Effect of Additional Aluminum Filtration on the Image Quality in Cone Beam Computed Tomographic Studies of Equine Distal Limbs Using Visual Grading Characteristics Analysis: A Pilot Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
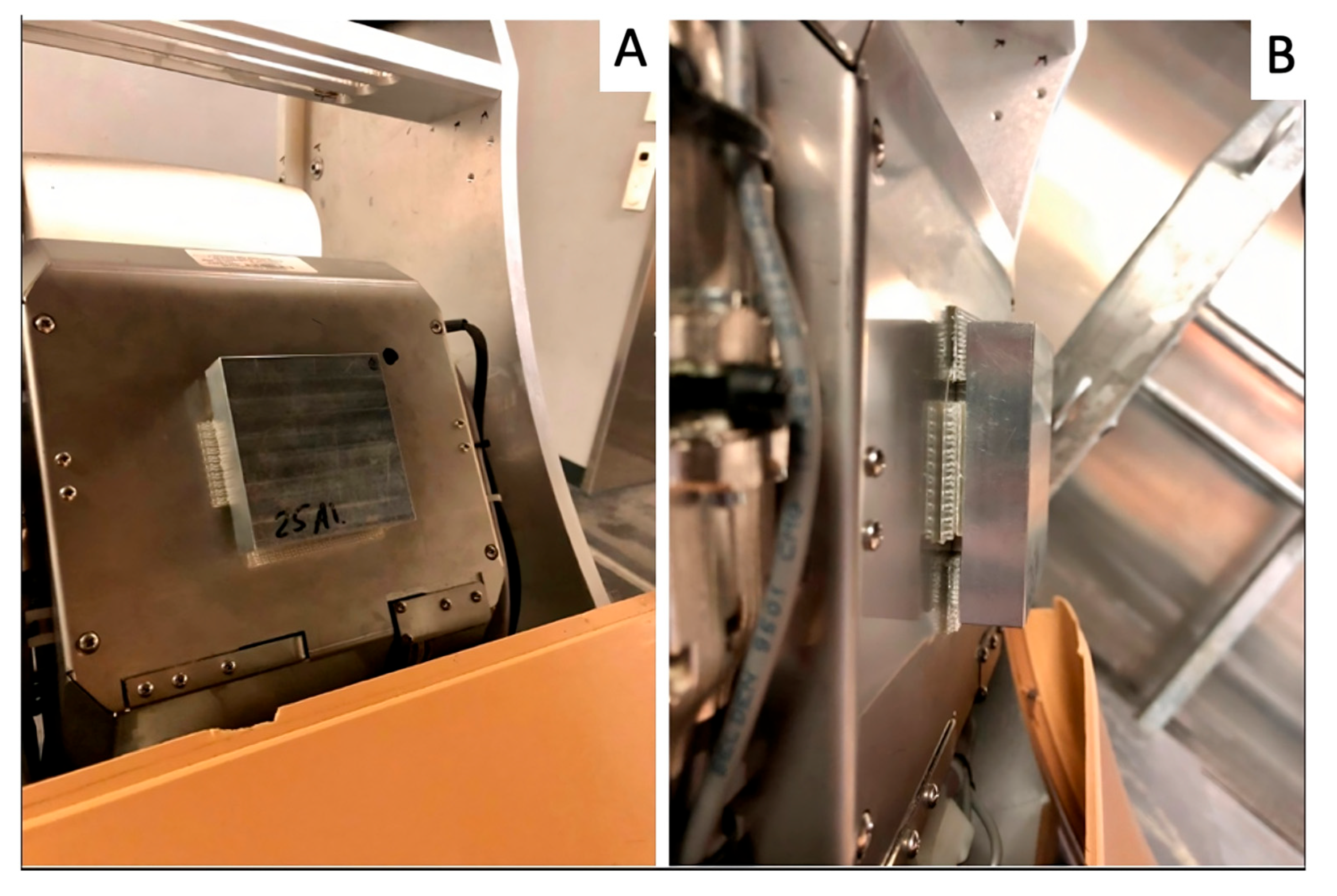
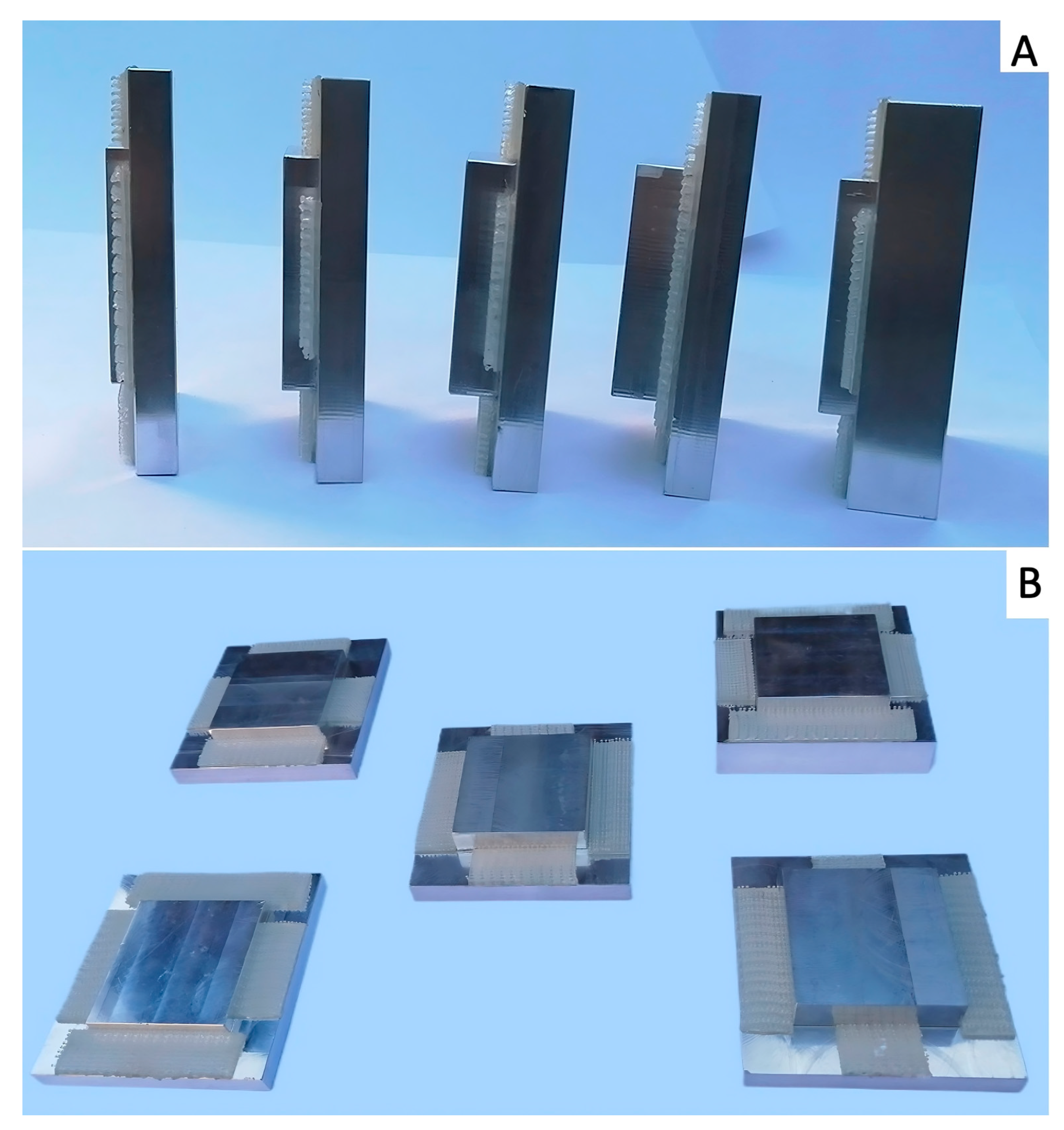
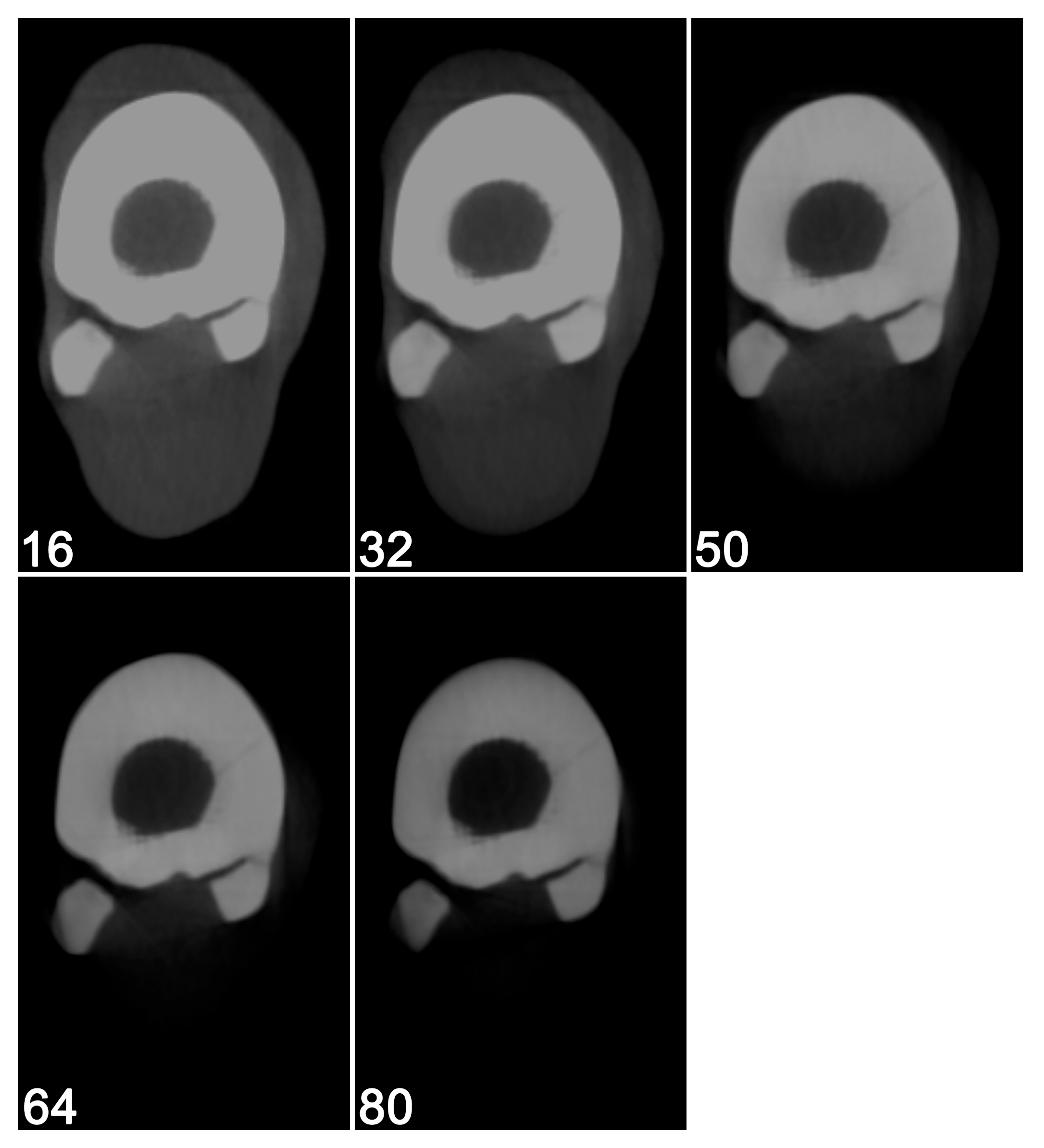
2.1. Image Acquisition and Reconstruction
2.2. Data Recording and Analysis
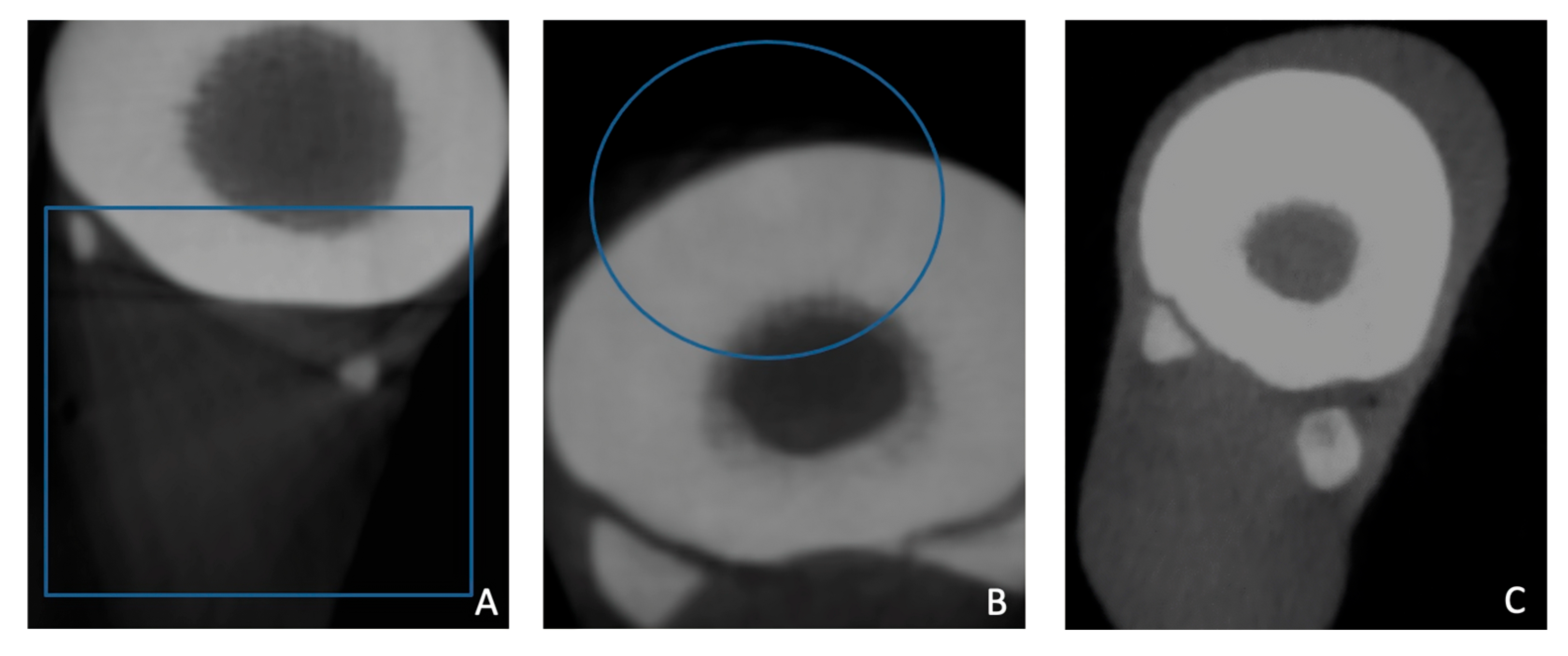
2.2.1. Visual Image Quality Assessment
2.2.2. Clinical Case
2.2.3. Statistics
3. Results
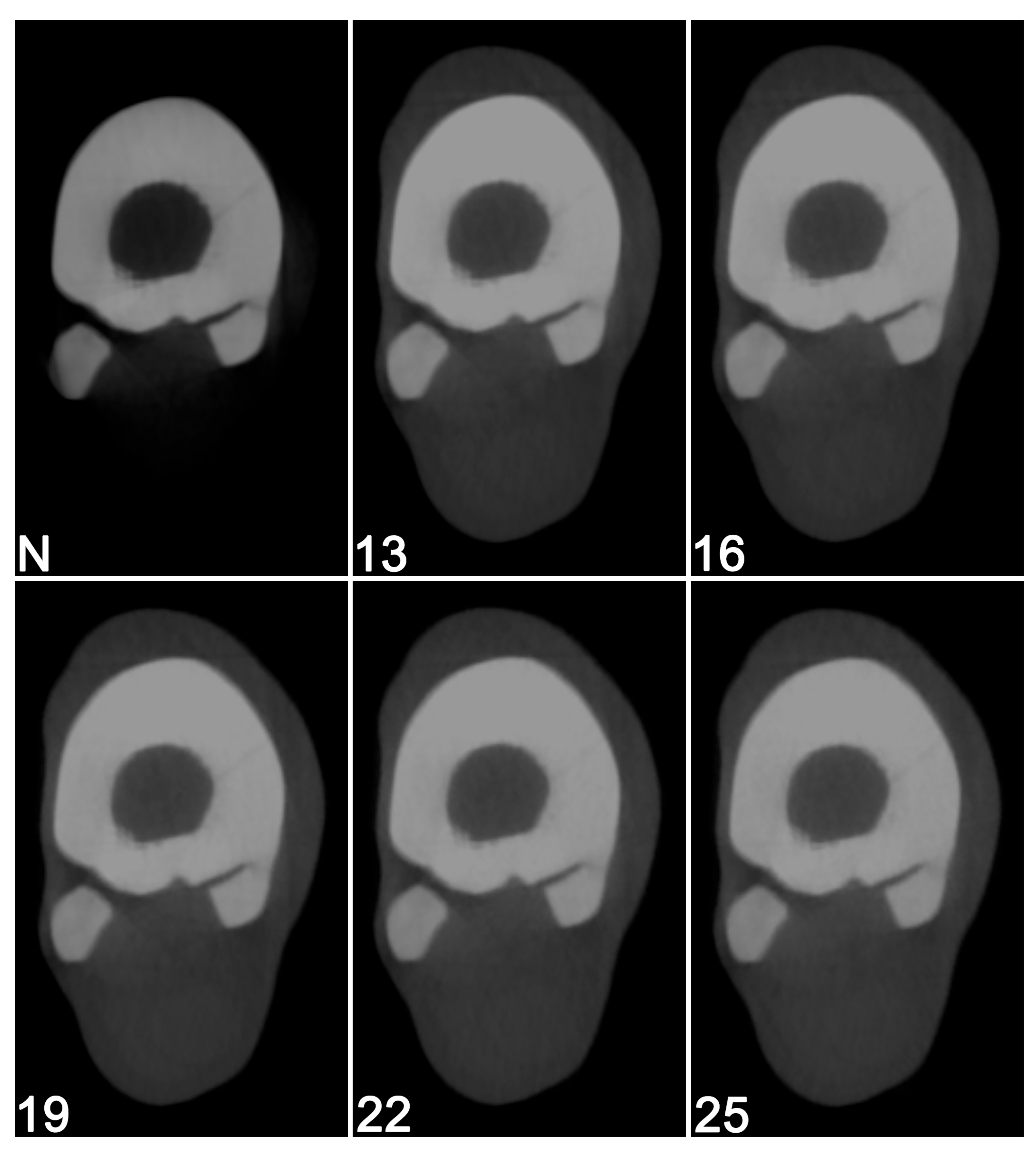
3.1. Visual Image Quality Assessment
3.2. Clinical Case–Visual Image Quality Assessment
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CBCT | Cone beam computed tomography |
| IS | Internal structure |
| MT | Metatarsus |
| MC | Metacarpus |
| MC-S | Metacarpus small |
| P1 | Proximal phalanx |
References
- de Preux, M.; Klopfenstein Bregger, M.D.; Brunisholz, H.P.; Van der Vekens, E.; Schweizer-Gorgas, D.; Koch, C. Clinical use of computer-assisted orthopedic surgery in horses. Vet. Surg. 2020, 49, 1075–1087. [Google Scholar] [CrossRef]
- Dakin, S.G.; Lam, R.; Rees, E.; Mumby, C.; West, C.; Weller, R. Technical set-up and radiation exposure for standing computed tomography of the equine head. Equine Vet. Educ. 2014, 26, 208–215. [Google Scholar] [CrossRef]
- Keane, M.; Paul, E.; Sturrock, C.J.; Rauch, C.; Rutland, C.S. Computed Tomography in Veterinary Medicine: Currently Published and Tomorrow’s Vision. In Computed Tomography: Advanced Applications; Halefoğlu, A.M., Ed.; InTechOpen: Rijeka, Croatia, 2017; pp. 271–289. [Google Scholar]
- Puchalski, S.M. Advances in equine computed tomography and use of contrast media. Vet. Clin. N. Am. Equine Pract. 2012, 28, 563–581. [Google Scholar] [CrossRef]
- Bregger, M.K.; Koch, C.; Zimmermann, R.; Sangiorgio, D.; Schweizer-Gorgas, D. Cone-beam computed tomography of the head in standing equids. BMC Vet. Res. 2019, 15, 289. [Google Scholar] [CrossRef]
- Pauwels, F.E.; Van der Vekens, E.; Christan, Y.; Koch, C.; Schweizer, D. Feasibility, indications, and radiographically confirmed diagnoses of standing extremity cone beam computed tomography in the horse. Vet. Surg. 2021, 50, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Stewart, H.L.; Siewerdsen, J.H.; Nelson, B.B.; Kawcak, C.E. Use of cone-beam computed tomography for advanced imaging of the equine patient. Equine Vet. J. 2021, 53, 872–885. [Google Scholar] [CrossRef]
- Crijns, C.P.; Martens, A.; Bergman, H.J.; van der Veen, H.; Duchateau, L.; van Bree, H.J.; Gielen, I.M. Intramodality and intermodality agreement in radiography and computed tomography of equine distal limb fractures. Equine Vet. J. 2014, 46, 92–96. [Google Scholar] [CrossRef]
- Perks, T.D.; Dendere, R.; Irving, B.; Hartley, T.; Scholtz, P.; Lawson, A.; Trauernicht, C.; Steiner, S.; Douglas, T.S. Filtration to reduce paediatric dose for a linear slot-scanning digital X-ray machine. Radiat. Prot. Dosim. 2015, 167, 552–561. [Google Scholar] [CrossRef]
- Perks, T.D.; Trauernicht, C.; Hartley, T.; Hobson, C.; Lawson, A.; Scholtz, P.; Dendere, R.; Steiner, S.; Douglas, T.S. Effect of aluminium filtration on dose and image quality in paediatric slot-scanning radiography. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 2332–2335. [Google Scholar]
- Hansson, B.; Finnbogason, T.; Schuwert, P.; Persliden, J. Added copper filtration in digital paediatric double-contrast colon examinations: Effects on radiation dose and image quality. Eur. Radiol. 1997, 7, 1117–1122. [Google Scholar] [CrossRef]
- Ay, M.R.; Mehranian, A.; Maleki, A.; Ghadiri, H.; Ghafarian, P.; Zaidi, H. Experimental assessment of the influence of beam hardening filters on image quality and patient dose in volumetric 64-slice X-ray CT scanners. Phys. Med. 2013, 29, 249–260. [Google Scholar] [CrossRef]
- Prionas, N.D.; Huang, S.Y.; Boone, J.M. Experimentally determined spectral optimization for dedicated breast computed tomography. Med. Phys. 2011, 38, 646–655. [Google Scholar] [CrossRef]
- Davies, T.; Skelly, C.; Puggioni, A.; D’Helft, C.; Connolly, S.; Hoey, S. Standing CT of the equine head: Reducing radiation dose maintains image quality. Vet. Radiol. Ultrasound 2020, 61, 137–146. [Google Scholar] [CrossRef]
- Demehri, S.; Muhit, A.; Zbijewski, W.; Stayman, J.W.; Yorkston, J.; Packard, N.; Senn, R.; Yang, D.; Foos, D.; Thawait, G.K.; et al. Assessment of image quality in soft tissue and bone visualization tasks for a dedicated extremity cone-beam CT system. Eur. Radiol. 2015, 25, 1742–1751. [Google Scholar] [CrossRef]
- Lang, H.; Neubauer, J.; Fritz, B.; Spira, E.M.; Strube, J.; Langer, M.; Kotter, E. A retrospective, semi-quantitative image quality analysis of cone beam computed tomography (CBCT) and MSCT in the diagnosis of distal radius fractures. Eur. Radiol. 2016, 26, 4551–4561. [Google Scholar] [CrossRef]
- Bierau, J.; Cruz, A.M.; Koch, C.; Manso-Diaz, G.; Büttner, K.; Staszyk, C.; Röcken, M. Visualization of anatomical structures in the fetlock region of the horse using cone beam computed tomography in comparison with conventional multidetector computed tomography. Front. Vet. Sci. 2024, 10, 1278148. [Google Scholar] [CrossRef]
- Siewerdsen, J.H.; Uneri, A.; Hernandez, A.M.; Burkett, G.W.; Boone, J.M. Cone-beam CT dose and imaging performance evaluation with a modular, multipurpose phantom. Med. Phys. 2020, 47, 467–479. [Google Scholar] [CrossRef]
- Stewart, H.L.; Siewerdsen, J.H.; Selberg, K.T.; Bills, K.W.; Kawcak, C.E. Cone-beam computed tomography produces images of numerically comparable diagnostic quality for bone and inferior quality for soft tissues compared with fan-beam computed tomography in cadaveric equine metacarpophalangeal joints. Vet. Radiol. Ultrasound 2023, 64, 1033–1036. [Google Scholar] [CrossRef]
- McQuillan, S.; Kearney, C.; Hoey, S.; Connolly, S.; Rowan, C. A threshold volume of 10 mL is suggested for detecting articular cartilage defects in equine carpal joints using CT arthrography: Ex vivo pilot study. Vet. Radiol. Ultrasound 2022, 63, 54–63. [Google Scholar] [CrossRef]
- Ogden, N.K.E.; Winderickx, K.; Bennell, A.; Stack, J.D. Computed tomography of the equine caudal spine and pelvis: Technique, image quality and anatomical variation in 56 clinical cases (2018–2023). Equine Vet. J. 2025, 5, 1265–1278. [Google Scholar] [CrossRef]
- Gabriel, A.; Jolly, S.; Detilleux, J.; Dessy-Doize, C.; Collin, B.; Reginster, J.-Y. Morphometric study of the equine navicular bone: Variations with breeds and types of horse and influence of exercise. J. Anat. 1998, 193, 535–549. [Google Scholar] [CrossRef]
- Abdunnabi, A.H.; Ahmed, Y.A.; Philip, C.J.; Davies, H.M.S. Morphometrical Variations of the Carpal Bones in Thoroughbreds and Ponies. Anat. Histol. Embryol. 2012, 41, 139–148. [Google Scholar] [CrossRef]
- Goldstein, D.M.; Engiles, J.B.; Rezabek, G.B.; Ruff, C.B. Locomotion on the edge: Structural properties of the third metacarpal in Thoroughbred and Quarter Horse racehorses and feral Assateague Island ponies. Anat. Rec. 2021, 304, 771–786. [Google Scholar] [CrossRef] [PubMed]
- Carrino, J.A.; Muhit, A.A.; Zbijewski, W.; Thawait, G.K.; Stayman, J.W.; Packard, N.; Senn, R.; Yang, D.; Foos, D.H.; Yorkston, J.; et al. Dedicated Cone-Beam CT System for Extremity Imaging. Radiology 2014, 270, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, P.P.; Chan, J.P. Advances in imaging (Intraop Cone-Beam Computed Tomography, Synthetic Computed Tomography, Bone Scan, Low-Dose Protocols). Neurosurg. Clin. 2024, 35, 161–172. [Google Scholar] [CrossRef] [PubMed]


| Variable | Rating |
|---|---|
| Distinctness of anatomical structure |
|
| |
| |
| |
| CT artifacts |
|
| |
| |
|
| mA | 50 mA | 64 mA | 80 mA | 100 mA | |
|---|---|---|---|---|---|
| Filter | |||||
| N | 0.610 | 0.601 | 0.321 | 0.048 | |
| F13 | 0.177 | 0.168 | −0.112 | −0.385 | |
| F16 | 0.188 | 0.179 | −0.101 | −0.374 | |
| F19 | 0.078 | 0.069 | −0.211 | −0.484 | |
| F22 | 0.179 | 0.170 | −0.110 | −0.383 | |
| F25 | 0.003 | −0.006 | −0.286 | −0.559 | |
| Region | Recommended Tube Current | Filter |
|---|---|---|
| Metacarpus/Metatarsus | 50–64 mA | None (native scan) |
| Proximal Phalanx (P1) | 50 mA | Aluminum filter (19–25 mm) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papini, L.; de Preux, M.; Pauwels, F.; Missotten, J.; Van der Vekens, E. Effect of Additional Aluminum Filtration on the Image Quality in Cone Beam Computed Tomographic Studies of Equine Distal Limbs Using Visual Grading Characteristics Analysis: A Pilot Study. Vet. Sci. 2025, 12, 1051. https://doi.org/10.3390/vetsci12111051
Papini L, de Preux M, Pauwels F, Missotten J, Van der Vekens E. Effect of Additional Aluminum Filtration on the Image Quality in Cone Beam Computed Tomographic Studies of Equine Distal Limbs Using Visual Grading Characteristics Analysis: A Pilot Study. Veterinary Sciences. 2025; 12(11):1051. https://doi.org/10.3390/vetsci12111051
Chicago/Turabian StylePapini, Luca, Mathieu de Preux, Frederik Pauwels, Joris Missotten, and Elke Van der Vekens. 2025. "Effect of Additional Aluminum Filtration on the Image Quality in Cone Beam Computed Tomographic Studies of Equine Distal Limbs Using Visual Grading Characteristics Analysis: A Pilot Study" Veterinary Sciences 12, no. 11: 1051. https://doi.org/10.3390/vetsci12111051
APA StylePapini, L., de Preux, M., Pauwels, F., Missotten, J., & Van der Vekens, E. (2025). Effect of Additional Aluminum Filtration on the Image Quality in Cone Beam Computed Tomographic Studies of Equine Distal Limbs Using Visual Grading Characteristics Analysis: A Pilot Study. Veterinary Sciences, 12(11), 1051. https://doi.org/10.3390/vetsci12111051






