Determination of H5N1 Avian Influenza Virus Persistence Following a 2024 Backyard Poultry Outbreak in Romania
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
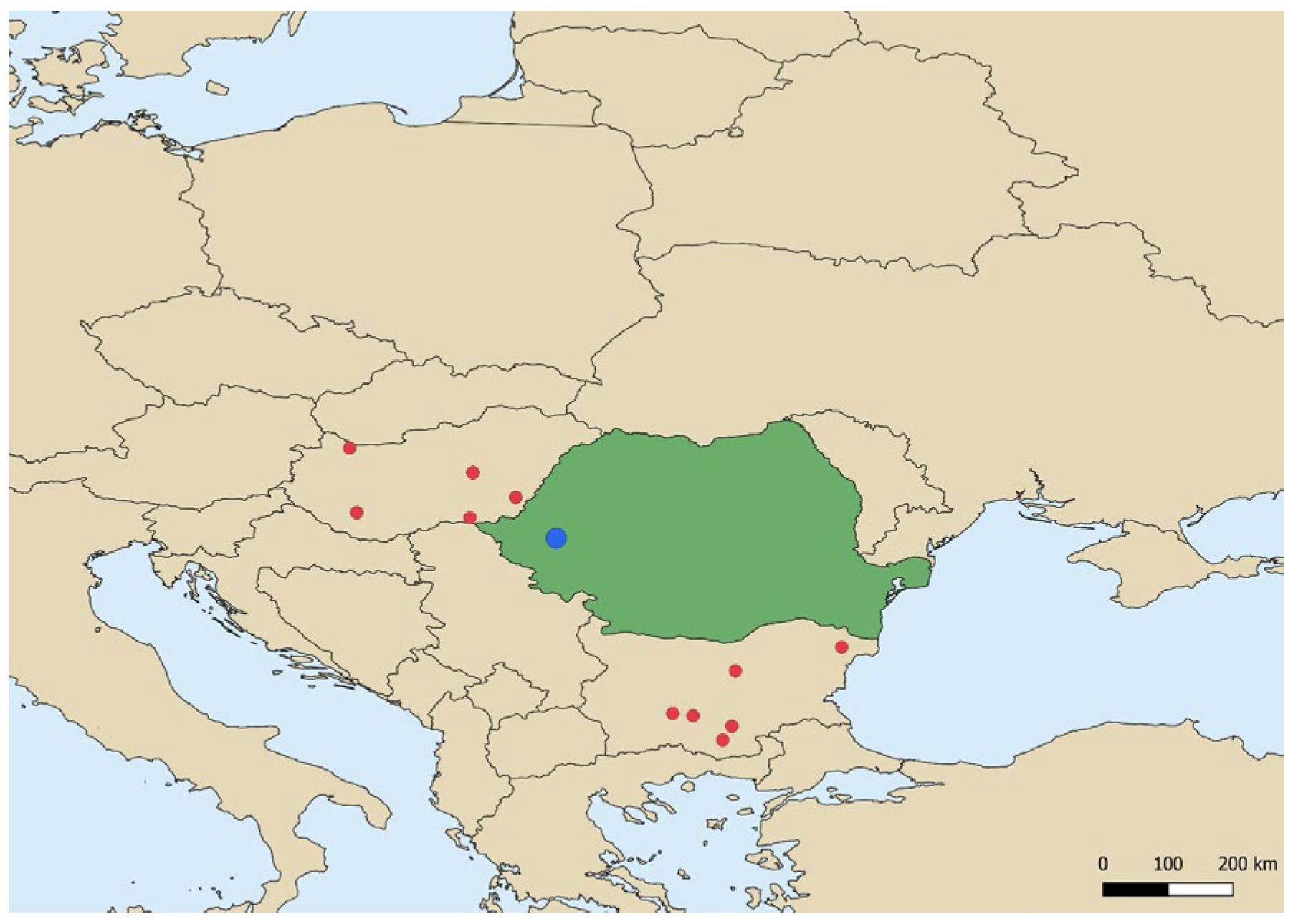
2.1. Outbreak Location and Sampling Strategy
2.2. Sample Preparation and RNA Extraction
2.3. Molecular Detection by Real-Time RT-PCR
2.4. Subtype Identification and Sequencing
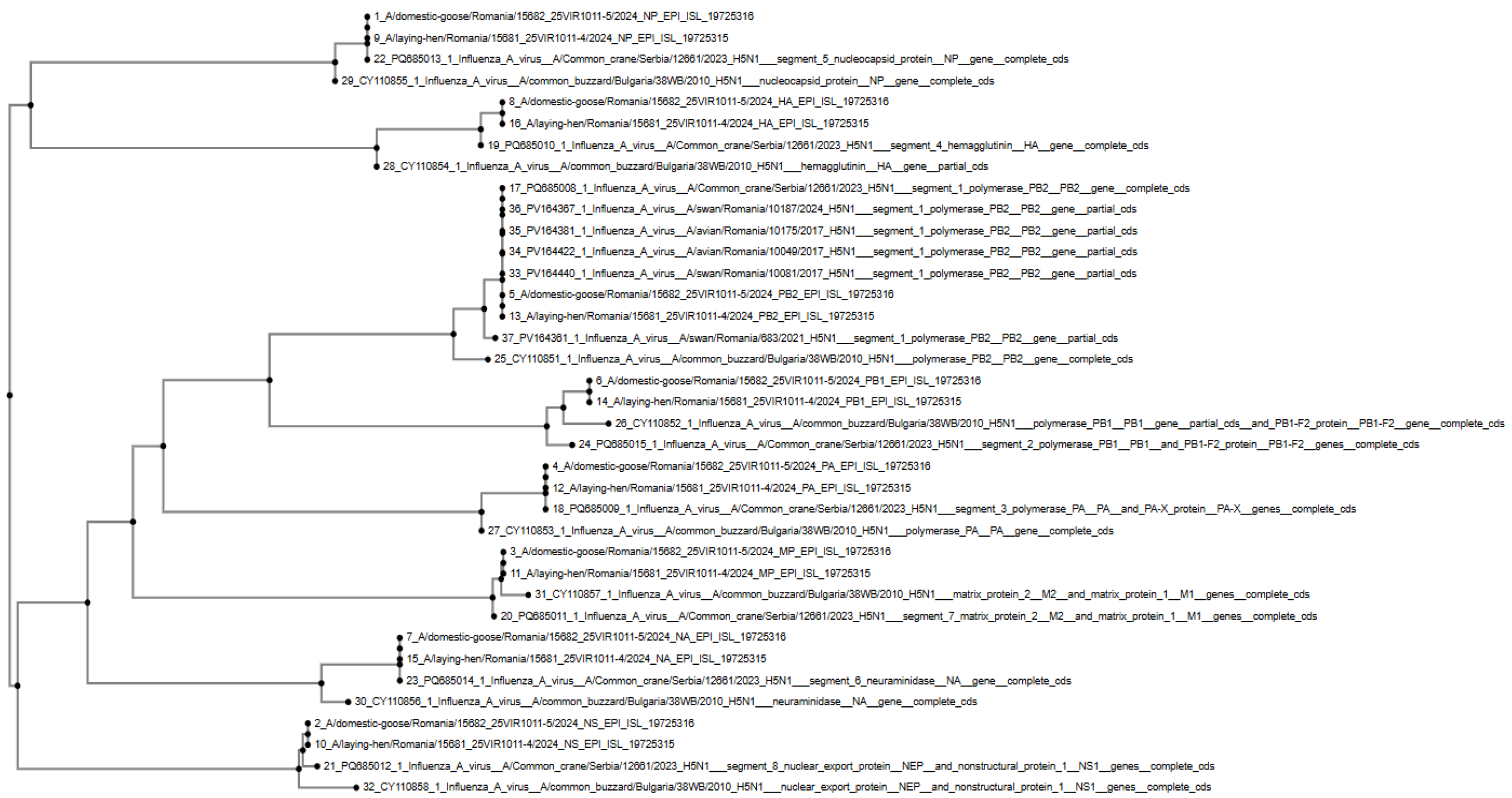
2.5. Phylogenetic Analysis
3. Results
3.1. HPAI H5N1 Confirmation in Outbreak Samples
3.2. Absence of AIV Detection in Persistence Study Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xie, Z.; Yang, J.; Jiao, W.; Li, X.; Iqbal, M.; Liao, M.; Dai, M. Clade 2.3.4.4b Highly Pathogenic Avian Influenza H5N1 Viruses: Knowns, Unknowns, and Challenges. J. Virol. 2025, 99, e0042425. [Google Scholar] [CrossRef]
- Webby, R.; Uyeki, T. An Update on Highly Pathogenic Avian Influenza A(H5N1) Virus, Clade 2.3.4.4b. J. Infect. Dis. 2024, 230, 533–542. [Google Scholar] [CrossRef]
- Burrough, E.; Magstadt, D.; Petersen, B.; Timmermans, S.; Gauger, P.; Zhang, J.; Siepker, C.; Mainenti, M.; Li, G.; Thompson, A.; et al. Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4b Virus Infection in Domestic Dairy Cattle and Cats, United States, 2024. Emerg. Infect. Dis. 2024, 30, 1335–1343. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Z.; Liu, X.; Wei, S.; Zhang, Y.; Fuxiang, Y.; Qiao, J.; Zhang, H.; Xiao, C. Highly Pathogenic Avian Influenza A(H5N1) Virus Infection in Dairy Cattle: Threat of Bird Flu Has Expanded to Open-Air Farmed Livestock. J. Infect. 2024, 89, 106311. [Google Scholar] [CrossRef] [PubMed]
- Steinsiepe, V.; Cruz, C.; Icochea, M.; Espejo, V.; Troncos, G.; Castro-Sanguinetti, G.; Schilling, M.; Tinoco, Y. Highly Pathogenic Avian Influenza A(H5N1) from Wild Birds, Poultry, and Mammals, Peru. Emerg. Infect. Dis. 2023, 29, 2572–2576. [Google Scholar] [CrossRef]
- Rimondi, A.; Vanstreels, R.; Olivera, V.; Donini, A.; Lauriente, M.; Uhart, M. Highly Pathogenic Avian Influenza A(H5N1) Viruses from Multispecies Outbreak, Argentina, August 2023. Emerg. Infect. Dis. 2024, 30, 812–814. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Bai, X.; Li, M.; Zeng, X.; Xu, J.; Li, P.; Wang, M.; Song, X.; Zhao, Z.; Tian, G.; et al. Highly Pathogenic Avian Influenza Virus (H5N1) Clade 2.3.4.4b Introduced by Wild Birds, China, 2021. Emerg. Infect. Dis. 2023, 29, 1367–1375. [Google Scholar] [CrossRef]
- Leguía, M.; Garcia-Glaessner, A.; Muñoz-Saavedra, B.; Juárez, D.; Barrera, P.; Calvo-Mac, C.; Jara, J.; Silva, W.; Ploog, K.; Amaro, L.; et al. Highly Pathogenic Avian Influenza A (H5N1) in Marine Mammals and Seabirds in Peru. Nat. Commun. 2023, 14, 5489. [Google Scholar] [CrossRef]
- Ariyama, N.; Pardo-Roa, C.; Muñoz, G.; Aguayo, C.; Ávila, C.; Mathieu, C.; Almonacid, L.; Medina, R.; Brito, B.; Johow, M.; et al. Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4b Virus in Wild Birds, Chile. Emerg. Infect. Dis. 2023, 29, 1842–1845. [Google Scholar] [CrossRef]
- Bevins, S.; Shriner, S.; Cumbee, J.; Dilione, K.; Douglass, K.; Ellis, J.; Killian, M.; Torchetti, M.; Lenoch, J. Intercontinental Movement of Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4 Virus to the United States, 2021. Emerg. Infect. Dis. 2022, 28, 1006–1011. [Google Scholar] [CrossRef]
- El-Shesheny, R.; Moatasim, Y.; Mahmoud, S.; Song, Y.; Taweel, A.; Gomaa, M.; Kamel, M.; Sayes, M.; Kandeil, A.; Lam, T.; et al. Highly Pathogenic Avian Influenza A(H5N1) Virus Clade 2.3.4.4b in Wild Birds and Live Bird Markets, Egypt. Pathogens 2022, 12, 36. [Google Scholar] [CrossRef]
- Hall, V.; Cardona, C.; Mendoza, K.; Torchetti, M.; Lantz, K.; Bueno, I.; Franzen-Klein, D. Surveillance for Highly Pathogenic Avian Influenza A (H5N1) in a Raptor Rehabilitation Center—2022. PLoS ONE 2024, 19, e0299330. [Google Scholar] [CrossRef]
- Jimenez-Bluhm, P.; Siegers, J.; Tan, S.; Sharp, B.; Freiden, P.; Johow, M.; Orozco, K.; Ruiz, S.; Baumberger, C.; Galdames, P.; et al. Detection and Phylogenetic Analysis of Highly Pathogenic A/H5N1 Avian Influenza Clade 2.3.4.4b Virus in Chile, 2022. Emerg. Microbes Infect. 2023, 12, 2220569. [Google Scholar] [CrossRef]
- Puryear, W.; Sawatzki, K.; Hill, N.; Foss, A.; Stone, J.; Doughty, L.; Walk, D.; Gilbert, K.; Murray, M.; Cox, E.; et al. Highly Pathogenic Avian Influenza A(H5N1) Virus Outbreak in New England Seals, United States. Emerg. Infect. Dis. 2023, 29, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Briand, F.; Souchaud, F.; Pierre, I.; Beven, V.; Hirchaud, E.; Hérault, F.; Planel, R.; Rigaudeau, A.; Bernard-Stoecklin, S.; Van Der Werf, S.; et al. Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4b Virus in Domestic Cat, France, 2022. Emerg. Infect. Dis. 2023, 29, 1696–1698. [Google Scholar] [CrossRef]
- Thorsson, E.; Zohari, S.; Roos, A.; Banihashem, F.; Bröjer, C.; Neimanis, A. Highly Pathogenic Avian Influenza A(H5N1) Virus in a Harbor Porpoise, Sweden. Emerg. Infect. Dis. 2023, 29, 852–855. [Google Scholar] [CrossRef]
- WOAH. Avian Influenza (Infection with Avian Influenza Viruses). In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, 8th ed.; World Organisation for Animal Health: Paris, France, 2021; Available online: https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/3.03.04_AI.pdf (accessed on 24 August 2025).
- European Union. Commission Implementing Regulation (EU) 2020/687 of 17 December 2019 Laying Down Rules for the Application of Regulation (EU) 2016/429 of the European Parliament and the Council as Regards the Prevention and Control of Certain Listed Diseases. Off. J. Eur. Union 2020, L174, 64–153. [Google Scholar]
- Autoritatea Națională Sanitară Veterinară și pentru Siguranța Alimentelor (ANSVSA). Ordinul Nr. 145/2018 pentru Aprobarea Normei Sanitare Veterinare Privind Supravegherea, Prevenirea, Controlul și Eradicarea Gripei Aviare. Monitorul Oficial al României 2018, Partea I, Nr. 642/24.VII.2018. Available online: https://legislatie.just.ro/Public/DetaliiDocument/207313 (accessed on 12 December 2024).
- Autoritatea Națională Sanitară Veterinară și pentru Siguranța Alimentelor (ANSVSA). Ordinul Nr. 36/2010 pentru Aprobarea Normei Sanitare Veterinare Privind Notificarea și Raportarea Bolilor la Animale. Monitorul Oficial al României 2010, Partea I, Nr. 223/09.IV.2010. Available online: https://legislatie.just.ro/public/DetaliiDocument/118165 (accessed on 12 December 2024).
- Heine, H.G.; Foord, A.J.; Wang, J.; Valdeter, S.; Walker, S.; Morrissy, C.; Wong, F.Y.; Meehan, B. Detection of Highly Pathogenic Zoonotic Influenza Virus H5N6 by Reverse-Transcriptase Quantitative Polymerase Chain Reaction. Virol. J. 2015, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- EURL (European Union Reference Laboratory for Avian Influenza). EURL Diagnostic Protocols for Avian Influenza Virus Detection and Subtyping; IZSVe: Legnaro, Italy, 2022; Available online: https://www.izsvenezie.com/reference-laboratories/avian-influenza-newcastle-disease/diagnostic-protocols/ (accessed on 8 December 2024).
- Van der Goot, J.A.; Koch, G.; De Jong, M.C.; Van Boven, M. Transmission of Highly Pathogenic Avian Influenza H5N1 Virus in Pekin Ducks Is Significantly Reduced by a Genetically Distant H5N2 Vaccine. Virology 2005, 339, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Slomka, M.J.; Coward, V.J.; Banks, J.; Löndt, B.Z.; Brown, I.H.; Voermans, J.; Koch, G.; Handberg, K.J.; Jørgensen, P.H.; Cherbonnel-Pansart, M.; et al. Validated H5 Eurasian Real-Time Reverse Transcriptase Polymerase Chain Reaction and Its Application in H5N1 Outbreaks in 2005–2006. Avian Dis. 2007, 51 (Suppl. S1), 373–377. [Google Scholar] [CrossRef]
- Hassan, K.E.; Balz, K.; Tuppurainen, E.; El Zowalaty, M.E.; Ulrich, R.; Beer, M.; Hoffmann, B. Improved Subtyping of Avian Influenza Viruses Using an RT-qPCR Based Low Density Array: Riems Influenza A Typing Array (RITA 2). Viruses 2022, 14, 415. [Google Scholar] [CrossRef]
- Lee, D.H.; Bertran, K.; Kwon, J.H.; Swayne, D.E. Evolution, Global Spread, and Pathogenicity of Highly Pathogenic Avian Influenza H5Nx Clade 2.3.4.4. J. Vet. Sci. 2017, 18 (Suppl. S1), 269–280. [Google Scholar] [CrossRef] [PubMed]
- Adlhoch, C.; Fusaro, A.; Kuiken, T.; Niqueux, É.; Staubach, C.; Terregino, C.; Aznar, I.; Guajardo, I.M.; Baldinelli, F. Avian Influenza Overview December 2022–March 2023. EFSA J. 2023, 21, e07976. [Google Scholar] [CrossRef]
- Gupta, S.; Hoque, M.; Fournié, G.; Henning, J. Patterns of Avian Influenza A (H5) and A (H9) Virus Infection in Backyard, Commercial Broiler and Layer Chicken Farms in Bangladesh. Transbound. Emerg. Dis. 2020, 68, 137–151. [Google Scholar] [CrossRef]
- Rehman, S.; Effendi, M.; Witaningruma, A.; Nnabuike, U.; Bilal, M.; Abbas, A.; Abbas, R.; Hussain, K. Avian Influenza (H5N1) Virus, Epidemiology and Its Effects on Backyard Poultry in Indonesia: A Review. F1000Research 2022, 11, 12878. [Google Scholar] [CrossRef]
- Hidayat, M.; Dewi, A.; Schoonman, L.; Wibawa, H.; Lubis, E.; Lockhart, C.; Setiaji, G.; McGrane, J.; Vink, W. Investigating the Endemic Presence and Persistence of HPAI H5N1 Virus on Java, Indonesia. Authorea 2020. [Google Scholar] [CrossRef]
- Ward, M.; Maftei, D.; Apostu, C.; Suru, A. Association between outbreaks of highly pathogenic avian influenza subtype H5N1 and migratory waterfowl (family Anatidae) populations. Zoonoses Public Health 2009, 56, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Stoimenov, G.; Goujgoulova, G.; Hristov, K. Analysis of a highly pathogenic avian influenza (H5N1) virus causing the first outbreak in domestic poultry in Bulgaria in January 2015. Vet. Med. 2020, 65, 435–444. [Google Scholar] [CrossRef]
- Lewis, N.; Banyard, A.; Whittard, E.; Karibayev, T.; Kafagi, T.; Chvala, I.; Byrne, A.; Akberovna, S.; King, J.; Harder, T.; et al. Emergence and spread of novel H5N8, H5N5 and H5N1 clade 2.3.4.4 highly pathogenic avian influenza in 2020. Emerg. Microbes Infect. 2021, 10, 148–151. [Google Scholar] [CrossRef]
- Li, Y.; An, Q.; Sun, Z.; Gao, X.; Wang, H. Multifaceted analysis of temporal and spatial distribution and risk factors of global poultry HPAI-H5N1, 2005–2023. Animals 2024, 18, 3. [Google Scholar] [CrossRef]
- Adlhoch, C.; Fusaro, A.; Gonzáles, J.; Kuiken, T.; Marangon, S.; Niqueux, E.; Staubach, C.; Terregino, C.; Aznar, I.; Guajardo, I.; et al. Avian influenza overview May–September 2021. EFSA J. 2022, 20, e07018. [Google Scholar] [CrossRef]
- Adlhoch, C.; Fusaro, A.; Gonzáles, J.; Kuiken, T.; Marangon, S.; Niqueux, E.; Staubach, C.; Terregino, C.; Aznar, I.; Guajardo, I.; et al. Avian influenza overview September–December 2021. EFSA J. 2021, 19, e07108. [Google Scholar] [CrossRef]
- Adlhoch, C.; Fusaro, A.; Gonzáles, J.; Kuiken, T.; Marangon, S.; Niqueux, E.; Staubach, C.; Terregino, C.; Aznar, I.; Guajardo, I.; et al. Avian influenza overview March–June 2022. EFSA J. 2022, 20, e07415. [Google Scholar] [CrossRef] [PubMed]
- Adlhoch, C.; Fusaro, A.; Gonzáles, J.; Kuiken, T.; Marangon, S.; Niqueux, E.; Staubach, C.; Terregino, C.; Aznar, I.; Guajardo, I.; et al. Avian influenza overview December 2021–March 2022. EFSA J. 2022, 20, e07289. [Google Scholar] [CrossRef]
- Adlhoch, C.; Fusaro, A.; Gonzáles, J.; Kuiken, T.; Marangon, S.; Niqueux, E.; Staubach, C.; Terregino, C.; Guajardo, I.; Chuzhakina, K.; et al. Avian influenza overview June–September 2022. EFSA J. 2022, 20, e07589. [Google Scholar] [CrossRef]
- Fusaro, A.; Gonzales, J.; Kuiken, T.; Mirinaviciute, G.; Niqueux, E.; Ståhl, K.; Staubach, C.; Svartström, O.; Terregino, C.; Willgert, K.; et al. Avian influenza overview December 2023–March 2024. EFSA J. 2024, 22, e8754. [Google Scholar] [CrossRef] [PubMed]
- Verhagen, J.; Fouchier, R.; Lewis, N. Highly pathogenic avian influenza viruses at the wild–domestic bird interface in Europe: Future directions for research and surveillance. Viruses 2021, 13, 212. [Google Scholar] [CrossRef]
- Gurău, M.R.; Oțelea, F.; Negru, E.; Șonea, C.; Beșleagă, S.; Bărbucianu, F.; Herman, V.; Iancu, I.; Baraitareanu, S.; Daneș, D. PCR method and Sanger sequencing for PB2 fragment detection in influenza virus strains from Romania. Rev. Rom. Med. Vet. 2024, 34, 86–90. [Google Scholar]
- Adlhoch, C.; Fusaro, A.; Gonzales, J.; Kuiken, T.; Mirinaviciute, G.; Niqueux, E.; Ståhl, K.; Staubach, C.; Terregino, C.; Willgert, K.; et al. Avian influenza overview September–December 2023. EFSA J. 2023, 21, e8539. [Google Scholar] [CrossRef]
- Van Borm, S.; Ahrens, A.; Bachofen, C.; Banyard, A.; Bøe, C.; Briand, F.; Dirbáková, Z.; Engelsma, M.; Fusaro, A.; Germeraad, E.; et al. Genesis and spread of novel highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus genotype EA-2023-DG reassortant, Western Europe. Emerg. Infect. Dis. 2025, 31, 401–412. [Google Scholar] [CrossRef]
- King, J.; Harder, T.; Globig, A.; Stacker, L.; Günther, A.; Grund, C.; Beer, M.; Pohlmann, A. Highly pathogenic avian influenza virus incursions of subtype H5N8, H5N5, H5N1, H5N4, and H5N3 in Germany during 2020–21. Virus Evol. 2022, 8, veac035. [Google Scholar] [CrossRef] [PubMed]
- Adlhoch, C.; Fusaro, A.; Gonzáles, J.; Kuiken, T.; Marangon, S.; Niqueux, E.; Staubach, C.; Terregino, C.; Baldinelli, F. Avian influenza overview August–December 2020. EFSA J. 2020, 18, e06379. [Google Scholar] [CrossRef] [PubMed]
- Iancu, I.; Tirziu, E.; Pascu, C.; Costinar, L.; Degi, J.; Badea, C.; Gligor, A.; Bucur, I.; Popa, S.A.; Herman, V. Evolution of HPAI avian influenza virus strains in Europe between 2005 and 2023. Rev. Rom. Med. Vet. 2024, 34, 138–144. [Google Scholar]
- Rosone, F.; Bonfante, F.; Sala, M.; Maniero, S.; Cersini, A.; Ricci, I.; Garofalo, L.; Caciolo, D.; Denisi, A.; Napolitan, A.; et al. Seroconversion of a swine herd in a free-range rural multi-species farm against HPAI H5N1 2.3.4.4b clade virus. Microorganisms 2023, 11, 1162. [Google Scholar] [CrossRef] [PubMed]
| Sample ID | Species | Matrix Gene | H5 | N1 | H7 | Virus Isolation | HA Cleavage Site | Pathotype |
|---|---|---|---|---|---|---|---|---|
| 16890-1 | Chicken | Positive | + | + | – | Positive | PLREKRRKR/GLFG | HPAI |
| 16890-2 | Chicken | Positive | + | + | – | Positive | PLREKRRKR/GLFG | HPAI |
| 16890-3 | Chicken | Positive | + | + | – | Positive | PLREKRRKR/GLFG | HPAI |
| 16890-4 | Chicken | Positive | + | + | – | Positive | PLREKRRKR/GLFG | HPAI |
| 16890-5 | Chicken | Positive | + | + | – | Positive | PLREKRRKR/GLFG | HPAI |
| 16890-6 | Chicken | Positive | + | + | – | Positive | PLREKRRKR/GLFG | HPAI |
| 16890-7 | Chicken | Positive | + | + | – | Positive | PLREKRRKR/GLFG | HPAI |
| 16890-8 | Chicken | Positive | + | + | – | Positive | PLREKRRKR/GLFG | HPAI |
| 16889-1 | Domestic goose | Positive | + | + | – | Positive | PLREKRRKR/GLFG | HPAI |
| 16889-2 | Domestic goose | Positive | + | + | – | Positive | PLREKRRKR/GLFG | HPAI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iancu, I.; Bărbuceanu, F.; Tîrziu, E.; Pascu, C.; Costinar, L.; Degi, J.; Badea, C.; Gligor, A.; Bucur, I.; Popa, S.A.; et al. Determination of H5N1 Avian Influenza Virus Persistence Following a 2024 Backyard Poultry Outbreak in Romania. Vet. Sci. 2025, 12, 922. https://doi.org/10.3390/vetsci12100922
Iancu I, Bărbuceanu F, Tîrziu E, Pascu C, Costinar L, Degi J, Badea C, Gligor A, Bucur I, Popa SA, et al. Determination of H5N1 Avian Influenza Virus Persistence Following a 2024 Backyard Poultry Outbreak in Romania. Veterinary Sciences. 2025; 12(10):922. https://doi.org/10.3390/vetsci12100922
Chicago/Turabian StyleIancu, Ionica, Florica Bărbuceanu, Emil Tîrziu, Corina Pascu, Luminița Costinar, Janos Degi, Corina Badea, Alexandru Gligor, Iulia Bucur, Sebastian Alexandru Popa, and et al. 2025. "Determination of H5N1 Avian Influenza Virus Persistence Following a 2024 Backyard Poultry Outbreak in Romania" Veterinary Sciences 12, no. 10: 922. https://doi.org/10.3390/vetsci12100922
APA StyleIancu, I., Bărbuceanu, F., Tîrziu, E., Pascu, C., Costinar, L., Degi, J., Badea, C., Gligor, A., Bucur, I., Popa, S. A., Gurau, M., & Herman, V. (2025). Determination of H5N1 Avian Influenza Virus Persistence Following a 2024 Backyard Poultry Outbreak in Romania. Veterinary Sciences, 12(10), 922. https://doi.org/10.3390/vetsci12100922













