Overcoming Challenges in Avian Influenza Diagnosis: The Role of Surface-Enhanced Raman Spectroscopy in Poultry Health Monitoring
Simple Summary
Abstract
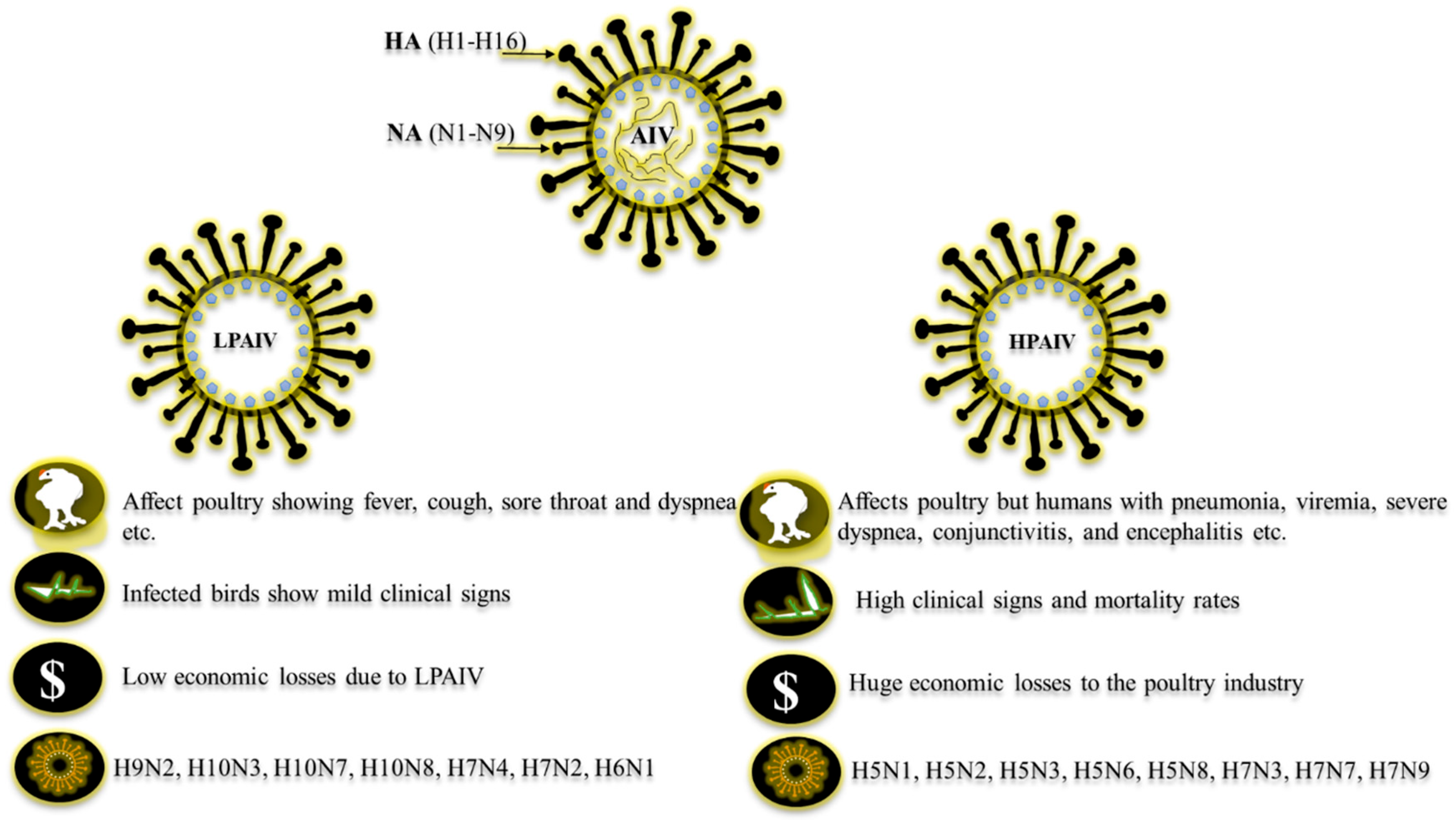
1. Introduction
2. Detection Methods
3. Public Health Importance of AIV
4. Factors Influencing the SERS-AIV Detection
4.1. Enhancement Mechanisms in SERS
4.1.1. Electromagnetic Enhancement
4.1.2. Chemical Enhancement
4.2. Sensitivity and Specificity of SERS in Detecting AIV Strains
5. SERS Applications for AIV Detection
5.1. SERS-Lateral Flow Immunoassay-Based AI Viral Detection
5.2. SERS-Antibody Probes for the Sensitive AIV Detection
5.3. SERS-Aptasensors for the Detection of Influenza Viruses
5.4. SERS-Based Immunoassay Platform for the Detection of Influenza Viruses
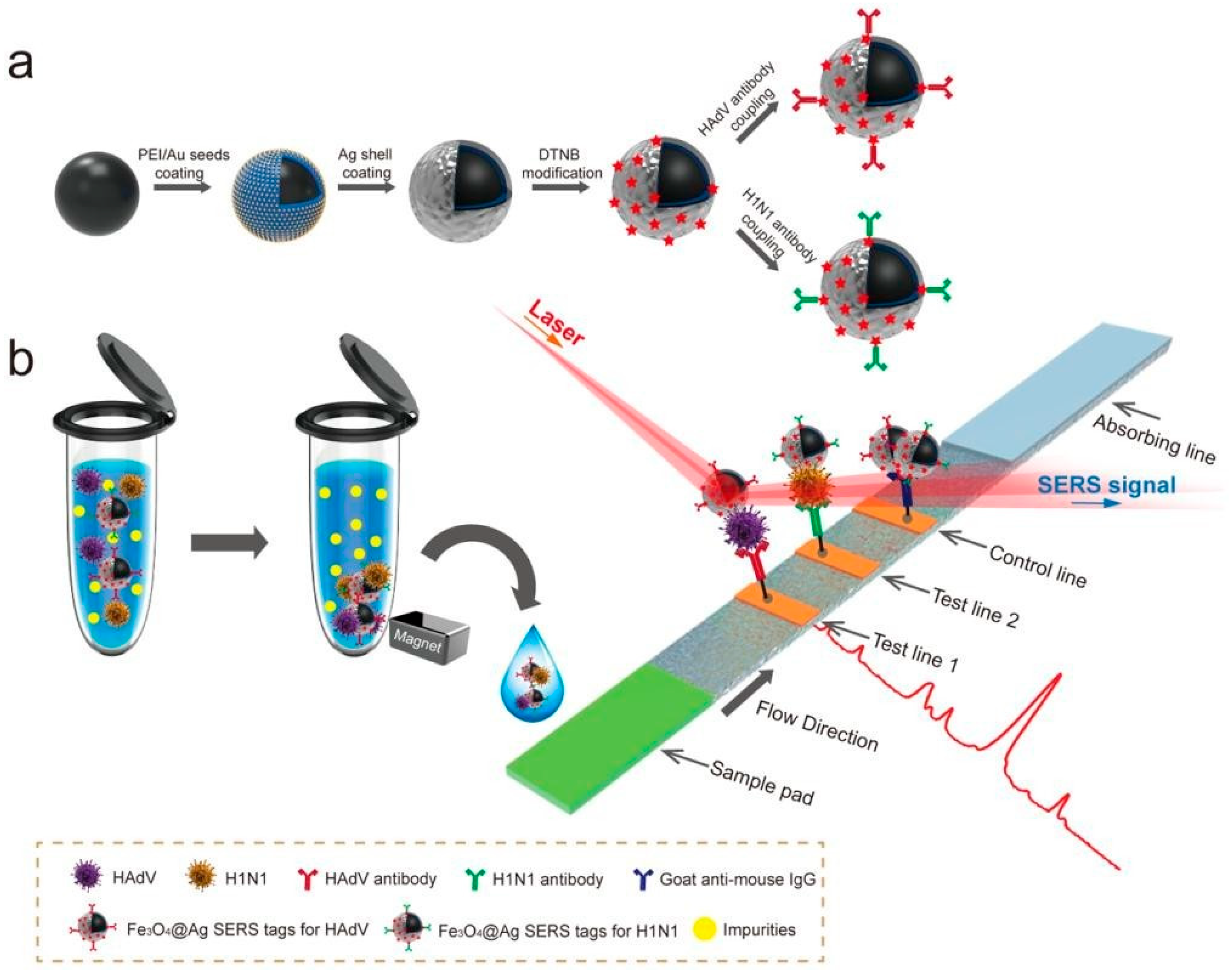
5.5. SERS-Immunomagnetic-Based AIV Detection
5.6. SERS Applications for Diverse Pathogen Detection
6. SERS-AIV Detection Challenges in Chickens and Future Prospects
7. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qadir, M.F.; Khan, A.; Saleemi, M.K.; Gul, S.T.; Khan, A.; Mujahid, Q. Epidemiological and pathological status of Mycoplasma gallisepticum in layer chicks at Faisalabad, Pakistan. Pak. J. Agri. Sci. 2021, 58, 213–218. [Google Scholar]
- Qadir, M.F.; Han, X.-Y.; Qiao, M.-L.; Wang, Y.; Zhang, D.; Bi, Y.-H.; Jahejo, A.R.; Cheng, Q.-Q.; Tian, W.-X. Expression of prostaglandins-related genes in erythrocytes of chickens infected with H9N2 subtype of avian influenza virus. Pak. J. Zool. 2021, 53, 1417–1424. [Google Scholar] [CrossRef]
- Duan, C.; Li, C.; Ren, R.; Bai, W.; Zhou, L. An overview of avian influenza surveillance strategies and modes. Sci. One Health 2023, 2, 100043. [Google Scholar]
- Liu, Z.; Wang, C.; Zheng, S.; Yang, X.; Han, H.; Dai, Y.; Xiao, R. Simultaneously ultrasensitive and quantitative detection of influenza A virus, SARS-CoV-2, and respiratory syncytial virus via multichannel magnetic SERS-based lateral flow immunoassay. Nanomedicine 2023, 47, 102624. [Google Scholar] [CrossRef]
- Scheibner, D.; Salaheldin, A.H.; Bagato, O.; Zaeck, L.M.; Mostafa, A.; Blohm, U.; Müller, C.; Eweas, A.F.; Franzke, K.; Karger, A.; et al. Phenotypic effects of mutations observed in the neuraminidase of human origin H5N1 influenza A viruses. PLoS Pathog. 2023, 19, e1011135. [Google Scholar]
- Sutton, T.C. The Pandemic Threat of Emerging H5 and H7 Avian Influenza Viruses. Viruses 2018, 10, 461. [Google Scholar] [CrossRef] [PubMed]
- Qadir, M.F.; Han, X.-Y.; Qiao, M.-L.; Cheng, Q.-Q.; Mangi, R.A.; Jahejo, A.R.; Khan, A.; Bi, Y.-H.; Tian, W.-X. Profiling of Apoptosis-Related Genes in Erythrocytes of Chickens Infected with Avian Influenza Virus (H9N2 Subtype). Pak. J. Zool. 2022, 54, 199–206. [Google Scholar]
- Charostad, J.; Rezaei Zadeh Rukerd, M.; Mahmoudvand, S.; Bashash, D.; Hashemi, S.M.A.; Nakhaie, M.; Zandi, K. A comprehensive review of highly pathogenic avian influenza (HPAI) H5N1: An imminent threat at doorstep. Travel Med. Infect. Dis. 2023, 55, 102638. [Google Scholar] [CrossRef] [PubMed]
- Perkins, L.E.; Swayne, D.E. Comparative susceptibility of selected avian and mammalian species to a Hong Kong-origin H5N1 high-pathogenicity avian influenza virus. Avian Dis. 2003, 47, 956–967. [Google Scholar] [CrossRef] [PubMed]
- Swayne, D.E.; Beck, J.R. Experimental study to determine if low-pathogenicity and high-pathogenicity avian influenza viruses can be present in chicken breast and thigh meat following intranasal virus inoculation. Avian Dis. 2005, 49, 81–85. [Google Scholar] [CrossRef]
- Swayne, D.E.; Kapczynski, D. Strategies and challenges for eliciting immunity against avian influenza virus in birds. Immunol. Rev. 2008, 225, 314–331. [Google Scholar] [CrossRef]
- Wakawa, A.; Sa’idu, L.; Kazeem, H.; Fatihu, M.; Adamu, J.; Mamman, P.; Abdu, P.; Bello, M.; Kwanahie, C.J.N.V.J. Highly pathogenic avian influenza I water fowls in Zaria, Nigeria. Niger. Vet. J. 2008, 29, 55–58. [Google Scholar] [CrossRef]
- Nakatani, H.; Nakamura, K.; Yamamoto, Y.; Yamada, M.; Yamamoto, Y. Epidemiology, pathology, and immunohistochemistry of layer hens naturally affected with H5N1 highly pathogenic avian influenza in Japan. Avian Dis. 2005, 49, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Röhm, C.; Horimoto, T.; Kawaoka, Y.; Süss, J.; Webster, R.G. Do hemagglutinin genes of highly pathogenic avian influenza viruses constitute unique phylogenetic lineages? Virology 1995, 209, 664–670. [Google Scholar] [CrossRef][Green Version]
- Blagodatski, A.; Trutneva, K.; Glazova, O.; Mityaeva, O.; Shevkova, L.; Kegeles, E.; Onyanov, N.; Fede, K.; Maznina, A.; Khavina, E.; et al. Avian Influenza in Wild Birds and Poultry: Dissemination Pathways, Monitoring Methods, and Virus Ecology. Pathogens 2021, 10, 630. [Google Scholar] [CrossRef]
- Simancas-Racines, A.; Cadena-Ullauri, S.; Guevara-Ramírez, P.; Zambrano, A.K.; Simancas-Racines, D. Avian Influenza: Strategies to Manage an Outbreak. Pathogens 2023, 12, 610. [Google Scholar] [CrossRef]
- Crawford, P.C.; Dubovi, E.J.; Castleman, W.L.; Stephenson, I.; Gibbs, E.P.; Chen, L.; Smith, C.; Hill, R.C.; Ferro, P.; Pompey, J.; et al. Transmission of equine influenza virus to dogs. Science 2005, 310, 482–485. [Google Scholar] [CrossRef]
- Chatziprodromidou, I.P.; Arvanitidou, M.; Guitian, J.; Apostolou, T.; Vantarakis, G.; Vantarakis, A. Global avian influenza outbreaks 2010–2016: A systematic review of their distribution, avian species and virus subtype. Syst. Rev. 2018, 7, 17. [Google Scholar] [CrossRef]
- Horimoto, T.; Kawaoka, Y. Pandemic threat posed by avian influenza A viruses. Clin. Microbiol. Rev. 2001, 14, 129–149. [Google Scholar] [CrossRef] [PubMed]
- Herfst, S.; Mok, C.K.P.; van den Brand, J.M.A.; van der Vliet, S.; Rosu, M.E.; Spronken, M.I.; Yang, Z.; de Meulder, D.; Lexmond, P.; Bestebroer, T.M.; et al. Human Clade 2.3.4.4 A/H5N6 Influenza Virus Lacks Mammalian Adaptation Markers and Does Not Transmit via the Airborne Route between Ferrets. mSphere 2018, 3, e00405–e00417. [Google Scholar] [CrossRef]
- Amonsin, A.; Payungporn, S.; Theamboonlers, A.; Thanawongnuwech, R.; Suradhat, S.; Pariyothorn, N.; Tantilertcharoen, R.; Damrongwantanapokin, S.; Buranathai, C.; Chaisingh, A.; et al. Genetic characterization of H5N1 influenza A viruses isolated from zoo tigers in Thailand. Virology 2006, 344, 480–491. [Google Scholar] [CrossRef]
- Cáceres, C.J.; Rajao, D.S.; Perez, D.R. Airborne Transmission of Avian Origin H9N2 Influenza A Viruses in Mammals. Viruses 2021, 13, 1919. [Google Scholar] [CrossRef]
- Cao, X.; Yang, F.; Wu, H.; Xu, L. Genetic characterization of novel reassortant H5N6-subtype influenza viruses isolated from cats in eastern China. Arch. Virol. 2017, 162, 3501–3505. [Google Scholar] [CrossRef] [PubMed]
- Gambaryan, A.S.; Matrosovich, M.N. What adaptive changes in hemagglutinin and neuraminidase are necessary for emergence of pandemic influenza virus from its avian precursor? Biochemistry 2015, 80, 872–880. [Google Scholar] [CrossRef]
- Keawcharoen, J.; Oraveerakul, K.; Kuiken, T.; Fouchier, R.A.; Amonsin, A.; Payungporn, S.; Noppornpanth, S.; Wattanodorn, S.; Theambooniers, A.; Tantilertcharoen, R.; et al. Avian influenza H5N1 in tigers and leopards. Emerg. Infect. Dis. 2004, 10, 2189–2191. [Google Scholar] [CrossRef]
- Kuiken, T.; Holmes, E.C.; McCauley, J.; Rimmelzwaan, G.F.; Williams, C.S.; Grenfell, B.T. Host species barriers to influenza virus infections. Science 2006, 312, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.I.; Kim, E.H.; Kim, Y.I.; Park, S.J.; Si, Y.J.; Lee, I.W.; Nguyen, H.D.; Yu, K.M.; Yu, M.A.; Jung, J.H.; et al. Comparison of the pathogenic potential of highly pathogenic avian influenza (HPAI) H5N6, and H5N8 viruses isolated in South Korea during the 2016–2017 winter season. Emerg. Microbes Infect. 2018, 7, 29. [Google Scholar] [CrossRef]
- Li, X.; Fu, Y.; Yang, J.; Guo, J.; He, J.; Guo, J.; Weng, S.; Jia, Y.; Liu, B.; Li, X.; et al. Genetic and biological characterization of two novel reassortant H5N6 swine influenza viruses in mice and chickens. Infect. Genet. Evol. 2015, 36, 462–466. [Google Scholar] [CrossRef]
- Marchenko, V.; Goncharova, N.; Susloparov, I.; Kolosova, N.; Gudymo, A.; Svyatchenko, S.; Danilenko, A.; Durymanov, A.; Gavrilova, E.; Maksyutov, R.; et al. Isolation and characterization of H5Nx highly pathogenic avian influenza viruses of clade 2.3.4.4 in Russia. Virology 2018, 525, 216–223. [Google Scholar] [CrossRef]
- Yoon, K.J.; Cooper, V.L.; Schwartz, K.J.; Harmon, K.M.; Kim, W.I.; Janke, B.H.; Strohbehn, J.; Butts, D.; Troutman, J. Influenza virus infection in racing greyhounds. Emerg. Infect. Dis. 2005, 11, 1974–1976. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Gao, X.; Wang, T.; Li, Y.; Li, Y.; Xu, Y.; Chu, D.; Sun, H.; Wu, C.; Li, S.; et al. Fatal H5N6 Avian Influenza Virus Infection in a Domestic Cat and Wild Birds in China. Sci. Rep. 2015, 5, 10704. [Google Scholar] [CrossRef] [PubMed]
- Quirk, M. Zoo tigers succumb to avian influenza. Lancet Infect. Dis. 2004, 4, 716. [Google Scholar] [CrossRef]
- Guy, S.; Hocking, B.A. Times of Pestilence: Would a Bill of Rights Assist Australian Citizens Who Are Quarantined in the Event of an Avian Influenza (Bird Flu) Pandemic? Curr. Issues Crim. Justice 2006, 17, 451–467. [Google Scholar] [CrossRef]
- Nabi, G.; Wang, Y.; Lü, L.; Jiang, C.; Ahmad, S.; Wu, Y.; Li, D. Bats and birds as viral reservoirs: A physiological and ecological perspective. Sci. Total Environ. 2021, 754, 142372. [Google Scholar]
- Yamaji, R.; Saad, M.D.; Davis, C.T.; Swayne, D.E.; Wang, D.; Wong, F.Y.K.; McCauley, J.W.; Peiris, J.S.M.; Webby, R.J.; Fouchier, R.A.M.; et al. Pandemic potential of highly pathogenic avian influenza clade 2.3.4.4 A(H5) viruses. Rev. Med. Virol. 2020, 30, e2099. [Google Scholar] [PubMed]
- Zhang, H.; Li, H.; Wang, W.; Wang, Y.; Han, G.Z.; Chen, H.; Wang, X. A unique feature of swine ANP32A provides susceptibility to avian influenza virus infection in pigs. PLoS Pathog. 2020, 16, e1008330. [Google Scholar]
- Hong, S.C.; Murale, D.P.; Jang, S.Y.; Haque, M.M.; Seo, M.; Lee, S.; Woo, D.H.; Kwon, J.; Song, C.S.; Kim, Y.K.; et al. Discrimination of Avian Influenza Virus Subtypes Using Host-Cell Infection Fingerprinting by a Sulfinate-based Fluorescence Superoxide Probe. Angew. Chem. Int. Ed. Engl. 2018, 57, 9716–9721. [Google Scholar] [CrossRef]
- Vemula, S.V.; Zhao, J.; Liu, J.; Wang, X.; Biswas, S.; Hewlett, I. Current Approaches for Diagnosis of Influenza Virus Infections in Humans. Viruses 2016, 8, 96. [Google Scholar] [CrossRef]
- Augustine, R.; Hasan, A.; Das, S.; Ahmed, R.; Mori, Y.; Notomi, T.; Kevadiya, B.D.; Thakor, A.S. Loop-Mediated Isothermal Amplification (LAMP): A Rapid, Sensitive, Specific, and Cost-Effective Point-of-Care Test for Coronaviruses in the Context of COVID-19 Pandemic. Biology 2020, 9, 182. [Google Scholar] [CrossRef]
- Vasilyeva, A.D.; Yurina, L.V.; Evtushenko, E.G.; Gavrilina, E.S.; Krylov, V.B.; Nifantiev, N.E.; Kurochkin, I.N. Increasing the Sensitivity of Aspergillus Galactomannan ELISA Using Silver Nanoparticle-Based Surface-Enhanced Raman Spectroscopy. Sensors 2025, 25, 4376. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, Y.; Fu, Q.; Lin, M.; He, J.; He, S.; Yang, M.; Chen, S.; Zhou, J. Reciprocating-flowing on-a-chip enables ultra-fast immunobinding for multiplexed rapid ELISA detection of SARS-CoV-2 antibody. Biosens. Bioelectron. 2021, 176, 112920. [Google Scholar] [CrossRef]
- Wang, R.; Li, Y. Hydrogel based QCM aptasensor for detection of avian influenza virus. Biosens. Bioelectron. 2013, 42, 148–155. [Google Scholar] [CrossRef]
- Yang, T.; Guo, X.; Wang, H.; Fu, S.; Wen, Y.; Yang, H. Magnetically optimized SERS assay for rapid detection of trace drug-related biomarkers in saliva and fingerprints. Biosens. Bioelectron. 2015, 68, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Wang, R.; Hargis, B.; Lu, H.; Li, Y. A SPR aptasensor for detection of avian influenza virus H5N1. Sensors 2012, 12, 12506–12518. [Google Scholar] [CrossRef]
- Li, Y.; Hong, M.; Qiu, B.; Lin, Z.; Chen, Y.; Cai, Z.; Chen, G. Highly sensitive fluorescent immunosensor for detection of influenza virus based on Ag autocatalysis. Biosens. Bioelectron. 2014, 54, 358–364. [Google Scholar] [CrossRef]
- Lee, N.; Wang, C.; Park, J. User-friendly point-of-care detection of influenza A (H1N1) virus using light guide in three-dimensional photonic crystal. RSC Adv. 2018, 8, 22991–22997. [Google Scholar] [CrossRef]
- Lei, K.F.; Huang, C.H.; Kuo, R.L.; Chang, C.K.; Chen, K.F.; Tsao, K.C.; Tsang, N.M. Paper-based enzyme-free immunoassay for rapid detection and subtyping of influenza A H1N1 and H3N2 viruses. Anal. Chim. Acta 2015, 883, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Duenas, L.; Gomez, F.A. Thread- and Capillary Tube-Based Electrodes for the Detection of Glucose and Acetylthiocholine. Micromachines 2020, 11, 920. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xu, L.; Zhang, F.; Song, Z.; Hu, Y.; Ji, Y.; Shen, J.; Li, B.; Lu, H.; Yang, H. A promising magnetic SERS immunosensor for sensitive detection of avian influenza virus. Biosens. Bioelectron. 2017, 89, 906–912. [Google Scholar] [CrossRef]
- Radziuk, D.; Moehwald, H. Prospects for plasmonic hot spots in single molecule SERS towards the chemical imaging of live cells. Phys. Chem. Chem. Phys. 2015, 17, 21072–21093. [Google Scholar] [CrossRef]
- Cao, X.; Hong, S.; Jiang, Z.; She, Y.; Wang, S.; Zhang, C.; Li, H.; Jin, F.; Jin, M.; Wang, J. SERS-active metal-organic frameworks with embedded gold nanoparticles. Analyst 2017, 142, 2640–2647. [Google Scholar] [CrossRef]
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A Review on Surface-Enhanced Raman Scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, S.; Zheng, J.; He, L. Surface-enhanced Raman spectroscopy (SERS) combined techniques for high-performance detection and characterization. Trends Anal. Chem. 2017, 90, 1–13. [Google Scholar] [CrossRef]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Ding, S.Y.; You, E.M.; Tian, Z.Q.; Moskovits, M. Electromagnetic theories of surface-enhanced Raman spectroscopy. Chem. Soc. Rev. 2017, 46, 4042–4076. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Esteban, R.; Borisov, A.G.; Baumberg, J.J.; Nordlander, P.; Lezec, H.J.; Aizpurua, J.; Crozier, K.B. Quantum mechanical effects in plasmonic structures with subnanometre gaps. Nat. Commun. 2016, 7, 11495. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, I.; Lombardi, J.R. Enhanced Raman Scattering with Dielectrics. Chem. Rev. 2016, 116, 14921–14981. [Google Scholar] [CrossRef]
- Zrimsek, A.B.; Wong, N.L.; Van Duyne, R.P. Single Molecule Surface-Enhanced Raman Spectroscopy: A Critical Analysis of the Bianalyte versus Isotopologue Proof. J. Phys. Chem. C 2016, 120, 5133–5142. [Google Scholar] [CrossRef]
- Pilot, R. SERS detection of food contaminants by means of portable Raman instruments. J. Raman Spectrosc. 2018, 49, 954–981. [Google Scholar] [CrossRef]
- Cialla-May, D.; Zheng, X.S.; Weber, K.; Popp, J. Recent progress in surface-enhanced Raman spectroscopy for biological and biomedical applications: From cells to clinics. Chem. Soc. Rev. 2017, 46, 3945–3961. [Google Scholar] [CrossRef]
- Xie, W.; Schlücker, S. Surface-enhanced Raman spectroscopic detection of molecular chemo- and plasmo-catalysis on noble metal nanoparticles. Chem. Commun. 2018, 54, 2326–2336. [Google Scholar] [CrossRef]
- Fikiet, M.A.; Khandasammy, S.R.; Mistek, E.; Ahmed, Y.; Halámková, L.; Bueno, J.; Lednev, I.K. Surface enhanced Raman spectroscopy: A review of recent applications in forensic science. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 197, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.-C.; Zhang, X.-G.; Briega-Martos, V.; Jin, X.; Yang, J.; Chen, S.; Yang, Z.-L.; Wu, D.-Y.; Feliu, J.M.; Williams, C.T.; et al. In situ Raman spectroscopic evidence for oxygen reduction reaction intermediates at platinum single-crystal surfaces. Nat. Energy 2019, 4, 60–67. [Google Scholar] [CrossRef]
- Song, C.; Yang, Y.; Yang, B.; Min, L.; Wang, L. Combination assay of lung cancer associated serum markers using surface-enhanced Raman spectroscopy. J. Mater. Chem. B 2016, 4, 1811–1817. [Google Scholar] [CrossRef]
- Zhan, L.; Zhen, S.J.; Wan, X.Y.; Gao, P.F.; Huang, C.Z. A sensitive surface-enhanced Raman scattering enzyme-catalyzed immunoassay of respiratory syncytial virus. Talanta 2016, 148, 308–312. [Google Scholar] [CrossRef]
- Karn-orachai, K.; Sakamoto, K.; Laocharoensuk, R.; Bamrungsap, S.; Songsivilai, S.; Dharakul, T.; Miki, K. Extrinsic surface-enhanced Raman scattering detection of influenza A virus enhanced by two-dimensional gold@silver core–shell nanoparticle arrays. RSC Adv. 2016, 6, 97791–97799. [Google Scholar] [CrossRef]
- Fu, X.; Wang, Q.; Ma, B.; Zhang, B.; Sun, K.; Yu, X.; Ye, Z.; Zhang, M. Advances in Detection Techniques for the H5N1 Avian Influenza Virus. Int. J. Mol. Sci. 2023, 24, 17157. [Google Scholar] [CrossRef]
- Burnet, F.M.; Bull, D.H. Changes in influenza virus associated with adaptation to passage in chick embryos. Aust. J. Exp. Biol. Med. 1943, 21, 55–69. [Google Scholar] [CrossRef]
- Yang, L.; Cheng, Y.; Zhao, X.; Wei, H.; Tan, M.; Li, X.; Zhu, W.; Huang, W.; Chen, W.; Liu, J.; et al. Mutations associated with egg adaptation of influenza A(H1N1)pdm09 virus in laboratory based surveillance in China, 2009–2016. Biosaf. Health 2019, 1, 41–45. [Google Scholar] [CrossRef]
- Hoffmann, E.; Stech, J.; Guan, Y.; Webster, R.G.; Perez, D.R. Universal primer set for the full-length amplification of all influenza A viruses. Arch. Virol. 2001, 146, 2275–2289. [Google Scholar] [CrossRef]
- Kode, S.S.; Pawar, S.D.; Tare, D.S.; Mullick, J. Application of frozen and stored glutaraldehyde-fixed turkey red blood cells for hemagglutination and hemagglutination inhibition assays for the detection and identification of influenza viruses. J. Virol. Methods 2021, 289, 114046. [Google Scholar] [CrossRef]
- Pawar, S.D.; Parkhi, S.S.; Koratkar, S.S.; Mishra, A.C. Receptor specificity and erythrocyte binding preferences of avian influenza viruses isolated from India. Virol. J. 2012, 9, 251. [Google Scholar] [CrossRef] [PubMed]
- Comin, A.; Toft, N.; Stegeman, A.; Klinkenberg, D.; Marangon, S. Serological diagnosis of avian influenza in poultry: Is the haemagglutination inhibition test really the ‘gold standard’? Influenza Other Respir. Viruses 2013, 7, 257–264. [Google Scholar] [CrossRef]
- Rimmelzwaan, G.F.; Verburgh, R.J.; Nieuwkoop, N.J.; Bestebroer, T.M.; Fouchier, R.A.; Osterhaus, A.D. Use of GFP-expressing influenza viruses for the detection of influenza virus A/H5N1 neutralizing antibodies. Vaccine 2011, 29, 3424–3430. [Google Scholar] [CrossRef]
- Rowe, T.; Abernathy, R.A.; Hu-Primmer, J.; Thompson, W.W.; Lu, X.; Lim, W.; Fukuda, K.; Cox, N.J.; Katz, J.M. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J. Clin. Microbiol. 1999, 37, 937–943. [Google Scholar] [CrossRef]
- Stelzer-Braid, S.; Wong, B.; Robertson, P.; Lynch, G.W.; Laurie, K.; Shaw, R.; Barr, I.; Selleck, P.W.; Baleriola, C.; Escott, R.; et al. A commercial ELISA detects high levels of human H5 antibody but cross-reacts with influenza A antibodies. J. Clin. Virol. 2008, 43, 241–243. [Google Scholar] [CrossRef]
- Adair, B.M.; Todd, D.; McKillop, E.R.; McNulty, M.S. Detection of influenza a type-specific antibodies in chicken and turkey sera by enzyme linked immunosorbent assay. Avian Pathol. 1989, 18, 455–463. [Google Scholar] [CrossRef]
- Fatunmbi, O.O.; Newman, J.A.; Sivanandan, V.; Halvorson, D.A. A broad-spectrum avian influenza subtype antigen for indirect enzyme-linked immunosorbent assay. Avian Dis. 1989, 33, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Snyder, D.B.; Marquardt, W.W.; Mallinson, E.T.; Allen, D.A.; Savage, P.K. An enzyme-linked immunosorbent assay method for the simultaneous measurement of antibody titer to multiple viral, bacterial or protein antigens. Vet. Immunol. Immunopathol. 1985, 9, 303–317. [Google Scholar] [CrossRef]
- Shafer, A.L.; Katz, J.B.; Eernisse, K.A. Development and validation of a competitive enzyme-linked immunosorbent assay for detection of type A influenza antibodies in avian sera. Avian Dis. 1998, 42, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Jin, M.; Liu, F.; Guo, X.; Hu, Q.; Han, L.; Tan, Y.; Chen, H. Development and evaluation of a DAS-ELISA for rapid detection of avian influenza viruses. Avian Dis. 2006, 50, 325–330. [Google Scholar] [CrossRef]
- Dong, J.; Sakurai, A.; Nomura, N.; Park, E.Y.; Shibasaki, F.; Ueda, H. Isolation of Recombinant Phage Antibodies Targeting the Hemagglutinin Cleavage Site of Highly Pathogenic Avian Influenza Virus. PLoS ONE 2013, 8, e61158. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.S.; Fleming, D.M.; Zambon, M.C. Multiplex reverse transcription-PCR for surveillance of influenza A and B viruses in England and Wales in 1995 and 1996. J. Clin. Microbiol. 1997, 35, 2076–2082. [Google Scholar] [CrossRef]
- He, F.; Soejoedono, R.D.; Murtini, S.; Goutama, M.; Kwang, J. Complementary monoclonal antibody-based dot ELISA for universal detection of H5 avian influenza virus. BMC Microbiol. 2010, 10, 330. [Google Scholar] [CrossRef]
- Ho, H.T.; Qian, H.L.; He, F.; Meng, T.; Szyporta, M.; Prabhu, N.; Prabakaran, M.; Chan, K.P.; Kwang, J. Rapid detection of H5N1 subtype influenza viruses by antigen capture enzyme-linked immunosorbent assay using H5- and N1-specific monoclonal antibodies. Clin. Vaccine Immunol. 2009, 16, 726–732. [Google Scholar]
- Sajid, M.; Kawde, A.-N.; Daud, M. Designs, formats and applications of lateral flow assay: A literature review. J. Saudi Chem. Soc. 2015, 19, 689–705. [Google Scholar] [CrossRef]
- Durairaj, K.; Than, D.D.; Nguyen, A.T.V.; Kim, H.S.; Yeo, S.J.; Park, H. Cysteamine-Gold Coated Carboxylated Fluorescent Nanoparticle Mediated Point-of-Care Dual-Modality Detection of the H5N1 Pathogenic Virus. Int. J. Mol. Sci. 2022, 23, 7957. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wu, D.; Wei, J.; Xiao, G. A new method for the detection of the H5 influenza virus by magnetic beads capturing quantum dot fluorescent signals. Biotechnol. Lett. 2010, 32, 1933–1937. [Google Scholar] [CrossRef]
- Yeo, S.J.; Kang, H.; Dao, T.D.; Cuc, B.T.; Nguyen, A.T.V.; Tien, T.T.T.; Hang, N.L.K.; Phuong, H.V.M.; Thanh, L.T.; Mai, L.Q.; et al. Development of a smartphone-based rapid dual fluorescent diagnostic system for the simultaneous detection of influenza A and H5 subtype in avian influenza A-infected patients. Theranostics 2018, 8, 6132–6148. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, W.P.; Zhang, Z.L.; He, R.L.; Lin, Y.; Xie, M.; Wang, H.Z.; Pang, D.W. Robust and highly sensitive fluorescence approach for point-of-care virus detection based on immunomagnetic separation. Anal. Chem. 2012, 84, 2358–2365. [Google Scholar] [CrossRef]
- Chen, X.; Wen, H.; He, F.; Li, J.; Chen, C.; Zhang, J.; Jin, G.; Shi, B. Partial Sequence Cloning of LHR Gene in Cynoglossus semilaevis and its tissue expression analysis. Period. Ocean Univ. China 2010, 40, 71–77. [Google Scholar]
- Doak, S.H.; Zair, Z.M. Real-time reverse-transcription polymerase chain reaction: Technical considerations for gene expression analysis. Methods Mol. Biol. 2012, 817, 251–270. [Google Scholar]
- Chen, W.; He, B.; Li, C.; Zhang, X.; Wu, W.; Yin, X.; Fan, B.; Fan, X.; Wang, J. Real-time RT-PCR for H5N1 avian influenza A virus detection. J. Med. Microbiol. 2007, 56, 603–607. [Google Scholar] [PubMed]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, E63. [Google Scholar] [CrossRef] [PubMed]
- Dinh, D.T.; Le, M.T.; Vuong, C.D.; Hasebe, F.; Morita, K. An Updated Loop-Mediated Isothermal Amplification Method for Rapid Diagnosis of H5N1 Avian Influenza Viruses. Trop. Med. Health 2011, 39, 3–7. [Google Scholar] [CrossRef]
- Imai, M.; Ninomiya, A.; Minekawa, H.; Notomi, T.; Ishizaki, T.; Tashiro, M.; Odagiri, T. Development of H5-RT-LAMP (loop-mediated isothermal amplification) system for rapid diagnosis of H5 avian influenza virus infection. Vaccine 2006, 24, 6679–6682. [Google Scholar] [CrossRef]
- Jayawardena, S.; Cheung, C.Y.; Barr, I.; Chan, K.H.; Chen, H.; Guan, Y.; Peiris, J.S.; Poon, L.L. Loop-mediated isothermal amplification for influenza A (H5N1) virus. Emerg. Infect. Dis. 2007, 13, 899–901. [Google Scholar] [CrossRef]
- Jung, J.H.; Oh, S.J.; Kim, Y.T.; Kim, S.Y.; Kim, W.J.; Jung, J.; Seo, T.S. Combination of multiplex reverse-transcription loop-mediated isothermal amplification with an immunochromatographic strip for subtyping influenza A virus. Anal. Chim. Acta 2015, 853, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Yu, X.; Chen, H.; Diao, Y. An immunoassay-based reverse-transcription loop-mediated isothermal amplification assay for the rapid detection of avian influenza H5N1 virus viremia. Biosens. Bioelectron. 2016, 86, 255–261. [Google Scholar] [CrossRef]
- Kievits, T.; van Gemen, B.; van Strijp, D.; Schukkink, R.; Dircks, M.; Adriaanse, H.; Malek, L.; Sooknanan, R.; Lens, P. NASBA isothermal enzymatic in vitro nucleic acid amplification optimized for the diagnosis of HIV-1 infection. J. Virol. Methods 1991, 35, 273–286. [Google Scholar] [CrossRef]
- Malek, L.; Sooknanan, R.; Compton, J. Nucleic acid sequence-based amplification (NASBA). Methods Mol. Biol. 1994, 28, 253–260. [Google Scholar]
- Shan, S.; Ko, L.S.; Collins, R.A.; Wu, Z.; Chen, J.; Chan, K.Y.; Xing, J.; Lau, L.T.; Yu, A.C. Comparison of nucleic acid-based detection of avian influenza H5N1 with virus isolation. Biochem. Biophys. Res. Commun. 2003, 302, 377–383. [Google Scholar] [CrossRef]
- Chantratita, W.; Sukasem, C.; Kaewpongsri, S.; Srichunrusami, C.; Pairoj, W.; Thitithanyanont, A.; Chaichoune, K.; Ratanakron, P.; Songserm, T.; Damrongwatanapokin, S.; et al. Qualitative detection of avian influenza A (H5N1) viruses: A comparative evaluation of four real-time nucleic acid amplification methods. Mol. Cell. Probes 2008, 22, 287–293. [Google Scholar]
- Collins, R.A.; Ko, L.S.; So, K.L.; Ellis, T.; Lau, L.T.; Yu, A.C. Detection of highly pathogenic and low pathogenic avian influenza subtype H5 (Eurasian lineage) using NASBA. J. Virol. Methods 2002, 103, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.; Telles, J.N.; Corden, S.; Gao, R.B.; Vernet, G.; Van Aarle, P.; Shu, Y.L. Development and validation of a commercial real-time NASBA assay for the rapid confirmation of influenza A H5N1 virus in clinical samples. J. Virol. Methods 2010, 170, 173–176. [Google Scholar]
- Song-hua, S.; Le-ting, L.; Jia-hua, C.; Zhong-liang, W. Detection of Avian Influenza Virus Subtype H5 Using NASBA. Virol. Sin. 2005, 20, 288–292. [Google Scholar]
- Deiman, B.; van Aarle, P.; Sillekens, P. Characteristics and applications of nucleic acid sequence-based amplification (NASBA). Mol. Biotechnol. 2002, 20, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Dawson, E.D.; Moore, C.L.; Dankbar, D.M.; Mehlmann, M.; Townsend, M.B.; Smagala, J.A.; Smith, C.B.; Cox, N.J.; Kuchta, R.D.; Rowlen, K.L. Identification of A/H5N1 influenza viruses using a single gene diagnostic microarray. Anal. Chem. 2007, 79, 378–384. [Google Scholar] [CrossRef]
- Kessler, N.; Ferraris, O.; Palmer, K.; Marsh, W.; Steel, A. Use of the DNA flow-thru chip, a three-dimensional biochip, for typing and subtyping of influenza viruses. J. Clin. Microbiol. 2004, 42, 2173–2185. [Google Scholar] [CrossRef]
- Kwon, N.Y.; Ahn, J.J.; Kim, J.-H.; Kim, S.Y.; Lee, J.H.; Kwon, J.-H.; Song, C.-S.; Hwang, S.Y. Rapid Subtyping and Pathotyping of Avian Influenza Virus using Chip-based RT-PCR. BioChip J. 2019, 13, 333–340. [Google Scholar] [CrossRef]
- Lee, D.-H.; Kim, J.-H.; Lee, Y.-N.; Park, J.-K.; Yuk, S.-S.; Jung, J.-W.; Hwang, S.Y.; Lee, Y.-J.; Kang, H.-M.; Choi, J.-G.; et al. Simultaneous subtyping and pathotyping of the 2010–2011 South Korean HPAI outbreak strain by using a diagnostic microarray. BioChip J. 2011, 5, 369–374. [Google Scholar] [CrossRef]
- Shi, L.; Sun, J.S.; Yang, Z.P.; Bao, H.M.; Jiang, Y.P.; Xiong, Y.Z.; Cao, D.; Yu, X.W.; Chen, H.L.; Zheng, S.M.; et al. Development of a DNA microarray-based multiplex assay of avian influenza virus subtypes H5, H7, H9, N1, and N2. Acta Virol. 2014, 58, 14–19. [Google Scholar] [CrossRef]
- Huang, X.; Shi, Y.; Fu, Y.; Jiang, H.; Huang, Z.; Yin, G. Research progress in the application of gene chip technology in animal disease detection. Guizhou J. Anim. Husb. Vet. 2020, 44, 49–51. [Google Scholar]
- Sanger, F.; Air, G.M.; Barrell, B.G.; Brown, N.L.; Coulson, A.R.; Fiddes, C.A.; Hutchison, C.A.; Slocombe, P.M.; Smith, M. Nucleotide sequence of bacteriophage phi X174 DNA. Nature 1977, 265, 687–695. [Google Scholar] [CrossRef]
- Deyde, V.M.; Gubareva, L.V. Influenza genome analysis using pyrosequencing method: Current applications for a moving target. Expert. Rev. Mol. Diagn. 2009, 9, 493–509. [Google Scholar] [CrossRef] [PubMed]
- Chiu, N.-F. The Current Status and Future Promise of SPR Biosensors. Biosensors 2022, 12, 933. [Google Scholar] [CrossRef]
- Nylander, C.; Liedberg, B.; Lind, T. Gas detection by means of surface plasmon resonance. Sen. Actuators 1982, 3, 79–88. [Google Scholar] [CrossRef]
- Wong, C.L.; Chua, M.; Mittman, H.; Choo, L.X.; Lim, H.Q.; Olivo, M. A Phase-Intensity Surface Plasmon Resonance Biosensor for Avian Influenza A (H5N1) Detection. Sensors 2017, 17, 2363. [Google Scholar] [CrossRef]
- Gao, A.; Lu, N.; Dai, P.; Li, T.; Pei, H.; Gao, X.; Gong, Y.; Wang, Y.; Fan, C. Silicon-nanowire-based CMOS-compatible field-effect transistor nanosensors for ultrasensitive electrical detection of nucleic acids. Nano Lett. 2011, 11, 3974–3978. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Zhuo, M.; Zhang, X.; Xu, C.; Jiang, J.; Gao, F.; Wan, Q.; Li, Q.; Wang, T. Indium-tin-oxide thin film transistor biosensors for label-free detection of avian influenza virus H5N1. Anal. Chim. Acta 2013, 773, 83–88. [Google Scholar] [CrossRef]
- Kwon, J.; Lee, Y.; Lee, T.; Ahn, J.H. Aptamer-Based Field-Effect Transistor for Detection of Avian Influenza Virus in Chicken Serum. Anal. Chem. 2020, 92, 5524–5531. [Google Scholar] [CrossRef]
- Jarocka, U.; Sawicka, R.; Góra-Sochacka, A.; Sirko, A.; Zagórski-Ostoja, W.; Radecki, J.; Radecka, H. Electrochemical immunosensor for detection of antibodies against influenza A virus H5N1 in hen serum. Biosens. Bioelectron. 2014, 55, 301–306. [Google Scholar] [CrossRef]
- Kukol, A.; Li, P.; Estrela, P.; Ko-Ferrigno, P.; Migliorato, P. Label-free electrical detection of DNA hybridization for the example of influenza virus gene sequences. Anal. Biochem. 2008, 374, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cheng, Z.; Fan, H.; Ai, S.; Han, R. Electrochemical detection of avian influenza virus H5N1 gene sequence using a DNA aptamer immobilized onto a hybrid nanomaterial-modified electrode. Electrochim. Acta 2011, 56, 6266–6270. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Chen, Y.; Song, L.J.; Su, Z.Q.; Zhang, H.Y. Advances in the application of field effect transistor biosensor in biomedical detection. China Biotechnol. 2021, 41, 73–88. [Google Scholar] [CrossRef]
- Shi, L.; Chu, Z.; Dong, X.; Jin, W.; Dempsey, E. A highly oriented hybrid microarray modified electrode fabricated by a template-free method for ultrasensitive electrochemical DNA recognition. Nanoscale 2013, 5, 10219–10225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiao, K.; Liu, C. Electrochemical Biosensor. J. Qingdao Inst. Chem. Technol. 1992, 2, 99–105. [Google Scholar]
- Fu, Y.; Romay, V.; Liu, Y.; Ibarlucea, B.; Baraban, L.; Khavrus, V.; Oswald, S.; Bachmatiuk, A.; Ibrahim, I.; Rümmeli, M.; et al. Chemiresistive biosensors based on carbon nanotubes for label-free detection of DNA sequences derived from avian influenza virus H5N1. Sens. Actuators B Chem. 2017, 249, 691–699. [Google Scholar] [CrossRef]
- Grabowska, I.; Malecka, K.; Stachyra, A.; Gora-Sochacka, A.; Sirko, A.; Zagorski-Ostoja, W.; Radecka, H.; Radecki, J. Single electrode genosensor for simultaneous determination of sequences encoding hemagglutinin and neuraminidase of avian influenza virus type H5N1. Anal. Chem. 2013, 85, 10167–10173. [Google Scholar] [CrossRef]
- Grabowska, I.; Stachyra, A.; Gora-Sochacka, A.; Sirko, A.; Olejniczak, A.B.; Lesnikowski, Z.J.; Radecki, J.; Radecka, H. DNA probe modified with 3-iron bis(dicarbollide) for electrochemical determination of DNA sequence of Avian Influenza Virus H5N1. Biosens. Bioelectron. 2014, 51, 170–176. [Google Scholar] [CrossRef]
- Malecka, K.; Stachyra, A.; Góra-Sochacka, A.; Sirko, A.; Zagórski-Ostoja, W.; Dehaen, W.; Radecka, H.; Radecki, J. New redox-active layer create via epoxy-amine reaction—The base of genosensor for the detection of specific DNA and RNA sequences of avian influenza virus H5N1. Biosens. Bioelectron. 2015, 65, 427–434. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Malecka, K.; Stachyra, A.; Góra-Sochacka, A.; Sirko, A.; Zagórski-Ostoja, W.; Radecka, H.; Radecki, J. Electrochemical genosensor based on disc and screen printed gold electrodes for detection of specific DNA and RNA sequences derived from Avian Influenza Virus H5N1. Sens. Actuators B Chem. 2016, 224, 290–297. [Google Scholar] [CrossRef]
- Lin, J.; Lum, J.; Wang, R.; Tung, S.; Hargis, B.; Li, Y.B.; Lu, H.G.; Berghman, L. A portable impedance biosensor instrument for rapid detection of avian influenza virus. In Proceedings of the SENSORS, 2010 IEEE, Waikoloa, HI, USA, 1–4 November 2010; pp. 1558–1563. [Google Scholar]
- Lin, J.; Wang, R.; Jiao, P.; Li, Y.; Li, Y.; Liao, M.; Yu, Y.; Wang, M. An impedance immunosensor based on low-cost microelectrodes and specific monoclonal antibodies for rapid detection of avian influenza virus H5N1 in chicken swabs. Biosens. Bioelectron. 2015, 67, 546–552. [Google Scholar] [CrossRef]
- Lum, J.; Wang, R.; Hargis, B.; Tung, S.; Bottje, W.; Lu, H.; Li, Y. An Impedance Aptasensor with Microfluidic Chips for Specific Detection of H5N1 Avian Influenza Virus. Sensors 2015, 15, 18565–18578. [Google Scholar] [CrossRef]
- Lum, J.; Wang, R.; Lassiter, K.; Srinivasan, B.; Abi-Ghanem, D.; Berghman, L.; Hargis, B.; Tung, S.; Lu, H.; Li, Y. Rapid detection of avian influenza H5N1 virus using impedance measurement of immuno-reaction coupled with RBC amplification. Biosens. Bioelectron. 2012, 38, 67–73. [Google Scholar] [CrossRef]
- Yan, X.F.; Wang, M.H.; Wen, X.H.; An, D. Rapid Detection of Avian Influenza Virus Using Immunomagnetic Separation and Impedance Measurement. Appl. Mech. Mater. 2013, 239, 367–371. [Google Scholar] [CrossRef]
- Kukushkin, V.I.; Ivanov, N.M.; Novoseltseva, A.A.; Gambaryan, A.S.; Yaminsky, I.V.; Kopylov, A.M.; Zavyalova, E.G. Highly sensitive detection of influenza virus with SERS aptasensor. PLoS ONE 2019, 14, e0216247. [Google Scholar] [CrossRef]
- Deschaines, T.O.; Wieboldt, D. Practical Applications of Surface-Enhanced Raman Scattering (SERS); Technical Note: 51874; Thermo Fisher Scientific: Madison, WI, USA, 2010; Available online: https://documents.thermofisher.com/TFS-Assets/CAD/Product-Bulletins/D19663~.pdf (accessed on 1 September 2025).
- Lin, D.-Y.; Yu, C.-Y.; Ku, C.-A.; Chung, C.-K. Design, Fabrication, and Applications of SERS Substrates for Food Safety Detection: Review. Micromachines 2023, 14, 1343. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; He, L. Recent advance in SERS techniques for food safety and quality analysis: A brief review. Curr. Opin. Food Sci. 2019, 28, 82–87. [Google Scholar] [CrossRef]
- Li, C.; Huang, Y.; Li, X.; Zhang, Y.; Chen, Q.; Ye, Z.; Alqarni, Z.; Bell, S.E.J.; Xu, Y. Towards practical and sustainable SERS: A review of recent developments in the construction of multifunctional enhancing substrates. J. Mater. Chem. C 2021, 9, 11517–11552. [Google Scholar] [CrossRef]
- Gribanyov, D.; Zhdanov, G.; Olenin, A.; Lisichkin, G.; Gambaryan, A.; Kukushkin, V.; Zavyalova, E. SERS-Based Colloidal Aptasensors for Quantitative Determination of Influenza Virus. Int. J. Mol. Sci. 2021, 22, 1842. [Google Scholar] [CrossRef]
- Xia, J.; Li, W.; Sun, M.; Wang, H. Application of SERS in the Detection of Fungi, Bacteria and Viruses. Nanomaterials 2022, 12, 3572. [Google Scholar] [CrossRef]
- Ambartsumyan, O.; Gribanyov, D.; Kukushkin, V.; Kopylov, A.; Zavyalova, E. SERS-Based Biosensors for Virus Determination with Oligonucleotides as Recognition Elements. Int. J. Mol. Sci. 2020, 21, 3373. [Google Scholar] [CrossRef]
- Chen, H.; Park, S.G.; Choi, N.; Moon, J.I.; Dang, H.; Das, A.; Lee, S.; Kim, D.G.; Chen, L.; Choo, J. SERS imaging-based aptasensor for ultrasensitive and reproducible detection of influenza virus A. Biosens. Bioelectron. 2020, 167, 112496. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, I.; Jackson, N.; Denning, D.; O’Neill, L.; Byrne, H.J. Contributions of vibrational spectroscopy to virology: A review. Clin. Spectrosc. 2022, 4, 100022. [Google Scholar] [CrossRef]
- Shanmukh, S.; Jones, L.; Driskell, J.; Zhao, Y.; Dluhy, R.; Tripp, R.A. Rapid and sensitive detection of respiratory virus molecular signatures using a silver nanorod array SERS substrate. Nano Lett. 2006, 6, 2630–2636. [Google Scholar] [CrossRef]
- Driskell, J.D.; Zhu, Y.; Kirkwood, C.D.; Zhao, Y.; Dluhy, R.A.; Tripp, R.A. Rapid and sensitive detection of rotavirus molecular signatures using surface enhanced Raman spectroscopy. PLoS ONE 2010, 5, e10222. [Google Scholar] [CrossRef]
- Lozano Gómez, H.; Pascual Bielsa, A.; Arche Banzo, M.J. Fulminant myocarditis and cardiogenic shock during SARS-CoV-2 infection. Med. Clínica 2020, 155, 463–464. [Google Scholar] [CrossRef]
- Adlhoch, C.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Marangon, S.; Niqueux, É.; Staubach, C.; Terregino, C.; Aznar, I. European Food Safety Authority, European Centre for Disease Prevention, Control, European Union Reference Laboratory for Avian Influenza; Avian influenza overview December 2021–March 2022. EFSA J. 2022, 20, e07289. [Google Scholar]
- Agüero, M.; Monne, I.; Sánchez, A.; Zecchin, B.; Fusaro, A.; Ruano, M.J.; del Valle Arrojo, M.; Fernández-Antonio, R.; Souto, A.M.; Tordable, P. Highly pathogenic avian influenza A (H5N1) virus infection in farmed minks, Spain, October 2022. Eurosurveillance 2023, 28, 2300001. [Google Scholar] [CrossRef]
- Sun, X.; Belser, J.A.; Pappas, C.; Pulit-Penaloza, J.A.; Brock, N.; Zeng, H.; Creager, H.M.; Le, S.; Wilson, M.; Lewis, A.; et al. Risk Assessment of Fifth-Wave H7N9 Influenza A Viruses in Mammalian Models. J. Virol. 2019, 93, 10–128. [Google Scholar] [CrossRef]
- Bui, C.; Bethmont, A.; Chughtai, A.A.; Gardner, L.; Sarkar, S.; Hassan, S.; Seale, H.; MacIntyre, C.R. A Systematic Review of the Comparative Epidemiology of Avian and Human Influenza A H5N1 and H7N9—Lessons and Unanswered Questions. Transbound. Emerg. Dis. 2016, 63, 602–620. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, H. H7N9 Influenza Virus in China. Cold Spring Harb. Perspect. Med. 2021, 11, a038349. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Yu, Z.; Li, G.; Zhang, Z. Advanced sample preparation techniques for rapid surface-enhanced Raman spectroscopy analysis of complex samples. J. Chromatogr. A 2022, 1675, 463181. [Google Scholar] [CrossRef]
- Kitahama, Y.; Pancorbo, P.M.; Segawa, H.; Marumi, M.; Xiao, T.-H.; Hiramatsu, K.; Yang, W.; Goda, K. Place & Play SERS: Sample collection and preparation-free surface-enhanced Raman spectroscopy. Anal. Methods 2023, 15, 1028–1036. [Google Scholar] [PubMed]
- Liszewska, M.; Bartosewicz, B.; Budner, B.; Nasiłowska, B.; Szala, M.; Weyher, J.L.; Dzięcielewski, I.; Mierczyk, Z.; Jankiewicz, B.J. Evaluation of selected SERS substrates for trace detection of explosive materials using portable Raman systems. Vib. Spectrosc. 2019, 100, 79–85. [Google Scholar] [CrossRef]
- Krajczewski, J.; Ambroziak, R.; Kudelski, A. Substrates for Surface-Enhanced Raman Scattering Formed on Nanostructured Non-Metallic Materials: Preparation and Characterization. Nanomaterials 2020, 11, 75. [Google Scholar]
- Babaei, R.; Savaloni, H. Influence of laser wavelength on surface enhanced Raman spectroscopy using Mn based nano-particles produced by laser ablation synthesis in 4,4′ Bipyridine solution (LASiS). Optik 2021, 242, 167276. [Google Scholar] [CrossRef]
- Mayr, F.; Zimmerleiter, R.; Farias, P.M.A.; Bednorz, M.; Salinas, Y.; Galembek, A.; Cardozo, O.D.F.; Wielend, D.; Oliveira, D.; Milani, R.; et al. Sensitive and high laser damage threshold substrates for surface-enhanced Raman scattering based on gold and silver nanoparticles. Anal. Sci. Adv. 2023, 4, 335–346. [Google Scholar]
- Maneeprakorn, W.; Bamrungsap, S.; Apiwat, C.; Wiriyachaiporn, N. Surface-enhanced Raman scattering based lateral flow immunochromatographic assay for sensitive influenza detection. RSC Adv. 2016, 6, 112079–112085. [Google Scholar] [CrossRef]
- Goel, R.; Chakraborty, S.; Awasthi, V.; Bhardwaj, V.; Kumar Dubey, S. Exploring the various aspects of Surface enhanced Raman spectroscopy (SERS) with focus on the recent progress: SERS-active substrate, SERS-instrumentation, SERS-application. Sensor Actuat. A-Phys. 2024, 376, 115555. [Google Scholar] [CrossRef]
- Yang, C.-W.; Zhang, X.; Yuan, L.; Wang, Y.-K.; Sheng, G.-P. Deciphering the microheterogeneous repartition effect of environmental matrix on surface-enhanced Raman spectroscopy (SERS) analysis for pollutants in natural waters. Water Res. 2023, 232, 119668. [Google Scholar] [CrossRef]
- Tahir, M.A.; Dina, N.E.; Cheng, H.; Valev, V.K.; Zhang, L. Surface-enhanced Raman spectroscopy for bioanalysis and diagnosis. Nanoscale 2021, 13, 11593–11634. [Google Scholar] [CrossRef]
- Beeram, R.; Vepa, K.R.; Soma, V.R. Recent Trends in SERS-Based Plasmonic Sensors for Disease Diagnostics, Biomolecules Detection, and Machine Learning Techniques. Biosensors 2023, 13, 328. [Google Scholar] [CrossRef]
- Guan, W.; Yang, Z.; Wu, N.C.; Lee, H.H.Y.; Li, Y.; Jiang, W.; Shen, L.; Wu, D.C.; Chen, R.; Zhong, N.; et al. Clinical Correlations of Transcriptional Profile in Patients Infected With Avian Influenza H7N9 Virus. J. Infect. Dis. 2018, 218, 1238–1248. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.A.; Mohamed, M.E.M.; Erfan, A.M.; Abdelkarim, L.; Awadallah, M.A.I.J.S.V.R. Investigating the biosecurity measures’ applications in poultry farms and its relationship with the occurence of avian influenza. Slov. Vet. Res. 2021, 58, 315–321. [Google Scholar] [CrossRef]
- Parvin, R.; Nooruzzaman, M.; Kabiraj, C.K.; Begum, J.A.; Chowdhury, E.H.; Islam, M.R.; Harder, T. Controlling Avian Influenza Virus in Bangladesh: Challenges and Recommendations. Viruses 2020, 12, 751. [Google Scholar] [CrossRef]
- Tilli, G.; Laconi, A.; Galuppo, F.; Mughini-Gras, L.; Piccirillo, A. Assessing Biosecurity Compliance in Poultry Farms: A Survey in a Densely Populated Poultry Area in North East Italy. Animals 2022, 12, 1409. [Google Scholar] [CrossRef]
- Byrne, A.M.P.; Reid, S.M.; Seekings, A.H.; Núñez, A.; Obeso Prieto, A.B.; Ridout, S.; Warren, C.J.; Puranik, A.; Ceeraz, V.; Essen, S.; et al. H7N7 Avian Influenza Virus Mutation from Low to High Pathogenicity on a Layer Chicken Farm in the UK. Viruses 2021, 13, 259. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zhao, M.; Yu, Z.; Li, Y.; Zhang, X.; Feng, N.; Wang, T.; Wang, H.; He, H.; Zhao, Y.; et al. Cross-species infection potential of avian influenza H13 viruses isolated from wild aquatic birds to poultry and mammals. Emerg. Microbes Infect. 2023, 12, e2184177. [Google Scholar] [CrossRef]
- Giannini, V.; Fernandez-Dominguez, A.I.; Heck, S.C.; Maier, S.A. Plasmonic nanoantennas: Fundamentals and their use in controlling the radiative properties of nanoemitters. Chem. Rev. 2011, 111, 3888–3912. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, H.; Han, X.X.; Zhao, B. Metal–semiconductor heterostructures for surface-enhanced Raman scattering: Synergistic contribution of plasmons and charge transfer. Mater. Horiz. 2021, 8, 370–382. [Google Scholar] [CrossRef]
- Liebhart, D.; Bilic, I.; Grafl, B.; Hess, C.; Hess, M. Diagnosing Infectious Diseases in Poultry Requires a Holistic Approach: A Review. Poultry 2023, 2, 252–280. [Google Scholar] [CrossRef]
- Xiao, M.; Xie, K.; Dong, X.; Wang, L.; Huang, C.; Xu, F.; Xiao, W.; Jin, M.; Huang, B.; Tang, Y. Ultrasensitive detection of avian influenza A (H7N9) virus using surface-enhanced Raman scattering-based lateral flow immunoassay strips. Anal. Chim. Acta 2019, 1053, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, C.; Wang, X.; Wang, K.; Zhu, Y.; Rong, Z.; Wang, W.; Xiao, R.; Wang, S. Magnetic SERS Strip for Sensitive and Simultaneous Detection of Respiratory Viruses. ACS Appl. Mater. Interfaces 2019, 11, 19495–19505. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Yi, S.Y.; Hwang, A.; Eom, G.; Sim, J.; Jeong, J.; Lim, E.-K.; Chung, B.H.; Kim, B.; Jung, J.; et al. Facile and sensitive detection of influenza viruses using SERS antibody probes. RSC Adv. 2016, 6, 84415–84419. [Google Scholar] [CrossRef]
- Pang, Y.; Wang, J.; Xiao, R.; Wang, S. SERS molecular sentinel for the RNA genetic marker of PB1-F2 protein in highly pathogenic avian influenza (HPAI) virus. Biosens. Bioelectron. 2014, 61, 460–465. [Google Scholar] [CrossRef]
- Wang, Y.; Ruan, Q.; Lei, Z.C.; Lin, S.C.; Zhu, Z.; Zhou, L.; Yang, C. Highly Sensitive and Automated Surface Enhanced Raman Scattering-based Immunoassay for H5N1 Detection with Digital Microfluidics. Anal. Chem. 2018, 90, 5224–5231. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Qu, H.; Hao, L.; Shao, T.; Wang, K.; Xia, Z.; Li, Z.; Li, Q. SERS-based immunomagnetic bead for rapid detection of H5N1 influenza virus. Influenza Other Respir. Viruses 2023, 17, e13114. [Google Scholar] [CrossRef]
- Chen, H.; Park, S.K.; Joung, Y.; Kang, T.; Lee, M.K.; Choo, J. SERS-based dual-mode DNA aptasensors for rapid classification of SARS-CoV-2 and influenza A/H1N1 infection. Sens. Actuators B Chem. 2022, 355, 131324. [Google Scholar] [CrossRef]
- Abuhelwa, M.; Singh, A.; Liu, J.; Almalaysha, M.; Carlson, A.V.; Trout, K.E.; Morey, A.; Kinzel, E.; Channaiah, L.H.; Almasri, M. Fiber optics-based surface enhanced Raman Spectroscopy sensors for rapid multiplex detection of foodborne pathogens in raw poultry. Microsyst. Nanoeng. 2024, 10, 199. [Google Scholar] [CrossRef]
- Muthukumar, D.; Shtenberg, G. SERS-based immunosensor for E. coli contaminants detection in milk using silver-coated nanoporous silicon substrates. Talanta 2023, 254, 124132. [Google Scholar] [CrossRef]
- Wang, D.; Chen, Y.; Zhang, Q.; Chen, J.; Li, C.; Luo, Y.; Jin, Y.; Qi, X. SERS-Based Immunochromatographic Assay for Sensitive Detection of Escherichia coli O157:H7 Using a Novel WS2-AuDTNB Nanotag. Sensors 2025, 25, 2457. [Google Scholar] [CrossRef]
- Zavyalova, E.; Ambartsumyan, O.; Zhdanov, G.; Gribanyov, D.; Gushchin, V.; Tkachuk, A.; Rudakova, E.; Nikiforova, M.; Kuznetsova, N.; Popova, L.; et al. SERS-Based Aptasensor for Rapid Quantitative Detection of SARS-CoV-2. Nanomaterials 2021, 11, 1394. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, A.; Gao, Y.; Patibandla, S.; Mitra, D.; McCandless, M.G.; Fassero, L.A.; Gates, K.; Tandon, R.; Chandra Ray, P. The rapid diagnosis and effective inhibition of coronavirus using spike antibody attached gold nanoparticles. Nanoscale Adv. 2021, 3, 1588–1596. [Google Scholar] [CrossRef]
- Leong, S.X.; Leong, Y.X.; Tan, E.X.; Sim, H.Y.F.; Koh, C.S.L.; Lee, Y.H.; Chong, C.; Ng, L.S.; Chen, J.R.T.; Pang, D.W.C.; et al. Noninvasive and Point-of-Care Surface-Enhanced Raman Scattering (SERS)-Based Breathalyzer for Mass Screening of Coronavirus Disease 2019 (COVID-19) under 5 min. ACS Nano 2022, 16, 2629–2639. [Google Scholar] [CrossRef]
- Zhao, T.; Liang, P.; Ren, J.; Zhu, J.; Yang, X.; Bian, H.; Li, J.; Cui, X.; Fu, C.; Xing, J.; et al. Gold-silver alloy hollow nanoshells-based lateral flow immunoassay for colorimetric, photothermal, and SERS tri-mode detection of SARS-CoV-2 neutralizing antibody. Anal. Chim. Acta 2023, 1255, 341102. [Google Scholar] [CrossRef] [PubMed]
- Atta, S.; Zhao, Y.; Li, J.Q.; Vo-Dinh, T. Dual-Modal Colorimetric and Surface-Enhanced Raman Scattering (SERS)-Based Lateral Flow Immunoassay for Ultrasensitive Detection of SARS-CoV-2 Using a Plasmonic Gold Nanocrown. Anal. Chem. 2024, 96, 4783–4790. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, H.; Gao, J.; Wang, J.; Jin, Z.; Lv, M.; Yan, S. Development and Biomedical Application of Non-Noble Metal Nanomaterials in SERS. Nanomaterials 2024, 14, 1654. [Google Scholar] [CrossRef]
- Feare, C.J.; Yasue, M. Asymptomatic infection with highly pathogenic avian influenza H5N1 in wild birds: How sound is the evidence? Virol. J. 2006, 3, 96. [Google Scholar] [CrossRef]
- Lee, C.-Y. Exploring Potential Intermediates in the Cross-Species Transmission of Influenza A Virus to Humans. Viruses 2024, 16, 1129. [Google Scholar] [CrossRef]
- Youk, S.S.; Leyson, C.M.; Seibert, B.A.; Jadhao, S.; Perez, D.R.; Suarez, D.L.; Pantin-Jackwood, M.J. Mutations in PB1, NP, HA, and NA Contribute to Increased Virus Fitness of H5N2 Highly Pathogenic Avian Influenza Virus Clade 2.3.4.4 in Chickens. J. Virol. 2021, 95, 10–1128. [Google Scholar] [CrossRef]
- Belkasmi, S.F.Z.; Fellahi, S.; Touzani, C.D.; Faraji, F.Z.; Maaroufi, I.; Delverdier, M.; Guérin, J.-L.; Fihri, O.F.; El Houadfi, M.; Ducatez, M.F. Co-infections of chickens with avian influenza virus H9N2 and Moroccan Italy 02 infectious bronchitis virus: Effect on pathogenesis and protection conferred by different vaccination programmes. Avian Pathol. 2020, 49, 21–28. [Google Scholar] [CrossRef]
- Arafat, N.; Abd El Rahman, S.; Naguib, D.; El-Shafei, R.A.; Abdo, W.; Eladl, A.H. Co-infection of Salmonella enteritidis with H9N2 avian influenza virus in chickens. Avian Pathol. 2020, 49, 496–506. [Google Scholar] [CrossRef]
- Spackman, E.; Pedersen, J.C.; McKinley, E.T.; Gelb, J. Optimal specimen collection and transport methods for the detection of avian influenza virus and Newcastle disease virus. BMC Vet. Res. 2013, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Spackman, E.; Stephens, C.B. Identification of optimal sample collection devices and sampling locations for the detection of environmental viral contamination in wire poultry cages. Transbound. Emerg. Dis. 2021, 68, 598–604. [Google Scholar] [CrossRef]
- Bai, Z.; Wei, H.; Yang, X.; Zhu, Y.; Peng, Y.; Yang, J.; Wang, C.; Rong, Z.; Wang, S. Rapid Enrichment and Ultrasensitive Detection of Influenza A Virus in Human Specimen using Magnetic Quantum Dot Nanobeads Based Test Strips. Sens. Actuators B Chem. 2020, 325, 128780. [Google Scholar] [CrossRef]
- Berus, S.M.; Nowicka, A.B.; Wieruszewska, J.; Niciński, K.; Kowalska, A.A.; Szymborski, T.R.; Dróżdż, I.; Borowiec, M.; Waluk, J.; Kamińska, A. SERS Signature of SARS-CoV-2 in Saliva and Nasopharyngeal Swabs: Towards Perspective COVID-19 Point-of-Care Diagnostics. Int. J. Mol. Sci. 2023, 24, 9706. [Google Scholar] [CrossRef]
- Kahraman, M.; Mullen, E.R.; Korkmaz, A.; Wachsmann-Hogiu, S. Fundamentals and applications of SERS-based bioanalytical sensing. Nanophotonics 2017, 6, 831–852. [Google Scholar] [CrossRef]
- Saviñon-Flores, F.; Méndez, E.; López-Castaños, M.; Carabarin-Lima, A.; López-Castaños, K.A.; González-Fuentes, M.A.; Méndez-Albores, A. A Review on SERS-Based Detection of Human Virus Infections: Influenza and Coronavirus. Biosensors 2021, 11, 66. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qadir, M.F.; Yang, Y. Overcoming Challenges in Avian Influenza Diagnosis: The Role of Surface-Enhanced Raman Spectroscopy in Poultry Health Monitoring. Vet. Sci. 2025, 12, 1052. https://doi.org/10.3390/vetsci12111052
Qadir MF, Yang Y. Overcoming Challenges in Avian Influenza Diagnosis: The Role of Surface-Enhanced Raman Spectroscopy in Poultry Health Monitoring. Veterinary Sciences. 2025; 12(11):1052. https://doi.org/10.3390/vetsci12111052
Chicago/Turabian StyleQadir, Muhammad Farhan, and Yukun Yang. 2025. "Overcoming Challenges in Avian Influenza Diagnosis: The Role of Surface-Enhanced Raman Spectroscopy in Poultry Health Monitoring" Veterinary Sciences 12, no. 11: 1052. https://doi.org/10.3390/vetsci12111052
APA StyleQadir, M. F., & Yang, Y. (2025). Overcoming Challenges in Avian Influenza Diagnosis: The Role of Surface-Enhanced Raman Spectroscopy in Poultry Health Monitoring. Veterinary Sciences, 12(11), 1052. https://doi.org/10.3390/vetsci12111052