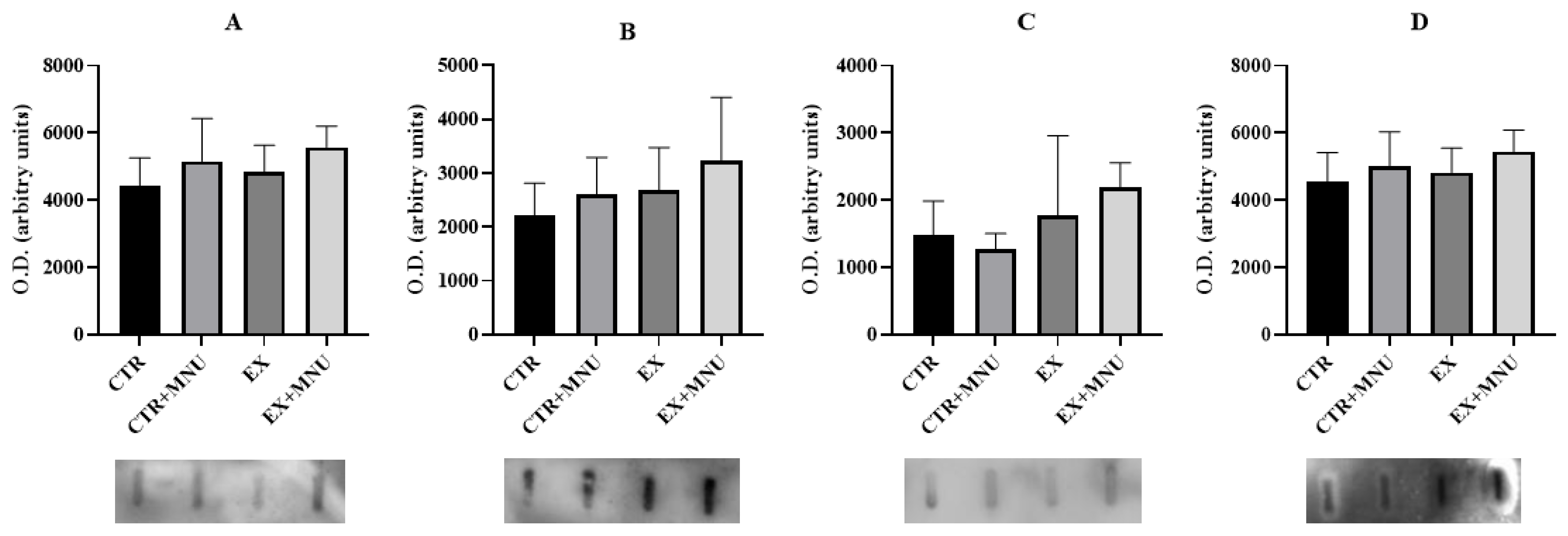
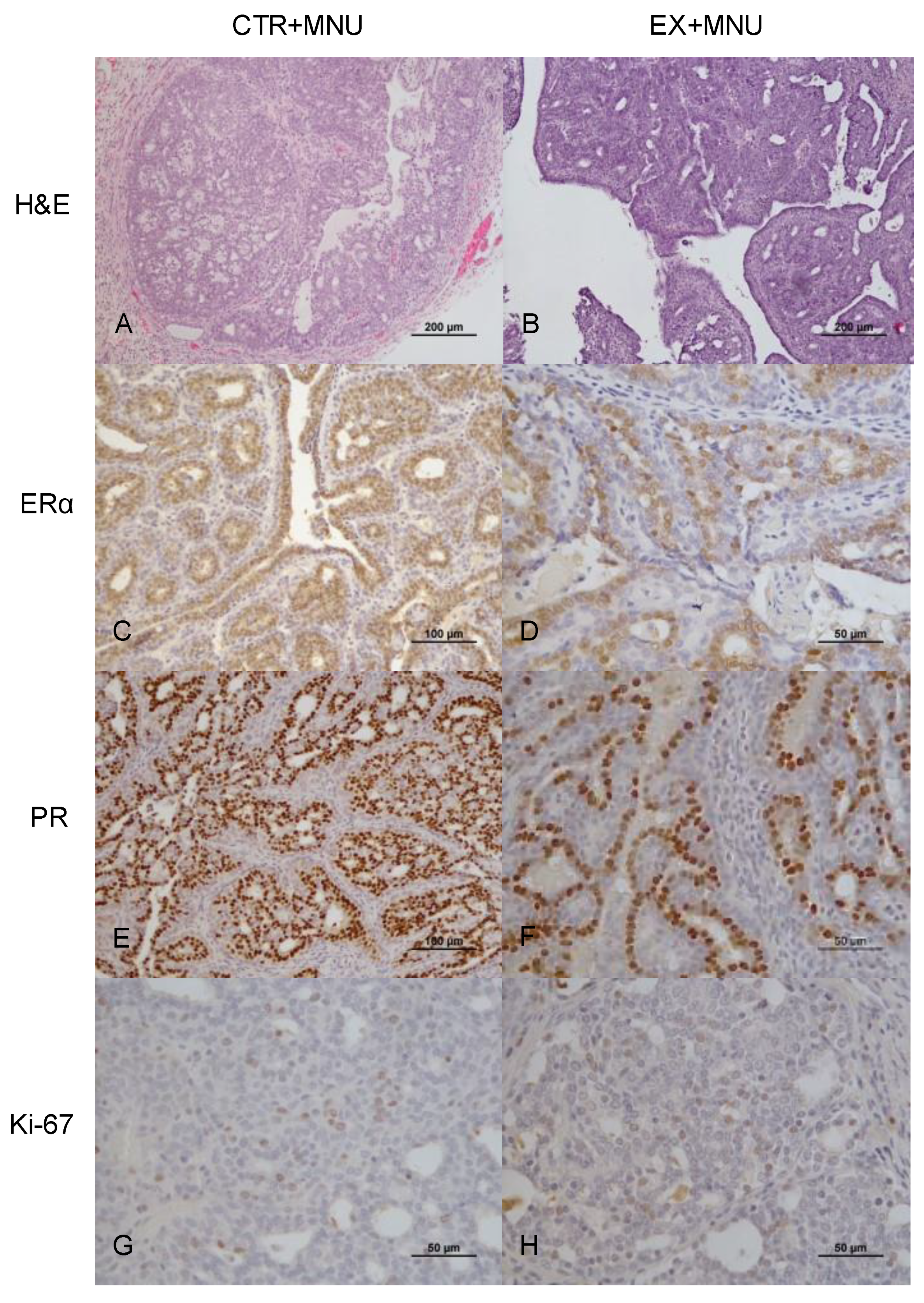
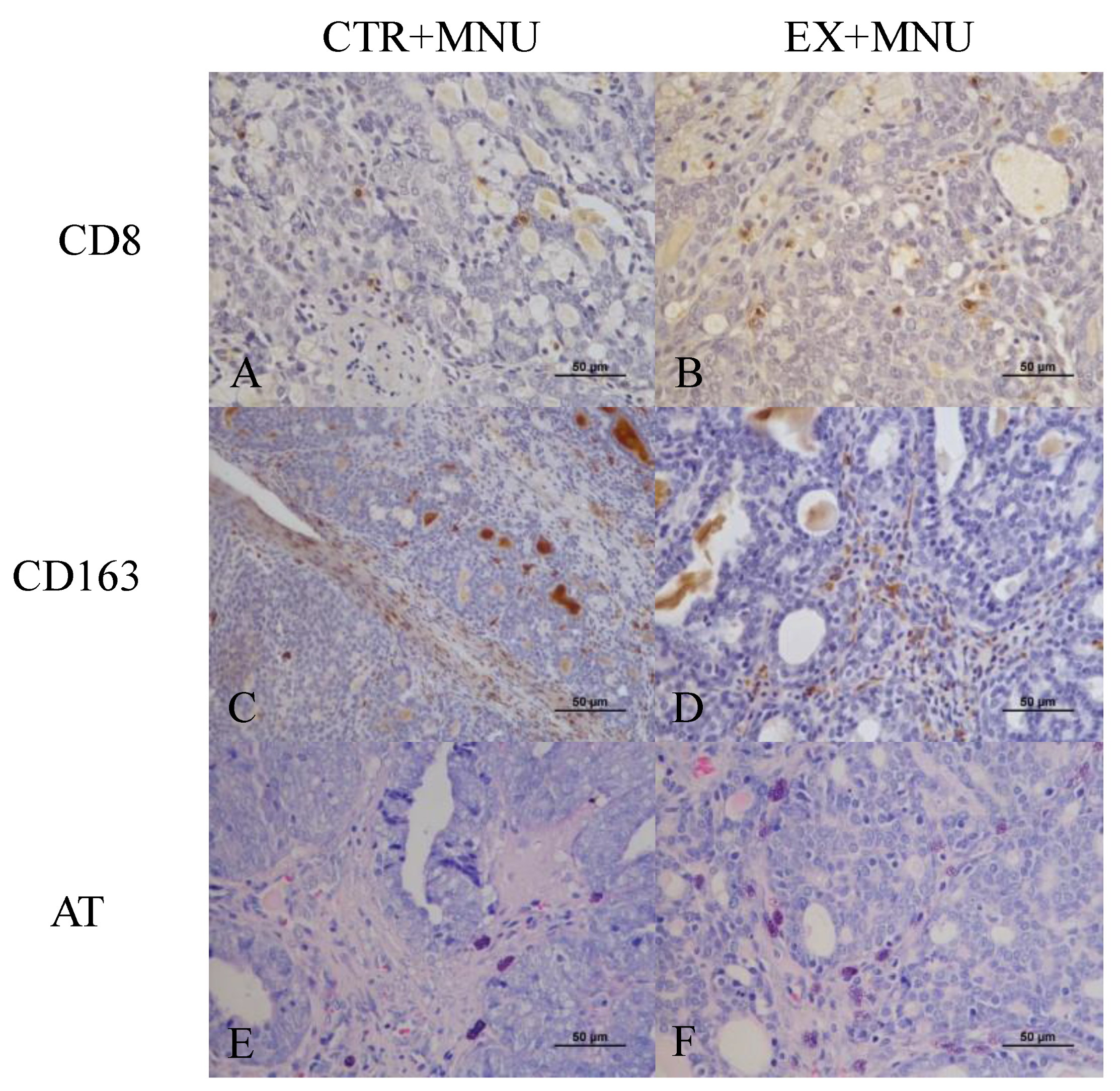
3.5. Histopathological, Histochemical and Immunohistochemistry Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CBC | Complete blood count |
| CK-MB | Creatinine kinase-MB |
| CRP | C-reactive protein |
| CTR | Sedentary control group |
| CTR+MNU | Sedentary induced group |
| ERα | Estrogen receptor alpha |
| EX | Exercised control |
| EX+MNU | Exercised induced group |
| H&E | Hematoxylin and eosin |
| IL-6 | Interleukin 6 |
| MCL | Maximal carrying load |
| MNU | N-methyl-N-nitrosourea |
| MPV | Mean platelet volume |
| NLR | Neutrophil-lymphocyte ratio |
| PDW | Platelet distribution width |
| PR | Progesterone receptor |
| RDW | Red cells distribution width |
| VEGF-A | Vascular endothelial growth factor-A |
References
- Ferreira, T.; Gama, A.; Seixas, F.; Faustino-Rocha, A.I.; Lopes, C.; Gaspar, V.M.; Mano, J.F.; Medeiros, R.; Oliveira, P.A. Mammary Glands of Women, Female Dogs and Female Rats: Similarities and Differences to Be Considered in Breast Cancer Research. Vet. Sci. 2023, 10, 379. [Google Scholar] [CrossRef] [PubMed]
- Barrios, C.H. Global Challenges in Breast Cancer Detection and Treatment. Breast 2022, 62, S3–S6. [Google Scholar] [CrossRef] [PubMed]
- Crosby, D.; Bhatia, S.; Brindle, K.M.; Coussens, L.M.; Dive, C.; Emberton, M.; Esener, S.; Fitzgerald, R.C.; Gambhir, S.S.; Kuhn, P.; et al. Early Detection of Cancer. Science 2022, 375, eaay9040. [Google Scholar] [CrossRef]
- Prasanth, B.K.; Alkhowaiter, S.; Sawarkar, G.; Dharshini, B.D.; Baskaran, A.R. Unlocking Early Cancer Detection: Exploring Biomarkers, Circulating DNA, and Innovative Technological Approaches. Cureus 2023, 15, e51090. [Google Scholar] [CrossRef]
- Panattoni, N.; Giannetta, N.; Dionisi, S.; Erguiza, B.; Sollazzo, F.; Cultrera, M.; Liquori, G.; De Leo, A.; Di Muzio, M.; Di Simone, E. Improving Sleep Quality in Cancer Patients: A Literature Review on Non-Pharmacologic Interventions. World Cancer Res. J. 2023, 10, 1–7. [Google Scholar] [CrossRef]
- Haywood, D.; Henneghan, A.M.; Chan, A.; Chan, R.J.; Dhillon, H.M.; Lustberg, M.B.; Vardy, J.L.; O’Connor, M.; Elvidge, N.; Dauer, E.; et al. The Effect of Non-Pharmacological Interventions on Cognitive Function in Cancer: An Overview of Systematic Reviews. Support. Care Cancer 2025, 33, 151. [Google Scholar] [CrossRef]
- Saeman, M.R.; DeSpain, K.; Liu, M.-M.; Carlson, B.A.; Song, J.; Baer, L.A.; Wade, C.E.; Wolf, S.E. Effects of Exercise on Soleus in Severe Burn and Muscle Disuse Atrophy. J. Surg. Res. 2015, 198, 19–26. [Google Scholar] [CrossRef]
- Krug, A.L.; Macedo, A.G.; Zago, A.S.; Rush, J.W.E.; Santos, C.F.; Amaral, S.L. High-Intensity Resistance Training Attenuates Dexamethasone-Induced Muscle Atrophy. Muscle Nerve 2016, 53, 779–788. [Google Scholar] [CrossRef]
- Morais, S.R.L.; Goya, A.G.; Urias, Ú.; Jannig, P.R.; Bacurau, A.V.N.; Mello, W.G.; Faleiros, P.L.; Oliveira, S.H.P.; Garcia, V.G.; Ervolino, E.; et al. Strength Training Prior to Muscle Injury Potentiates Low-Level Laser Therapy (LLLT)-Induced Muscle Regeneration. Lasers Med. Sci. 2017, 32, 317–325. [Google Scholar] [CrossRef]
- Marqueti, R.C.; Durigan, J.L.Q.; Oliveira, A.J.S.; Mekaro, M.S.; Guzzoni, V.; Aro, A.A.; Pimentel, E.R.; Selistre-de-Araujo, H.S. Effects of Aging and Resistance Training in Rat Tendon Remodeling. FASEB J. 2018, 32, 353–368. [Google Scholar] [CrossRef]
- Greene, N.P.; Nilsson, M.I.; Washington, T.A.; Lee, D.E.; Brown, L.A.; Papineau, A.M.; Shimkus, K.L.; Greene, E.S.; Crouse, S.F.; Fluckey, J.D. Impaired Exercise-Induced Mitochondrial Biogenesis in the Obese Zucker Rat, despite PGC-1α Induction, Is Due to Compromised Mitochondrial Translation Elongation. Am. J. Physiol. Endocrinol Metab. 2014, 306, E503–E511. [Google Scholar] [CrossRef] [PubMed]
- Padilha, C.S.; Marinello, P.C.; Galvão, D.A.; Newton, R.U.; Borges, F.H.; Frajacomo, F.; Deminice, R. Evaluation of Resistance Training to Improve Muscular Strength and Body Composition in Cancer Patients Undergoing Neoadjuvant and Adjuvant Therapy: A Meta-Analysis. J. Cancer Surviv. 2017, 11, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Li, W.; Chen, C.-S. Breast Cancer Animal Models and Applications. Zool. Res. 2020, 41, 477–494. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, A.; Faustino-Rocha, A.I.; Colaço, B.; Oliveira, P.A. Experimental Mammary Carcinogenesis-Rat Models. Life Sci. 2017, 173, 116–134. [Google Scholar] [CrossRef]
- Ferreira, T.; Azevedo, T.; Silva, J.; Faustino-Rocha, A.I.; Oliveira, P.A. Current Views on in Vivo Models for Breast Cancer Research and Related Drug Development. Expert Opin. Drug Discov. 2024, 19, 189–207. [Google Scholar] [CrossRef]
- Borowsky, A.D. Choosing a Mouse Model: Experimental Biology in Context—The Utility and Limitations of Mouse Models of Breast Cancer. Cold Spring Harb. Perspect. Biol. 2011, 3, a009670. [Google Scholar] [CrossRef]
- Fantozzi, A.; Christofori, G. Mouse Models of Breast Cancer Metastasis. Breast Cancer Res. 2006, 8, 212. [Google Scholar] [CrossRef]
- Lourenço, Í.; Krause Neto, W.; dos Santos Portella Amorim, L.; Moraes Munhoz Ortiz, V.; Lopes Geraldo, V.; Henrique da Silva Ferreira, G.; Chagas Caperuto, É.; Florencio Gama, E. Muscle Hypertrophy and Ladder-based Resistance Training for Rodents: A Systematic Review and Meta-analysis. Physiol. Rep. 2020, 8, e14502. [Google Scholar] [CrossRef]
- Nieman, D.C.; Wentz, L.M. The Compelling Link between Physical Activity and the Body’s Defense System. J. Sport Health Sci. 2019, 8, 201–217. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Ratamess, N.A. Hormonal Responses and Adaptations to Resistance Exercise and Training. Sports Med. 2005, 35, 339–361. [Google Scholar] [CrossRef]
- Xia, L.; Oyang, L.; Lin, J.; Tan, S.; Han, Y.; Wu, N.; Yi, P.; Tang, L.; Pan, Q.; Rao, S.; et al. The Cancer Metabolic Reprogramming and Immune Response. Mol. Cancer 2021, 20, 28. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.-L.; Gong, Y.; Qi, Y.-J.; Shao, Z.-M.; Jiang, Y.-Z. Effects of Dietary Intervention on Human Diseases: Molecular Mechanisms and Therapeutic Potential. Signal Transduct. Target. Ther. 2024, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.C.; Ellefsen, S.; Baar, K. Adaptations to Endurance and Strength Training. Cold Spring Harb. Perspect. Med. 2018, 8, a029769. [Google Scholar] [CrossRef] [PubMed]
- Krzysztofik, M.; Wilk, M.; Wojdała, G.; Gołaś, A. Maximizing Muscle Hypertrophy: A Systematic Review of Advanced Resistance Training Techniques and Methods. Int. J. Environ. Res. Public Health 2019, 16, 4897. [Google Scholar] [CrossRef]
- Faustino-Rocha, A.I.; Ginja, M.; Ferreira, R.; Oliveira, P.A. Studying Humane Endpoints in a Rat Model of Mammary Carcinogenesis. Iran. J. Basic Med. Sci. 2019, 22, 643–649. [Google Scholar] [CrossRef]
- Faustino-Rocha, A.I.; Silva, A.; Gabriel, J.; Gil da Costa, R.M.; Moutinho, M.; Oliveira, P.A.; Gama, A.; Ferreira, R.; Ginja, M. Long-Term Exercise Training as a Modulator of Mammary Cancer Vascularization. Biomed. Pharmacother. 2016, 81, 273–280. [Google Scholar] [CrossRef]
- Padilha, C.S.; Borges, F.H.; Costa Mendes da Silva, L.E.; Frajacomo, F.T.T.; Jordao, A.A.; Duarte, J.A.; Cecchini, R.; Guarnier, F.A.; Deminice, R. Resistance Exercise Attenuates Skeletal Muscle Oxidative Stress, Systemic pro-Inflammatory State, and Cachexia in Walker-256 Tumor-Bearing Rats. Appl. Physiol. Nutr. Metab. 2017, 42, 916–923. [Google Scholar] [CrossRef]
- Çelik, M.N.; Söğüt, M.Ü. Probiotics Improve Chemerin and Metabolic Syndrome Parameters in Obese Rats. Balk. Med. J. 2019, 36, 270–275. [Google Scholar] [CrossRef]
- Novelli, E.L.B.; Diniz, Y.S.; Galhardi, C.M.; Ebaid, G.M.X.; Rodrigues, H.G.; Mani, F.; Fernandes, A.A.H.; Cicogna, A.C.; Novelli Filho, J.L.V.B. Anthropometrical Parameters and Markers of Obesity in Rats. Lab. Anim. 2007, 41, 111–119. [Google Scholar] [CrossRef]
- Silva, J.; Duarte, J.A.; Oliveira, P.A. Realistic Aspects behind the Application of the Rat Model of Chemically-Induced Mammary Cancer: Practical Guidelines to Obtain the Best Results. Vet. World 2023, 16, 1222–1230. [Google Scholar] [CrossRef]
- Forbes, D.; Blom, H.; Kostomitsopoulos, N.; Moore, G.; Perretta, G. Euroguide: On the Accommodation and Care of Animals Used for Experimental and Other Scientific Purposes; Forbes, D., Blom, H., Kostomitsopoulos, N., Moore, G., Perretta, G., Eds.; Felasa: London, UK, 2007; ISBN 978-1-85315-751-6. [Google Scholar]
- Russo, J.; Russo, I.H. Atlas and Histologic Classification of Tumors of the Rat Mammary Gland. J. Mammary Gland. Biol. Neoplasia 2000, 5, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Lavalle, G.E.; Bertagnolli, A.C.; Tavares, W.L.F.; Ferreira, M.A.N.D.; Cassali, G.D. Mast Cells and Angiogenesis in Canine Mammary Tumor. Arq. Bras. Med. Vet. Zootec. 2010, 62, 1348–1351. [Google Scholar] [CrossRef]
- Arazi, H.; Rahmati, S.; Ghafoori, H. The Interaction Effects of Resistance Training and Sustanon Abuse on Liver Antioxidant Activities and Serum Enzymes in Male Rats. Interv. Med. Appl. Sci. 2017, 9, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.V.; Lino, A.D.S.; Ruffoni, L.G.D.; Domingos, M.M.; Barbosa, M.R.; Rodrigues, M.F.C.; Ferreira, F.C.; Tomaz, L.M.; Canevazzi, G.H.R.; Silva, N.S.; et al. Resistance Training and Hormone Replacement Increase MMP-2 Activity, Quality and Quantity of Bone in Ovariectomized Rats. Mot. Rev. Educ. Física 2017, 23, e1017107. [Google Scholar] [CrossRef][Green Version]
- Andreollo, N.A.; Santos, E.F.; Araújo, M.R.; Lopes, L.R. Rat’s Age versus Human’s Age: What Is the Relationship? Arq. Bras. Cir. Dig. 2012, 25, 49–51. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and Cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and Tumor Progression: Signaling Pathways and Targeted Intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Emam, K.K.; Abdel Fattah, M.E.; El Rayes, S.M.; Hebishy, M.A.; Dessouki, A.A. Assessment of Wheat Germ Oil Role in the Prevention of Induced Breast Cancer in Rats. ACS Omega 2022, 7, 13942–13952. [Google Scholar] [CrossRef]
- Danforth, D.N. The Role of Chronic Inflammation in the Development of Breast Cancer. Cancers 2021, 13, 3918. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, M.; Yang, Z.; Huang, X.; Lin, Y.; Yang, H. Healthy Lifestyles, Systemic Inflammation and Breast Cancer Risk: A Mediation Analysis. BMC Cancer 2024, 24, 208. [Google Scholar] [CrossRef]
- Faustino-Rocha, A.I.; Gama, A.; Oliveira, P.A.; Alvarado, A.; Neuparth, M.J.; Ferreira, R.; Ginja, M. Effects of Lifelong Exercise Training on Mammary Tumorigenesis Induced by MNU in Female Sprague–Dawley Rats. Clin. Exp. Med. 2017, 17, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Grubbs, C.J.; Hill, D.L.; Bland, K.I.; Beenken, S.W.; Lin, T.-H.; Eto, I.; Atigadda, V.R.; Vines, K.K.; Brouillette, W.J.; Muccio, D.D. 9cUAB30, an RXR Specific Retinoid, and/or Tamoxifen in the Prevention of Methylnitrosourea-Induced Mammary Cancers. Cancer Lett. 2003, 201, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Bigsby, R.M. Synergistic Tumor Promoter Effects of Estrone and Progesterone in Methylnitrosourea-Induced Rat Mammary Cancer. Cancer Lett. 2002, 179, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cho, Y.-Y.; Langfald, A.; Carper, A.; Lubet, R.A.; Grubbs, C.J.; Ericson, M.E.; Bode, A.M. Lapatinib, a Preventive/Therapeutic Agent against Mammary Cancer, Suppresses RTK-Mediated Signaling through Multiple Signaling Pathways. Cancer Prev. Res. 2011, 4, 1190–1197. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Cho, S.W. The Evasion Mechanisms of Cancer Immunity and Drug Intervention in the Tumor Microenvironment. Front. Pharmacol. 2022, 13, 868695. [Google Scholar] [CrossRef]
- Qin, B.; He, Z.; Xie, L.; Feng, S.; Ye, J.; Gui, J.; Sun, X.; Sang, M. The Augmentation of Cytotoxic Immune Cell Functionality through Physical Exertion Bolsters the Potency of Chemotherapy in Models of Mammary Carcinoma. Cancer Med. 2024, 13, e6951. [Google Scholar] [CrossRef]
- Bobeuf, F.; Labonté, M.; Khalil, A.; Dionne, I.J. Effect of Resistance Training on Hematological Blood Markers in Older Men and Women: A Pilot Study. Curr. Gerontol. Geriatr. Res. 2009, 2009, 156820. [Google Scholar] [CrossRef]

| Parameters | Experimental Groups | |||
|---|---|---|---|---|
| CTR (n = 7) | CTR+MNU (n = 5) | EX (n = 7) | EX+MNU (n = 6) | |
| Initial body weight (g) | 159.90 ± 13.11 | 148.27 ± 14.31 | 157.84 ± 18.75 | 156.08 ± 10.41 |
| Accurate final body weight (g) | 278.71 ± 10.47 | 274.32 ± 31.18 | 292.86 ± 20.79 | 260.67 ± 30.76 |
| Body mass index | 12.14 ± 1.35 | 12.84 ± 1.32 | 13.13 ± 0.75 | 12.21 ± 1.29 |
| Organs | Experimental Groups | |||
|---|---|---|---|---|
| CTR (n = 7) | CTR+MNU (n = 5) | EX (n = 7) | EX+MNU (n = 6) | |
| Spleen | 0.69 ± 0.11 | 0.68 ± 0.21 | 0.73 ± 0.11 | 0.69 ± 0.16 |
| Heart | 0.80 ± 0.05 | 0.78 ± 0.06 | 0.79 ± 0.09 | 0.75 ± 0.07 |
| Lungs | 1.43 ± 0.13 | 1.37 ± 0.15 | 1.46 ± 0.11 | 1.39 ± 0.09 |
| Liver | 7.15 ± 0.91 | 6.71 ± 0.91 | 6.30 ± 0.89 | 6.82 ± 1.19 |
| Soleus | 0.21 ± 0.05 | 0.20 ± 0.02 | 0.23 ± 0.02 | 0.20 ± 0.03 |
| Gastrocnemius | 3.20 ± 0.20 | 2.66 ± 0.99 | 3.56 ± 0.43 | 3.08 ± 0.24 |
| Biceps brachii | 0.28 ± 0.05 | 0.25 ± 0.06 | 0.30 ± 0.05 | 0.26 ± 0.02 |
| Femur | 3.41 ± 0.26 | 3.30 ± 0.07 | 3.59 ± 0.20 | 3.32 ± 0.10 |
| Tibia | 3.57 ± 0.30 a | 3.70 ± 0.19 | 3.96 ± 0.05 | 3.73 ± 0.15 |
| Parameters | Experimental Groups | |||
|---|---|---|---|---|
| CTR (n = 7) | CTR+MNU (n = 5) | EX (n = 7) | EX+MNU (n = 6) | |
| Erythrocytes (M/µL) | 8.27 ± 0.63 | 8.16 ± 0.68 a | 8.24 ± 0.44 a | 9.40 ± 0.38 |
| Hematocrit (%) | 44.60 ± 2.61 | 45.00 ± 4.86 a | 44.56 ± 1.86 a | 53.10 ± 2.06 |
| Hemoglobin (g/dL) | 15.17 ± 0.77 | 15.20 ± 1.28 a | 15.17 ± 0.55 a | 17.90 ± 0.60 |
| RDW (%) | 20.51 ± 1.51 | 19.32 ± 2.37 | 19.59 ± 1.74 | 20.64 ± 0.44 |
| Reticulocytes (K/µL) | 243.53 ± 50.14 | 267.16 ± 59.83 | 202.93 ± 32.34 | 250.90 ± 51.61 |
| Leucocytes (K/µL) | 1.86 ± 0.62 | 2.39 ± 0.66 | 1.93 ± 1.00 | 3.27 ± 1.09 |
| Neutrophils (K/µL) | 0.41 ± 0.14 | 0.56 ± 0.20 | 0.29 ± 0.10 e | 0.41 ± 0.09 |
| Lymphocytes (K/µL) | 1.34 ± 0.52 | 1.69 ± 0.52 | 1.55 ± 0.87 | 2.76 ± 0.99 |
| Monocytes (K/µL) | 0.10 ± 0.05 | 0.13 ± 0.05 | 0.09 ± 0.04 | 0.09 ± 0.03 |
| Eosinophils (K/µL) | 0.01 ± 0.00 | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.00 |
| Basophils (K/µL) | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| NLR | 0.34 ± 0.15 | 0.35 ± 0.12 | 0.21 ± 0.07 | 0.16 ± 0.03 |
| Platelets (K/µL) | 613.71 ± 83.87 | 489.40 ± 223.47 | 600.29 ± 100.12 | 636.20 ± 53.28 |
| MPV (fL) | 8.71 ± 0.21 | 8.96 ± 0.46 | 8.61 ± 0.41 | 8.64 ± 0.31 |
| PDW (fL) | 9.86 ± 0.42 | 10.26 ± 1.00 | 9.31 ± 0.72 | 9.84 ± 0.55 |
| Parameters | Experimental Groups | |||
|---|---|---|---|---|
| CTR (n = 7) | CTR+MNU (n = 5) | EX (n = 7) | EX+MNU (n = 6) | |
| Albumin (g/dL) | 4.77 ± 0.68 | 4.52 ± 0.02 | 4.10 ± 0.90 | 3.56 ± 1.05 |
| Cholesterol (mg/dL) | 104.77 ± 29.50 | 90.20 ± 6.64 | 85.20 ± 26.69 | 104.47 ± 29.24 |
| CK-MB (U/L) | 471.15 ± 160.00 | 558.94 ± 429.60 | 230.83 ± 166.20 | 202.66 ± 145.05 |
| Triglycerides (mg/dL) | 77.83 ± 36.38 | 122.45 ± 61.95 | 53.54 ± 11.11 | 88.77 ± 20.62 |
| Histological Patterns | Experimental Groups | |||||
|---|---|---|---|---|---|---|
| CTR (n = 7) | CTR+MNU (n = 5) | EX (n = 7) | EX+MNU (n = 6) | |||
| Malignant tumors | In situ carcinoma | Ductal solid and cribriform | 0 | 0 | 0 | 1 |
| Invasive carcinoma | Papillary | 0 | 1 | 0 | 2 | |
| Cribriform | 0 | 4 | 0 | 3 | ||
| Tubular | 0 | 0 | 0 | 1 | ||
| Total malignant tumors: | 0 | 5 | 0 | 7 | ||
| CTR+MNU (n = 5) | EX+MNU (n = 6) | |||||
|---|---|---|---|---|---|---|
| Invasive Carcinoma | In Situ Carcinoma | Invasive Carcinoma | ||||
| Papillary | Cribriform | Ductal solid and Cribriform | Papillary | Cribriform | Tubular | |
| ERα (%) | 39.25 ± 11.95 | 43.27 ± 11.78 a | 13.65 ± 7.28 | 21.13 ± 8.23 | 20.12 ± 6.77 a | 14.95 ± 2.33 |
| PR (%) | 60.15 ± 1.20 | 56.44 ± 9.98 b | 31.65 ± 9.12 | 34.43 ± 17.28 | 25.48 ± 12.75 b | 24.60 ± 7.78 |
| Ki-67 (%) | 16.15 ± 1.91 c,d | 2.00 ± 1.56 c | 1.30 ± 0.42 e | 7.15 ± 2.5 d,e | 6.22 ± 2.23 | 2.50 ± 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, J.; Azevedo, T.; Aires, I.; Medeiros, C.; Neuparth, M.J.; Seixas, F.; Ferreira, R.; Faustino-Rocha, A.I.; Oliveira, P.A.; Duarte, J.A. Effects of Ladder-Climbing Exercise on Mammary Cancer: Data from a Chemically Induced Rat Model. Vet. Sci. 2025, 12, 303. https://doi.org/10.3390/vetsci12040303
Silva J, Azevedo T, Aires I, Medeiros C, Neuparth MJ, Seixas F, Ferreira R, Faustino-Rocha AI, Oliveira PA, Duarte JA. Effects of Ladder-Climbing Exercise on Mammary Cancer: Data from a Chemically Induced Rat Model. Veterinary Sciences. 2025; 12(4):303. https://doi.org/10.3390/vetsci12040303
Chicago/Turabian StyleSilva, Jessica, Tiago Azevedo, Inês Aires, Catarina Medeiros, Maria J. Neuparth, Fernanda Seixas, Rita Ferreira, Ana I. Faustino-Rocha, Paula A. Oliveira, and José Alberto Duarte. 2025. "Effects of Ladder-Climbing Exercise on Mammary Cancer: Data from a Chemically Induced Rat Model" Veterinary Sciences 12, no. 4: 303. https://doi.org/10.3390/vetsci12040303
APA StyleSilva, J., Azevedo, T., Aires, I., Medeiros, C., Neuparth, M. J., Seixas, F., Ferreira, R., Faustino-Rocha, A. I., Oliveira, P. A., & Duarte, J. A. (2025). Effects of Ladder-Climbing Exercise on Mammary Cancer: Data from a Chemically Induced Rat Model. Veterinary Sciences, 12(4), 303. https://doi.org/10.3390/vetsci12040303









