Field Evaluation of a Ready-to-Use Porcine Circovirus Type 2 and Mycoplasma hyopneumoniae Vaccine in Naturally Infected Farms in Taiwan
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement for Experimental Procedures
2.2. Herd Information and Experimental Design
2.3. PCV2 Antibody and Viremia Testing
2.4. Lung Lesion Scoring
2.5. Statistical Analysis
3. Results
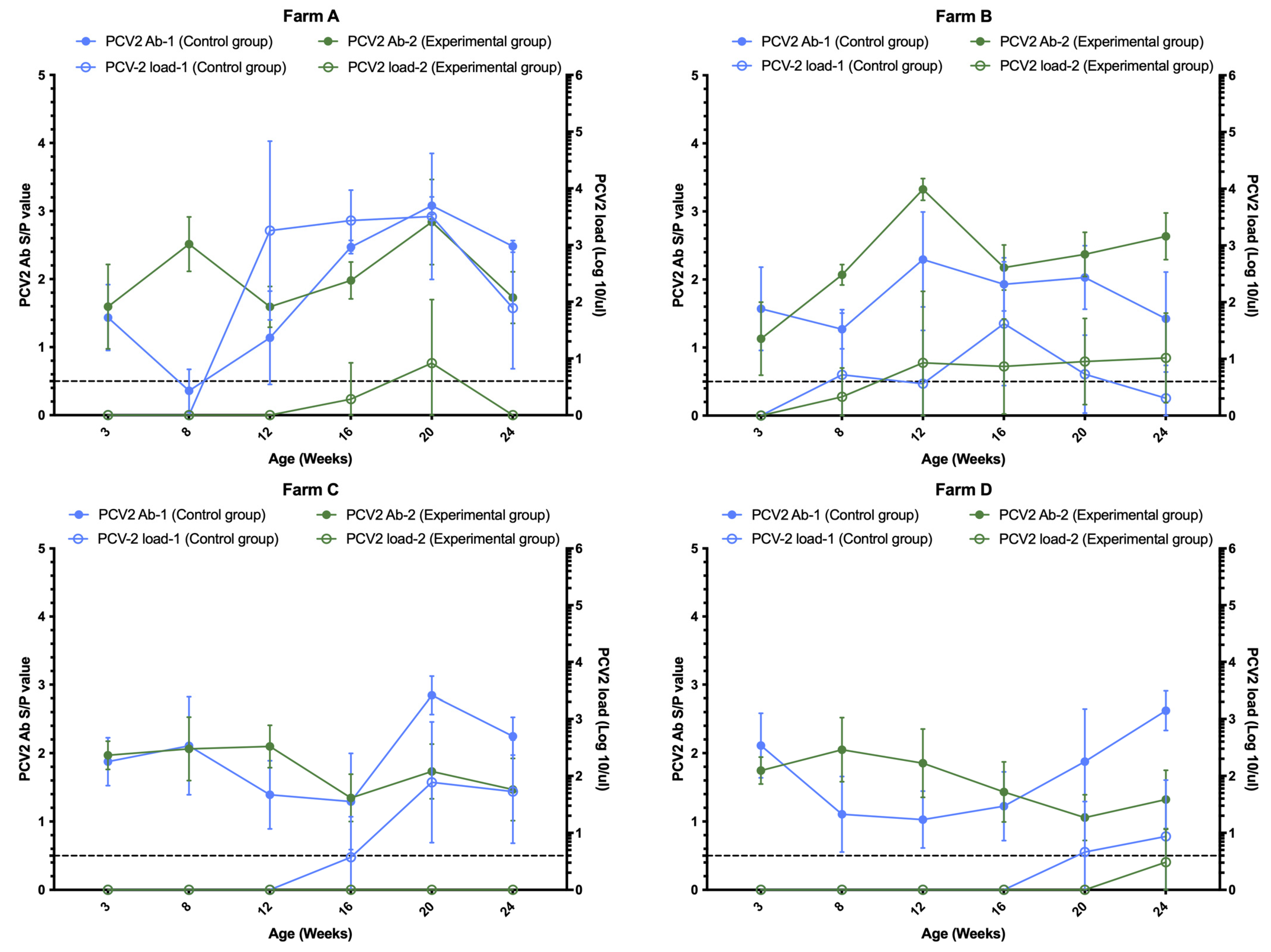
3.1. Comparison of PCV2 Antibodies
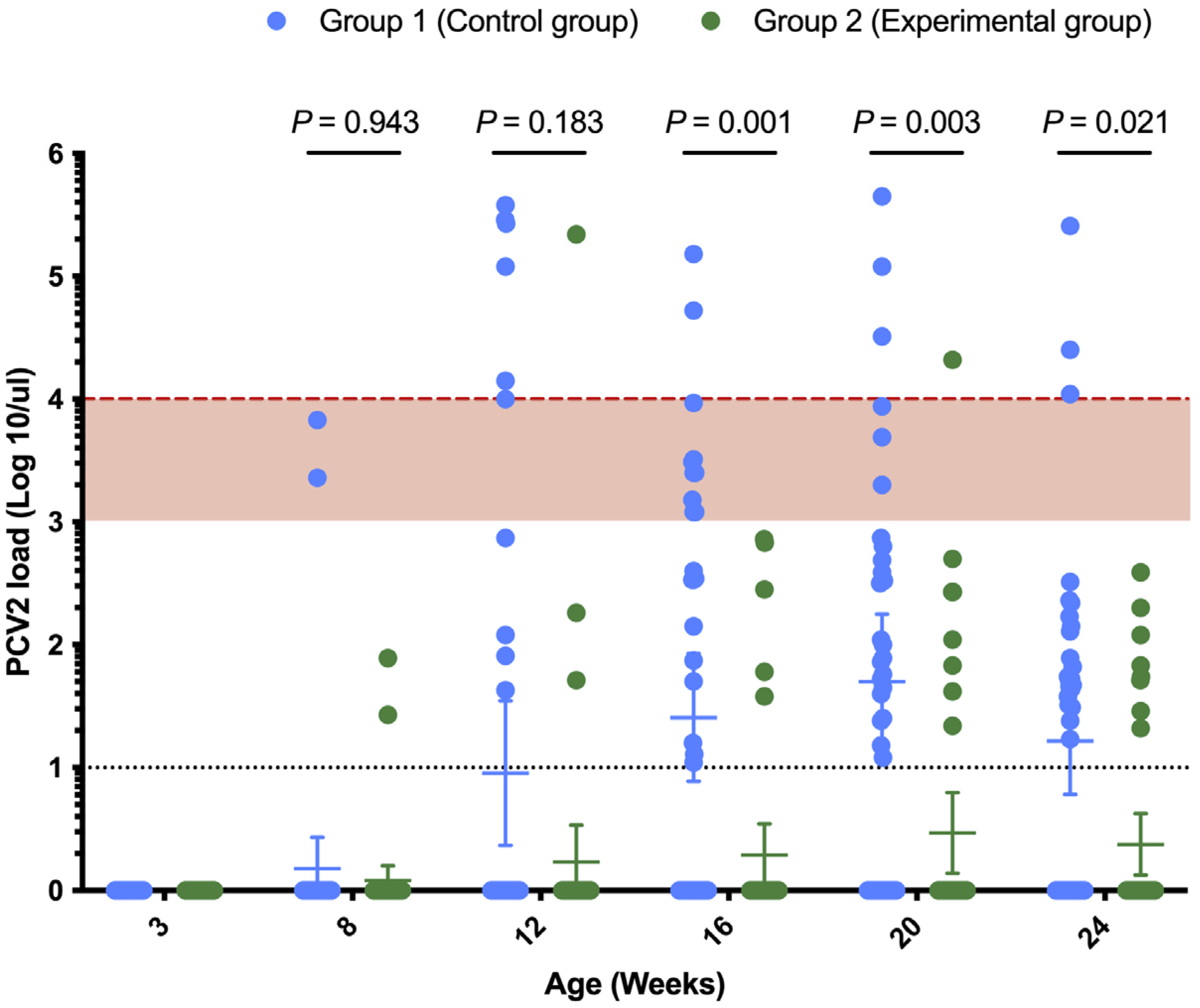
3.2. Comparison of PCV2 Viral Load and Positivity Rate
3.3. Lung Lesion Scoring
3.4. Comparison of Other Relevant Lesions in Slaughterhouses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Assavacheep, P.; Thanawongnuwech, R. Porcine respiratory disease complex: Dynamics of polymicrobial infections and management strategies after the introduction of the African swine fever. Front. Vet. Sci. 2022, 9, 1048861. [Google Scholar] [CrossRef] [PubMed]
- Chae, C. Porcine respiratory disease complex: Interaction of vaccination and porcine circovirus type 2, porcine reproductive and respiratory syndrome virus, and Mycoplasma hyopneumoniae. Vet. J. 2016, 212, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Segalés, J. Porcine circovirus type 2 (PCV2) infections: Clinical signs, pathology and laboratory diagnosis. Virus Res. 2012, 164, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Segalés, J.; Allan, G.M.; Domingo, M. Porcine circovirus diseases. Anim. Health Res. Rev. 2005, 6, 119–142. [Google Scholar] [CrossRef]
- Afghah, Z.; Webb, B.; Meng, X.J.; Ramamoorthy, S. Ten years of PCV2 vaccines and vaccination: Is eradication a possibility? Vet. Microbiol. 2017, 206, 21–28. [Google Scholar] [CrossRef]
- Alarcon, P.; Rushton, J.; Wieland, B. Cost of post-weaning multi-systemic wasting syndrome and porcine circovirus type-2 subclinical infection in England—An economic disease model. Prev. Vet. Med. 2013, 110, 88–102. [Google Scholar] [CrossRef]
- Gillespie, J.; Opriessnig, T.; Meng, X.J.; Pelzer, K.; Buechner-Maxwell, V. Porcine circovirus type 2 and porcine circovirus-associated disease. J. Vet. Intern. Med. 2009, 23, 1151–1163. [Google Scholar] [CrossRef]
- Meng, X.J. Porcine circovirus type 2 (PCV2): Pathogenesis and interaction with the immune system. Annu. Rev. Anim. Biosci. 2013, 1, 43–64. [Google Scholar] [CrossRef]
- Opriessnig, T.; McKeown, N.E.; Harmon, K.L.; Meng, X.J.; Halbur, P.G. Porcine circovirus type 2 infection decreases the efficacy of a modified live porcine reproductive and respiratory syndrome virus vaccine. Clin. Vaccine Immunol. 2006, 13, 923–929. [Google Scholar] [CrossRef]
- Ouyang, T.; Zhang, X.; Liu, X.; Ren, L. Co-infection of swine with porcine circovirus type 2 and other swine viruses. Viruses. 2019, 11, 185. [Google Scholar] [CrossRef]
- Thacker, E.L.; Minion, C.F. Mycoplasmosis. In Diseases of Swine; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Eds.; Iowa State University Press: Ames, IA, USA, 2010; pp. 779–797. [Google Scholar]
- Boeters, M.; Garcia-Morante, B.; van Schaik, G.; Segalés, J.; Rushton, J.; Steeneveld, W. The economic impact of endemic respiratory disease in pigs and related interventions—A systematic review. Porcine Health Manag. 2023, 9, 45. [Google Scholar]
- Ferraz, M.E.S.; Almeida, H.M.S.; Storino, G.Y.; Sonalio, K.; Souza, M.R.; Moura, C.A.A.; Costa, W.M.T.; Lunardi, L.; Linhares, D.C.L.; de Oliveira, L.G. Lung consolidation caused by Mycoplasma hyopneumoniae has a negative effect on productive performance and economic revenue in finishing pigs. Prev. Vet. Med. 2020, 182, 105091. [Google Scholar] [PubMed]
- Tonni, M.; Formenti, N.; Boniotti, M.B.; Guarneri, F.; Scali, F.; Romeo, C.; Pasquali, P.; Pieters, M.; Maes, D.; Alborali, G.L. The role of co-infections in M. hyopneumoniae outbreaks among heavy fattening pigs: A field study. Vet. Res. 2022, 53, 41. [Google Scholar] [PubMed]
- Stygar, A.H.; Jarkko, K.N.; Oliviero, C.; Laurila, T.; Heinonen, M. Economic value of mitigating Actinobacillus pleuropneumoniae infections in pig fattening herds. Agric. Syst. 2016, 144, 113–121. [Google Scholar] [CrossRef]
- Zhu, H.; Chang, X.; Zhou, J.; Wang, D.; Zhou, J.; Fan, B.; Ni, Y.; Yin, J.; Lv, L.; Zhao, Y.; et al. Co-infection analysis of bacterial and viral respiratory pathogens from clinically healthy swine in Eastern China. Vet. Med. Sci. 2021, 7, 1815–1819. [Google Scholar] [CrossRef]
- Plasencia-Muñoz, B.; Avelar-González, F.J.; De la Garza, M.; Jacques, M.; Moreno-Flores, A.; Guerrero-Barrera, A.L. Actinobacillus pleuropneumoniae interaction with swine endothelial cells. Front. Vet. Sci. 2020, 7, 569370. [Google Scholar] [CrossRef] [PubMed]
- Engle, T.B.; Jobman, E.E.; Moural, T.W.; McKnite, A.M.; Bundy, J.W.; Barnes, S.Y.; Davis, E.H.; Galeota, J.A.; Burkey, T.E.; Plastow, G.S.; et al. Variation in time and magnitude of immune response and viremia in experimental challenges with Porcine circovirus 2b. BMC Vet. Res. 2014, 10, 286. [Google Scholar]
- López-Soria, S.; Sibila, M.; Nofrarías, M.; Calsamiglia, M.; Manzanilla, E.G.; Ramírez-Mendoza, H.; Mínguez, A.; Serrano, J.M.; Marín, O.; Joisel, F.; et al. Effect of porcine circovirus type 2 (PCV2) load in serum on average daily weight gain during the postweaning period. Vet. Microbiol. 2014, 174, 296–301. [Google Scholar]
- Garcia-Morante, B.; Segalés, J.; Fraile, L.; Pérez de Rozas, A.; Maiti, H.; Coll, T.; Sibila, M. Assessment of Mycoplasma hyopneumoniae-induced pneumonia using different lung lesion scoring systems: A Comparative Review. J. Comp. Pathol. 2016, 154, 125–134. [Google Scholar]
- Merialdi, G.; Dottori, M.; Bonilauri, P.; Luppi, A.; Gozio, S.; Pozzi, P.; Spaggiari, B.; Martelli, P. Survey of pleuritis and pulmonary lesions in pigs at abattoir with a focus on the extent of the condition and herd risk factors. Vet. J. 2012, 193, 234–239. [Google Scholar]
- Cuccato, M.; Divari, S.; Ciaramita, S.; Sereno, A.; Campelli, D.; Biolatti, P.G.; Biolatti, B.; Meliota, F.; Bollo, E.; Cannizzo, F.T. Actinobacillus pleuropneumoniae serotypes by multiplex PCR identification and evaluation of lung lesions in pigs from Piedmont (Italy) Farms. Animals 2024, 14, 2255. [Google Scholar] [CrossRef] [PubMed]
- Tsai, G.T.; Lin, Y.C.; Lin, W.H.; Lin, J.H.; Chiou, M.T.; Liu, H.F.; Lin, C.N. Phylogeographic and genetic characterization of porcine circovirus type 2 in Taiwan from 2001–2017. Sci. Rep. 2019, 9, 10782. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.T. Investigation of infection status of Mycoplasma hyopneumoniae and Mycoplasma hyorhinis in nursery and finishing pigs. Master Thesis, National Pingtung University of Science and Technology, Pingtung, Taiwan, 2021. [Google Scholar] [CrossRef]
- Lin, C.N.; Ke, N.J.; Chiou, M.T. Cross-sectional study on the sero- and viral dynamics of porcine circovirus type 2 in the field. Vaccines 2020, 8, 339. [Google Scholar] [CrossRef]
- Pagot, E.; Rigaut, M.; Roudaut, D.; Panzavolta, L.; Jolie, R.; Duivo, D. Field efficacy of Porcilis® PCV M Hyo versus a licensed commercially available vaccine and placebo in the prevention of PRDC in pigs on a French farm: A randomized controlled trial. Porcine Health Manag. 2017, 3, 3. [Google Scholar]
- Tassis, P.D.; Tsakmakidis, I.; Papatsiros, V.G.; Koulialis, D.; Nell, T.; Brellou, G.; Tzika, E.D. A randomized controlled study on the efficacy of a novel combination vaccine against enzootic pneumonia (Mycoplasma hyopneumoniae) and porcine Circovirus type 2 (PCV2) in the presence of strong maternally derived PCV2 immunity in pigs. BMC Vet Res. 2017, 13, 91. [Google Scholar] [CrossRef]
- López-Lorenzo, G.; Prieto, A.; López-Novo, C.; Díaz, P.; López, C.M.; Morrondo, P.; Fernández, G.; Díaz-Cao, J.M. Efficacy of two commercial ready-to-use PCV2 and Mycoplasma hyopneumoniae vaccines under field Conditions. Animals 2021, 11, 1553. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Oh, T.; Suh, J.; Chae, C. A Comparative Field Evaluation of the Effect of Growth Performance Between Porcine Circovirus Type 2a (PCV2a)- and PCV2b-Based Bivalent Vaccines Containing PCV2 and Mycoplasma hyopneumoniae. Front. Vet. Sci. 2022, 9, 859344. [Google Scholar] [CrossRef]
- Nielsen, G.B.; Haugegaard, J.; Jolie, R. Field evaluation of a ready-to-use combined Porcine circovirus type 2 and Mycoplasma hyopneumoniae vaccine in Denmark—A historical comparison of productivity parameters in 20 nursery and 23 finishing herds. Porcine Health Manag. 2018, 4, 29. [Google Scholar] [CrossRef]
- European Medicines Agency Summary of Opinion (Initial Authorisation)—Porcilis PCV M Hyo. 2014. Available online: https://www.ema.europa.eu/en/medicines/veterinary/EPAR/porcilis-pcv-m-hyo (accessed on 14 November 2014).
- Olvera, A.; Sibila, M.; Calsamiglia, M.; Segalés, J.; Domingo, M. Comparison of porcine circovirus type 2 load in serum quantified by a real time PCR in postweaning multisystemic wasting syndrome and porcine dermatitis and nephropathy syndrome naturally affected pigs. J. Virol. Methods. 2004, 117, 75–80. [Google Scholar] [CrossRef]
- Opriessnig, T.; Meng, X.J.; Halbur, P.G. Porcine circovirus type 2 associated disease: Update on current terminology, clinical manifestations, pathogenesis, diagnosis, and intervention strategies. J. Vet. Diagn. Investig. 2007, 19, 591–615. [Google Scholar] [CrossRef]
- Ke, C.H.; Du, M.Y.; Hsieh, W.J.; Lin, C.C.; Ting, J.M.; Chiou, M.T.; Lin, C.N. Implementation of point-of-care platforms for rapid detection of porcine circovirus type 2. J. Vet. Sci. 2024, 25, e28. [Google Scholar] [PubMed]
- McKeown, N.E.; Opriessnig, T.; Thomas, P.; Guenette, D.K.; Elvinger, F.; Fenaux, M.; Halbur, P.G.; Meng, X.J. Effects on porcine circovirus type 2 (PCV2) maternal antibodies on experimental infection of piglets with PCV2. Clin. Diagn. Lab. Immunol. 2005, 12, 1347–1351. [Google Scholar] [PubMed]
- Madec, F.; Derrien, H. Fréquence, intensité et localization des lesion pulmonaires chez le porc charcutier: Resultants d’une premiére série d’observations en abattoir. J. Rech. Porc. Fr. 1981, 13, 231–236. [Google Scholar]
- Có-Rives, I.; Chen, Y.A.; Moore, A.C. Skin-based vaccination: A systematic mapping review of the types of vaccines and methods used and immunity and protection elicited in pigs. Vaccines 2023, 11, 450. [Google Scholar] [CrossRef] [PubMed]
- Owen, K.; Blackie, N.; Gibson, T.J. The effect of needle reuse on piglet skin puncture force. Vet. Sci. 2022, 9, 90. [Google Scholar] [CrossRef]
- Pileri, E.; Mateu, E. Review on the transmission porcine reproductive and respiratory syndrome virus between pigs and farms and impact on vaccination. Vet. Res. 2016, 47, 108. [Google Scholar]
- Madapong, A.; Saeng-Chuto, K.; Tantituvanont, A.; Nilubol, D. Safety of PRRSV-2 MLV vaccines administrated via the intramuscular or intradermal route and evaluation of PRRSV transmission upon needle-free and needle delivery. Sci. Rep. 2021, 11, 23107. [Google Scholar]
- Salman, M.; Lin, H.; Suntisukwattana, R.; Watcharavongtip, P.; Jermsutjarit, P.; Tantituvanont, A.; Nilubol, D. Intradermal needle-free injection prevents African Swine Fever transmission, while intramuscular needle injection does not. Sci. Rep. 2023, 13, 4600. [Google Scholar]
- Patterson, A.R.; Ramamoorthy, S.; Madson, D.M.; Meng, X.J.; Halbur, P.G.; Opriessnig, T. Shedding and infection dynamics of porcine circovirus type 2 (PCV2) after experimental infection. Vet. Microbiol. 2011, 149, 91–98. [Google Scholar]
- Imeah, B.; Penz, E.; Rana, M.; Trask, C. Needle-less Injector Study Team. Economic analysis of new workplace technology including productivity and injury: The case of needle-less injection in swine. PLoS ONE 2020, 15, e0233599. [Google Scholar]
- Daniels, C.S.; Funk, J.A. Prevalence of carcass defects in market swine at harvest. In Proceedings of the International Conference on the Epidemiology and Control of Biological, Chemical and Physical Hazards in Pigs and Pork, Quebec, QC, Canada, 30 September–2 October 2009; pp. 121–123. [Google Scholar]
- King, D.; Painter, T.; Holtkamp, D.; DuBois, P.; Wang, C. Effect of injection tool on incidence of head and neck abscesses at slaughter. J. Swine Health Prod. 2010, 18, 290–293. [Google Scholar]
- Pileri, E.; Cortey, M.; Rodríguez, F.; Sibila, M.; Fraile, L.; Segalés, J. Comparison of the immunoperoxidase monolayer assay and three commercial ELISAs for detection of antibodies against porcine circovirus type 2. Vet. J. 2014, 201, 429–432. [Google Scholar] [PubMed]
- Liu, Y.; Barrett, C.B.; Pham, T.; Violette, W. The intertemporal evolution of agriculture and labor over a rapid structural transformation: Lessons from Vietnam. Food Policy 2020, 94, 101913. [Google Scholar] [PubMed]
- Marois, C.; Gottschalk, M.; Morvan, H.; Fablet, C.; Madec, F.; Kobisch, M. Experimental infection of SPF pigs with Actinobacillus pleuropneumoniae serotype 9 alone or in association with Mycoplasma hyopneumoniae. Vet. Microbiol. 2009, 135, 283–291. [Google Scholar]
- Chang, C.L.; McAleer, M.; Chen, M.G.; Huang, B.W. Modelling the Asymmetric Volatility in Hog Prices in Taiwan: The Impact of Joining the WTO. Available online: https://ssrn.com/abstract=1355869 (accessed on 9 March 2009).
| Farm | Area | Number of Sows | Vaccination Program for PCV2 and Mycoplasma hyopneumoniae (MHP) in Sows | Vaccination Program for PCV2 and MHP in Piglets | Vaccine Brand |
|---|---|---|---|---|---|
| A | Tainan | 750 | No immunity | 1. PCV2:10 days old/MHP: 10 and 24 days of age 2. PCV2: 3 weeks old/MHP: 3 weeks of age | 1. PCV2-1/MHP-2 2. Porcilis® PCV M Hyo |
| B | Yunlin | 1550 | No immunity | 1. PCV2: 3 weeks old/MHP: 3 days old 2. PCV2: 3 weeks old/MHP: 3 weeks of age | 1. PCV2-1/MHP-2 2. Porcilis® PCV M Hyo |
| C | Chiayi | 3000 | PCV2: 5 weeks before delivery | 1. PCV2 + MHP: 21–24 days old 2. PCV2: 3 weeks old/MHP: 3 weeks of age | 1. PCV2-1/MHP-1 2. Porcilis® PCV M Hyo |
| D | Changhua | 650 | PCV2 and MHP at weaning | 1. PCV2: 3 weeks old/MHP: 3 weeks old 2. PCV2: 3 weeks old/MHP: 3 weeks of age | 1. PCV2-1/MHP-3 2. Porcilis® PCV M Hyo |
| Farm | Group a | Area Under the Curve | p Value b |
|---|---|---|---|
| A | 1 | 445.58 | 0.0001 |
| 2 | 48.04 | ||
| B | 1 | 155.06 | 0.988 |
| 2 | 145.33 | ||
| C | 1 | 132.98 | 0.0001 |
| 2 | 0 | ||
| D | 1 | 45.28 | 0.054 |
| 2 | 9.72 |
| Weeks of Age | N | Group (%) a | p Value b | |
|---|---|---|---|---|
| 1 | 2 | |||
| 3 | 40 | 0 (0) | 0 (0) | - |
| 8 | 40 | 2 (5) | 2 (5) | 1 |
| 12 | 40 | 10 (25) | 3 (7.5) | 0.0338 |
| 16 | 40 | 20 (50) | 5 (12.5) | 0.0002 |
| 20 | 40 | 25 (62.5) | 8 (20) | 0.0001 |
| 24 | 40 | 22 (55) | 8 (20) | 0.0012 |
| Farm | Group (n) a | Lung Lesion Score | No. of Undetermined b | ||||
|---|---|---|---|---|---|---|---|
| 0 (%) | 1–4 (%) | 5–9 (%) | >10 (%) | Mean ± SD | |||
| B | 1 (30) | 8 (26.7) | 21 (70.0) | 1 (3.3) | 0 (0) | 1.30 ± 1.21 | 0 |
| 2 (30) | 11 (36.7) | 18 (60.0) | 1 (3.3) | 0 (0) | 1.30 ± 1.42 | 0 | |
| C | 1 (26) | 4 (15.4) | 10 (38.5) | 10 (38.5) | 2 (7.7) | 4.38 ± 3.35 | 0 |
| 2 (65) | 11 (16.9) | 52 (80.0) | 1 (1.5) | 1 (1.5) | 1.94 ± 2.22 | 0 | |
| D | 1 (11) | 2 (25.0) | 5 (62.5) | 1 (12.5) | 0 (0) | 1.88 ± 2.64 | 3 |
| 2 (11) | 5 (45.5) | 5 (45.5) | 1 (9.1) | 0 (0) | 1.00 ± 1.48 | 0 | |
| Farm | Group (n) a | Pleuritis | Pericarditis | APP |
|---|---|---|---|---|
| B | 1 (30) | 1 (3.3) | 0 (0) | 0 (0) |
| 2 (30) | 3 (10.0) | 0 (0) | 0 (0) | |
| C | 1 (26) | 9 (34.6) **b | 0 (0) | 14 (53.8) ** |
| 2 (65) | 4 (6.2) ** | 3 (4.6) | 0 (0) ** | |
| D | 1 (11) | 4 (36.4) | 0 (0) | 9 (81.8) ** |
| 2 (11) | 0 (0) | 0 (0) | 2 (18.2) ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsueh, F.-C.; Chien, C.-Y.; Chang, S.-W.; Lian, B.-R.; Lin, H.-Y.; Ellerma, L.; Chiou, M.-T.; Lin, C.-N. Field Evaluation of a Ready-to-Use Porcine Circovirus Type 2 and Mycoplasma hyopneumoniae Vaccine in Naturally Infected Farms in Taiwan. Vet. Sci. 2025, 12, 304. https://doi.org/10.3390/vetsci12040304
Hsueh F-C, Chien C-Y, Chang S-W, Lian B-R, Lin H-Y, Ellerma L, Chiou M-T, Lin C-N. Field Evaluation of a Ready-to-Use Porcine Circovirus Type 2 and Mycoplasma hyopneumoniae Vaccine in Naturally Infected Farms in Taiwan. Veterinary Sciences. 2025; 12(4):304. https://doi.org/10.3390/vetsci12040304
Chicago/Turabian StyleHsueh, Fu-Chun, Chia-Yi Chien, Shu-Wei Chang, Bo-Rong Lian, Hong-Yao Lin, Leonardo Ellerma, Ming-Tang Chiou, and Chao-Nan Lin. 2025. "Field Evaluation of a Ready-to-Use Porcine Circovirus Type 2 and Mycoplasma hyopneumoniae Vaccine in Naturally Infected Farms in Taiwan" Veterinary Sciences 12, no. 4: 304. https://doi.org/10.3390/vetsci12040304
APA StyleHsueh, F.-C., Chien, C.-Y., Chang, S.-W., Lian, B.-R., Lin, H.-Y., Ellerma, L., Chiou, M.-T., & Lin, C.-N. (2025). Field Evaluation of a Ready-to-Use Porcine Circovirus Type 2 and Mycoplasma hyopneumoniae Vaccine in Naturally Infected Farms in Taiwan. Veterinary Sciences, 12(4), 304. https://doi.org/10.3390/vetsci12040304







