Doxorubicin–Cyclophosphamide Protocol in Dogs with Splenic Haemangiosarcoma and Haemoabdomen: A Retrospective Case Series
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Eligibility Criteria and Case Definitions
2.3. Pre-Operative Stabilisation and Staging
2.4. Surgical Procedure
2.5. Chemotherapy Protocol (Anthracycline–Alkylator)
2.6. Maintenance Metronomic Therapy
2.7. Data Collection
2.8. Outcomes
2.9. Adverse Events and Grading
2.10. Statistical Analysis
3. Results
3.1. Baseline Cohort Characteristics
3.2. Peri-Operative Events and Treatment Delivery
3.3. Chemotherapy-Related Adverse Events (VCOG-CTCAE v2)
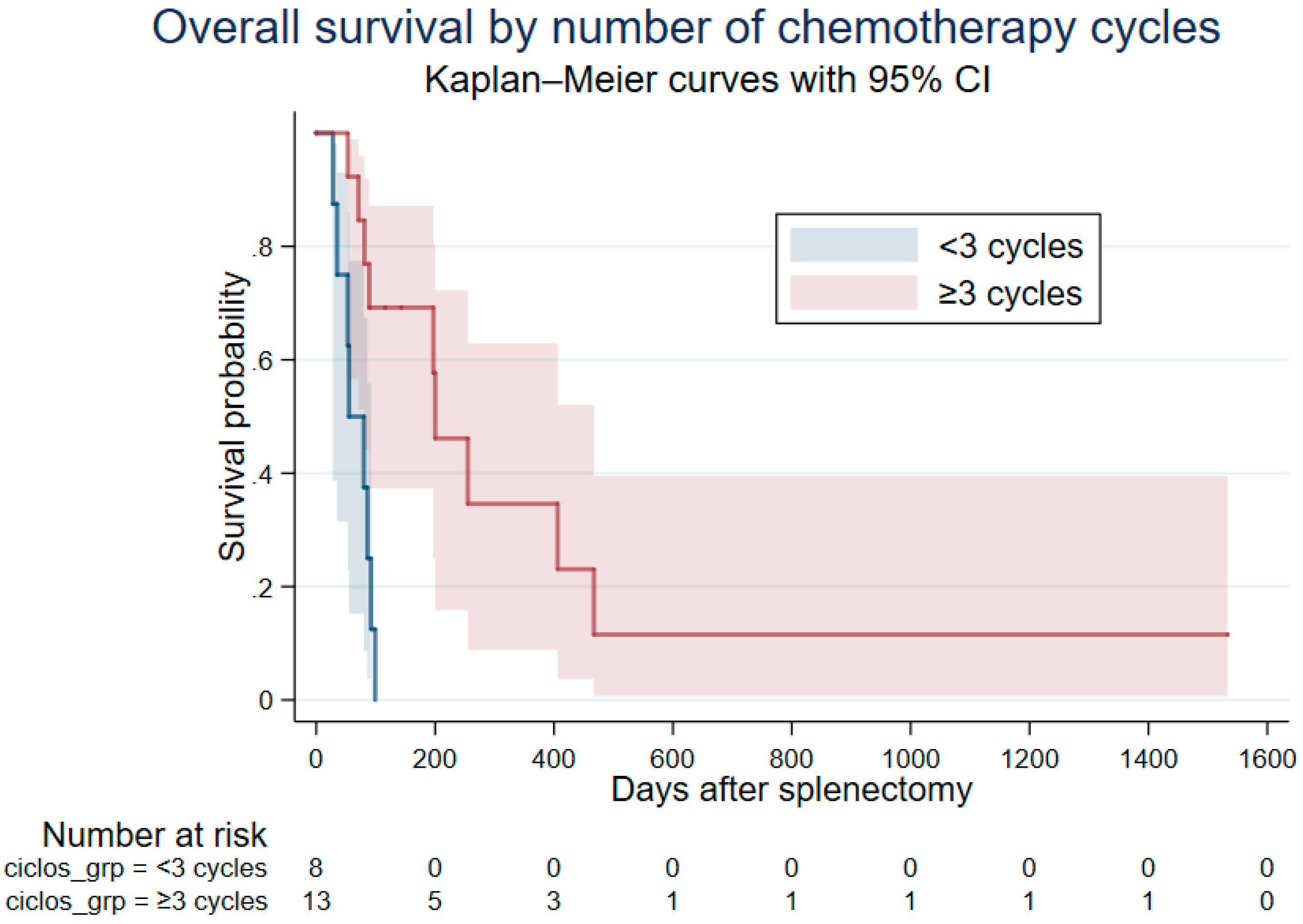
3.4. Survival and Time-Aware Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HSA | Haemangiosarcoma |
| VAC | Vincristine–doxorubicin–cyclophosphamide |
| AC | Doxorubicin–cyclophosphamide |
References
- Wong, R.W.; Gonsalves, M.N.; Huber, M.L.; Rich, L.; Strom, A. Erythrocyte and Biochemical Abnormalities as Diagnostic Markers in Dogs With Hemangiosarcoma Related Hemoabdomen. Vet. Surg. 2015, 44, 852–857. [Google Scholar] [CrossRef]
- Aronsohn, M.G.; Dubiel, B.; Roberts, B.; Powers, B.E. Prognosis for Acute Nontraumatic Hemoperitoneum in the Dog: A Retrospective Analysis of 60 Cases (2003–2006). J. Am. Anim. Hosp. Assoc. 2009, 45, 72–77. [Google Scholar] [CrossRef]
- Hammer, A.S.; Couto, C.G.; Filppi, J.; Getzy, D.; Shank, K. Efficacy and Toxicity of VAC Chemotherapy (Vincristine, Doxorubicin, and Cyclophosphamide) in Dogs with Hemangiosarcoma. J. Vet. Intern. Med. 1991, 5, 160–166. [Google Scholar] [CrossRef]
- Sorenmo, K.U.; Jeglum, K.A.; Helfand, S.C. Chemotherapy of Canine Hemangiosarcoma with Doxorubicin and Cyclophosphamide. J. Vet. Intern. Med. 1993, 7, 370–376. [Google Scholar] [CrossRef]
- Hillman, A.; Swafford, B.; Delavenne, C.; Fieten, H.; Boerkamp, K.; Tietje, K. Descriptive Analysis of Haemangiosarcoma Occurrence in Dogs Enrolled in the Golden Retriever Lifetime Study. Vet. Comp. Oncol. 2023, 21, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Pintar, J.; Breitschwerdt, E.B.; Hardie, E.M.; Spaulding, K.A. Acute Nontraumatic Hemoabdomen in the Dog: A Retrospective Analysis of 39 Cases (1987–2001). J. Am. Anim. Hosp. Assoc. 2003, 39, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Menard, J.V.; Sylvester, S.R.; Lopez, D.J. Assessing Major Influences on Decision-Making and Outcome for Dogs Presenting Emergently with Nontraumatic Hemoabdomen. J. Am. Vet. Med. Assoc. 2023, 261, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Batschinski, K.; Nobre, A.; Vargas-Mendez, E.; Tedardi, M.V.; Cirillo, J.; Cestari, G.; Ubukata, R.; Dagli, M.L.Z. Canine Visceral Hemangiosarcoma Treated with Surgery Alone or Surgery and Doxorubicin: 37 Cases (2005–2014). Can. Vet. J. 2018, 59, 967–972. [Google Scholar]
- Alvarez, F.J.; Hosoya, K.; Lara-Garcia, A.; Kisseberth, W.; Couto, G. VAC Protocol for Treatment of Dogs with Stage III Hemangiosarcoma. J. Am. Anim. Hosp. Assoc. 2013, 49, 370–377. [Google Scholar] [CrossRef]
- Wendelburg, K.M.; Price, L.L.; Burgess, K.E.; Lyons, J.A.; Lew, F.H.; Berg, J. Survival Time of Dogs with Splenic Hemangiosarcoma Treated by Splenectomy with or without Adjuvant Chemotherapy: 208 Cases (2001–2012). J. Am. Vet. Med. Assoc. 2015, 247, 393–403. [Google Scholar] [CrossRef]
- Finotello, R.; Stefanello, D.; Zini, E.; Marconato, L. Comparison of Doxorubicin-Cyclophosphamide with Doxorubicin-Dacarbazine for the Adjuvant Treatment of Canine Hemangiosarcoma. Vet. Comp. Oncol. 2017, 15, 25–35. [Google Scholar] [CrossRef]
- Faroni, E.; Sabattini, S.; Guerra, D.; Iannuzzi, C.; Chalfon, C.; Agnoli, C.; Stefanello, D.; Polton, G.; Ramos, S.; Aralla, M.; et al. Timely Adjuvant Chemotherapy Improves Outcome in Dogs with Non-Metastatic Splenic Hemangiosarcoma Undergoing Splenectomy. Vet. Comp. Oncol. 2023, 21, 123–130. [Google Scholar] [CrossRef]
- Matsuyama, A.; Woods, J.P.; Mutsaers, A.J. Evaluation of Toxicity of a Chronic Alternate Day Metronomic Cyclophosphamide Chemotherapy Protocol in Dogs with Naturally Occurring Cancer. Can. Vet. J. 2017, 58, 51–55. [Google Scholar]
- Treggiari, E.; Borrego, J.F.; Gramer, I.; Valenti, P.; Harper, A.; Finotello, R.; Toni, C.; Laomedonte, P.; Romanelli, G. Retrospective Comparison of First-Line Adjuvant Anthracycline vs Metronomic-Based Chemotherapy Protocols in the Treatment of Stage I and II Canine Splenic Haemangiosarcoma. Vet. Comp. Oncol. 2020, 18, 43–51. [Google Scholar] [CrossRef]
- Petrucci, G.N.; Magalhães, T.R.; Dias, M.; Queiroga, F.L. Metronomic Chemotherapy: Bridging Theory to Clinical Application in Canine and Feline Oncology. Front. Vet. Sci. 2024, 11, 1397376. [Google Scholar] [CrossRef]
- Lana, S.; U’ren, L.; Plaza, S.; Elmslie, R.; Gustafson, D.; Morley, P.; Dow, S. Continuous Low-Dose Oral Chemotherapy for Adjuvant Therapy of Splenic Hemangiosarcoma in Dogs. J. Vet. Intern. Med. 2007, 21, 764–769. [Google Scholar] [CrossRef]
- Suissa, S. Immortal Time Bias in Pharmaco-Epidemiology. Am. J. Epidemiol. 2008, 167, 492–499. [Google Scholar] [CrossRef]
- Lévesque, L.E.; Hanley, J.A.; Kezouh, A.; Suissa, S. Problem of Immortal Time Bias in Cohort Studies: Example Using Statins for Preventing Progression of Diabetes. BMJ 2010, 340, b5087. [Google Scholar] [CrossRef]
- De Nardi, A.B.; de Oliveira Massoco Salles Gomes, C.; Fonseca-Alves, C.E.; de Paiva, F.N.; Linhares, L.C.M.; Carra, G.J.U.; dos Santos Horta, R.; Ruiz Sueiro, F.A.; Jark, P.C.; Nishiya, A.T.; et al. Diagnosis, Prognosis, and Treatment of Canine Hemangiosarcoma: A Review Based on a Consensus Organized by the Brazilian Association of Veterinary Oncology, ABROVET. Cancers 2023, 15, 2025. [Google Scholar] [CrossRef]
- Mullin, C.; Clifford, C.A. Miscellaneous Tumours: Hemangiosarcoma. In Withrow and MacEwen’s Small Animal Clinical Oncology; Withrow, S.J., Vail, D.M., Page, R.L., Eds.; Elsevier Saunders: St. Louis, MO, USA, 2020; pp. 773–778. [Google Scholar]
- LeBlanc, A.K.; Atherton, M.; Bentley, R.T.; Boudreau, C.E.; Burton, J.H.; Curran, K.M.; Dow, S.; Giuffrida, M.A.; Kellihan, H.B.; Mason, N.J.; et al. Veterinary Cooperative Oncology Group—Common Terminology Criteria for Adverse Events (VCOG-CTCAE v2) Following Investigational Therapy in Dogs and Cats. Vet. Comp. Oncol. 2021, 19, 311–352. [Google Scholar] [CrossRef]
- Corvera, G.; Alegría-Morán, R.; Cifuentes, F.F.; Torres, C.G. Pathological Characterization and Risk Factors of Splenic Nodular Lesions in Dogs (Canis lupus familiaris). Animals 2024, 14, 802. [Google Scholar] [CrossRef]
- Stewart, S.D.; Ehrhart, E.J.; Davies, R.; Khanna, C. Prospective Observational Study of Dogs with Splenic Mass Rupture Suggests Potentially Lower Risk of Malignancy and More Favourable Perioperative Outcomes. Vet. Comp. Oncol. 2020, 18, 811–817. [Google Scholar] [CrossRef]
- Kelley, M.; Sinnott-Stutzman, V.; Whelan, M. Retrospective Analysis of the Use of Tranexamic Acid in Critically Ill Dogs and Cats (2018–2019): 266 Dogs and 28 Cats. J. Vet. Emerg. Crit. Care 2022, 32, 791–799. [Google Scholar] [CrossRef]
- Faulhaber, E.A.; Janik, E.; Thamm, D.H. Adjuvant Carboplatin for Treatment of Splenic Hemangiosarcoma in Dogs: Retrospective Evaluation of 18 Cases (2011–2016) and Comparison with Doxorubicin-Based Chemotherapy. J. Vet. Intern. Med. 2021, 35, 1929–1934. [Google Scholar] [CrossRef]
- Ziogaite, B.; Contreras, E.T.; Horgan, J.E. Incidence of Splenic Malignancy and Hemangiosarcoma in Dogs Undergoing Splenectomy Surgery at a Surgical Specialty Clinic: 182 Cases (2017–2021). PLoS ONE 2024, 19, e0314737. [Google Scholar] [CrossRef]
- Ruffoni, E.; Stewart, S.; Khanna, C.; Thomson, C.; Tougas, G.; Fenger, J.; Wilson-Robles, H.; Cawley, J. A Prospective Observational Study of 345 Canines with Ruptured Splenic Tumors Suggests Benign Lesions Are More Common than Previously Reported. J. Am. Vet. Med. Assoc. 2025, 263, 985–990. [Google Scholar] [CrossRef]
- Swanton, J.C.; Coleman, K.A.; Baldeon, M.A. The Association between the Number and Location of Splenic Masses and Malignancy: A Retrospective Study of 436 Canine Patients Undergoing Splenectomy. J. Am. Vet. Med. Assoc. 2025, 1, 1–7. [Google Scholar] [CrossRef]
- Millar, S.L.; Curley, T.L.; Monnet, E.L.; Zersen, K.M. Premature Death in Dogs with Nontraumatic Hemoabdomen and Splenectomy with Benign Histopathologic Findings. J. Am. Vet. Med. Assoc. 2021, 260, S9–S14. [Google Scholar] [CrossRef]
- Marconato, L.; Chalfon, C.; Finotello, R.; Polton, G.; Vasconi, M.E.; Annoni, M.; Stefanello, D.; Mesto, P.; Capitani, O.; Agnoli, C.; et al. Adjuvant Anthracycline-Based vs. Metronomic Chemotherapy vs. No Medical Treatment for Dogs with Metastatic Splenic Hemangiosarcoma: A Multi-Institutional Retrospective Study of the Italian Society of Veterinary Oncology. Vet. Comp. Oncol. 2019, 17, 537–544. [Google Scholar] [CrossRef]
- Alexander, C.K.; Cronin, K.L.; Silver, M.; Gardner, H.L.; London, C. The Addition of Metronomic Chemotherapy Does Not Improve Outcome for Canine Splenic Haemangiosarcoma. J. Small Anim. Pract. 2019, 60, 32–37. [Google Scholar] [CrossRef]
| Variable | n (%) |
|---|---|
| Sex | |
| Male | 10 (47.6%) |
| Female | 11 (52.4%) |
| Neuter status | |
| Neutered | 14 (66.7%) |
| Intact | 7 (33.3%) |
| Breed (most common) | |
| Mixed breed | 7 (33.3%) |
| Labrador Retriever | 5 (23.8%) |
| German Shepherd | 3 (14.3%) |
| Median age (years) | 10.2 (8.7–12.0) * |
| Median weight (kg) | 28.8 (24.8–37.2) * |
| Anaemia (Hct < 37%) | |
| Yes | 13 (68.4%) |
| No | 6 (31.6%) |
| Thrombocytopenia (<186 × 103/µL) | |
| Yes | 11 (57.9%) |
| No | 8 (42.1%) |
| Intraoperative complications | |
| Yes | 9 (42.9%) |
| No | 12 (57.1%) |
| Postoperative complications | |
| Yes | 0 (0%) |
| No | 21 (100%) |
| Metronomic chemotherapy | |
| Yes | 4 (19.0%) |
| No | 17 (81.0%) |
| System | Adverse Event | Grade 1 n (%) | Grade 2 n (%) | Grade 3–4 n (%) | Any Grade n (%) |
|---|---|---|---|---|---|
| Haematological | Neutropenia | 1 (4.8) | 0 | 1 (4.8) | 2 (9.5) |
| Gastrointestinal | Diarrhoea | 2 (9.5) | 0 | 0 | 2 (9.5) |
| Constitutional | Anorexia/Inappetence | 0 | 1 (4.8) | 0 | 1 (4.8) |
| Summary | Dogs with ≥1 adverse event | — | — | 1 (4.8) | 5 (23.8) |
| Variable | Scale/Contrast | HR (95% CI) | p-Value |
|---|---|---|---|
| Chemotherapy cycles | Per additional cycle | 0.60 (0.46–0.78) | <0.001 |
| Metronomic therapy † | Yes vs. No | 0.15 (0.03–0.78) | 0.024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
del Castillo, N.; Fuertes-Recuero, M.; De Angelis, E.; de la Riva, C.; García, C.; Rayón, N.; Márquez, S.; Ortiz-Díez, G. Doxorubicin–Cyclophosphamide Protocol in Dogs with Splenic Haemangiosarcoma and Haemoabdomen: A Retrospective Case Series. Vet. Sci. 2025, 12, 1053. https://doi.org/10.3390/vetsci12111053
del Castillo N, Fuertes-Recuero M, De Angelis E, de la Riva C, García C, Rayón N, Márquez S, Ortiz-Díez G. Doxorubicin–Cyclophosphamide Protocol in Dogs with Splenic Haemangiosarcoma and Haemoabdomen: A Retrospective Case Series. Veterinary Sciences. 2025; 12(11):1053. https://doi.org/10.3390/vetsci12111053
Chicago/Turabian Styledel Castillo, Noemí, Manuel Fuertes-Recuero, Elisabetta De Angelis, Claudia de la Riva, Cristina García, Noemí Rayón, Sandra Márquez, and Gustavo Ortiz-Díez. 2025. "Doxorubicin–Cyclophosphamide Protocol in Dogs with Splenic Haemangiosarcoma and Haemoabdomen: A Retrospective Case Series" Veterinary Sciences 12, no. 11: 1053. https://doi.org/10.3390/vetsci12111053
APA Styledel Castillo, N., Fuertes-Recuero, M., De Angelis, E., de la Riva, C., García, C., Rayón, N., Márquez, S., & Ortiz-Díez, G. (2025). Doxorubicin–Cyclophosphamide Protocol in Dogs with Splenic Haemangiosarcoma and Haemoabdomen: A Retrospective Case Series. Veterinary Sciences, 12(11), 1053. https://doi.org/10.3390/vetsci12111053







