Effects of Different Diluents and Freezing Methods on Cryopreservation of Hu Ram Semen
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Animals and Semen Collection
2.3. Preparation of Semen Diluents
2.4. Cryopreservation and Thawing of Sperm
2.5. Evaluation of Sperm Motility and Biokinetic Characteristics
2.6. Evaluation of Sperm Membrane Integrity
2.7. Evaluation of Sperm Acrosome Integrity
2.8. Evaluation of Sperm ROS Level
2.9. Statistical Analysis
3. Results
3.1. Effect of Different Diluents on Sperm Motility and Biokinetic Characteristics during Cryopreservation
3.2. Effect of Different Diluents on Sperm Membrane Integrity during Cryopreservation
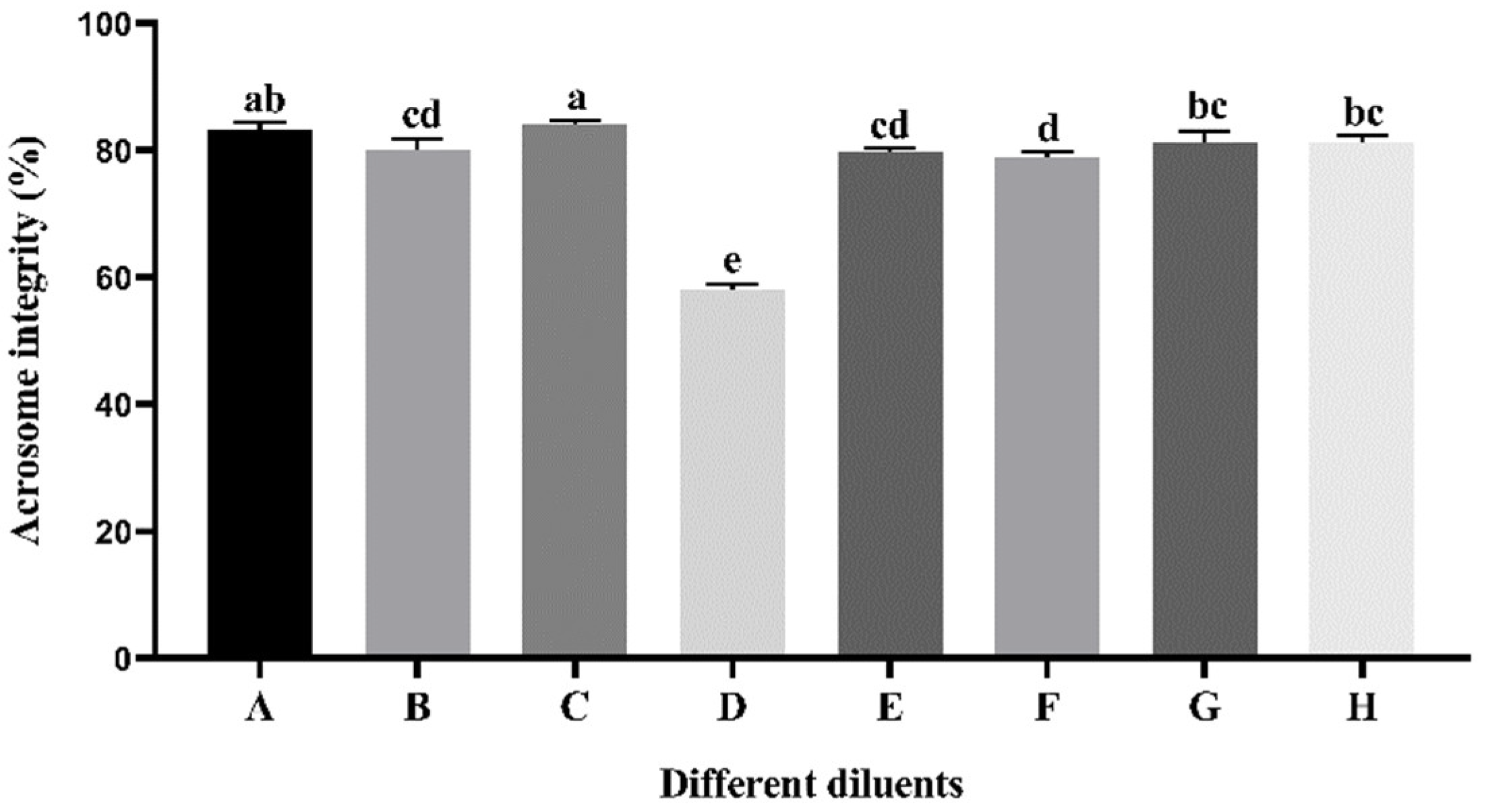
3.3. Effect of Different Diluents on Sperm Acrosome Integrity during Cryopreservation
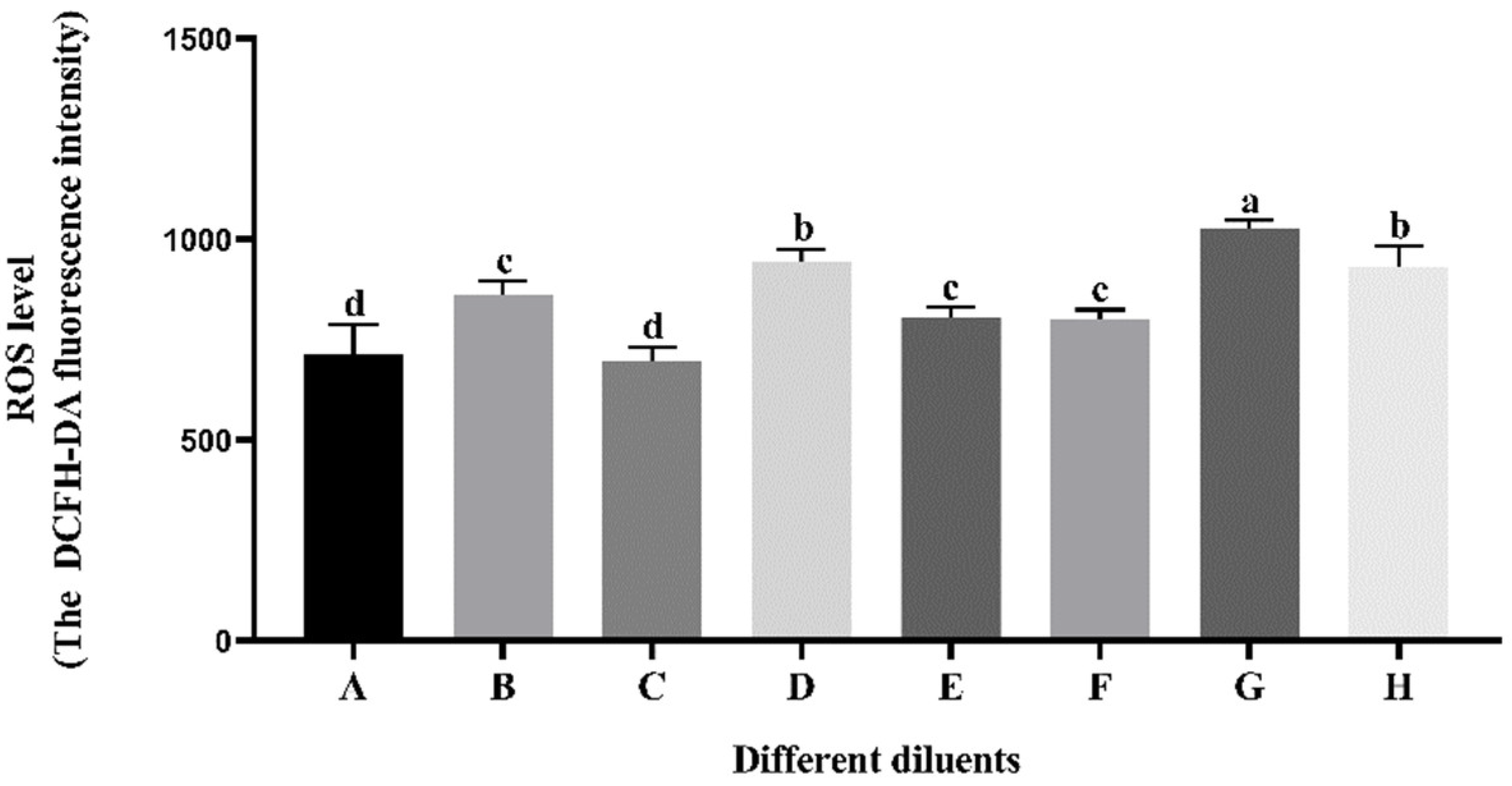
3.4. Effect of Different Diluents on Sperm ROS Level during Cryopreservation
3.5. Effect of Different Freezing Methods on Sperm Motility and Biokinetic Characteristics during Cryopreservation
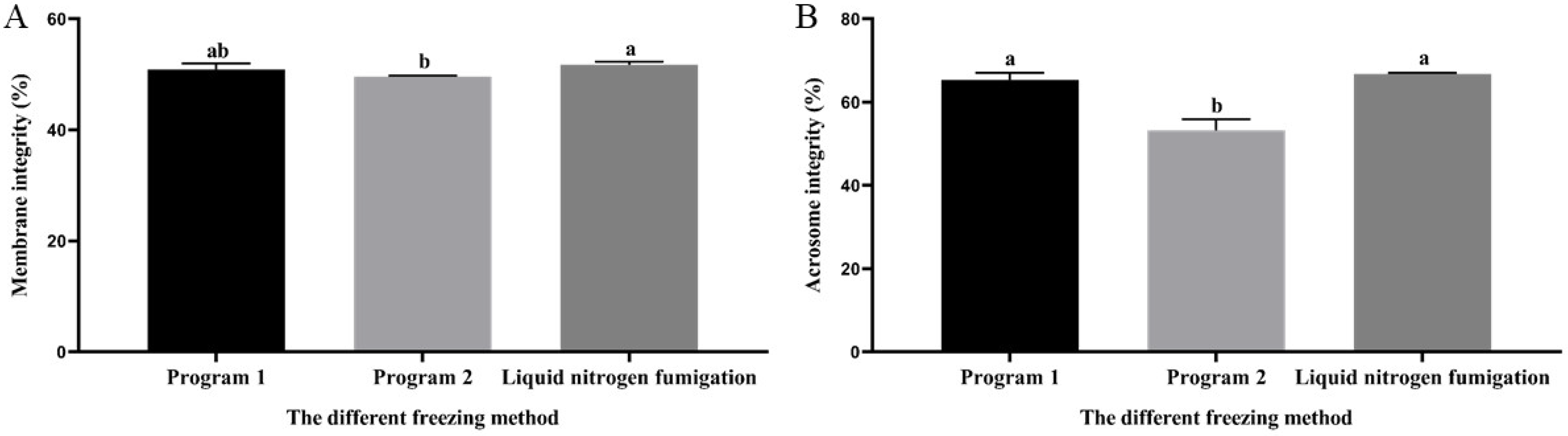
3.6. Effect of Different Freezing Methods on Sperm Membrane and Acrosome Integrity during Cryopreservation
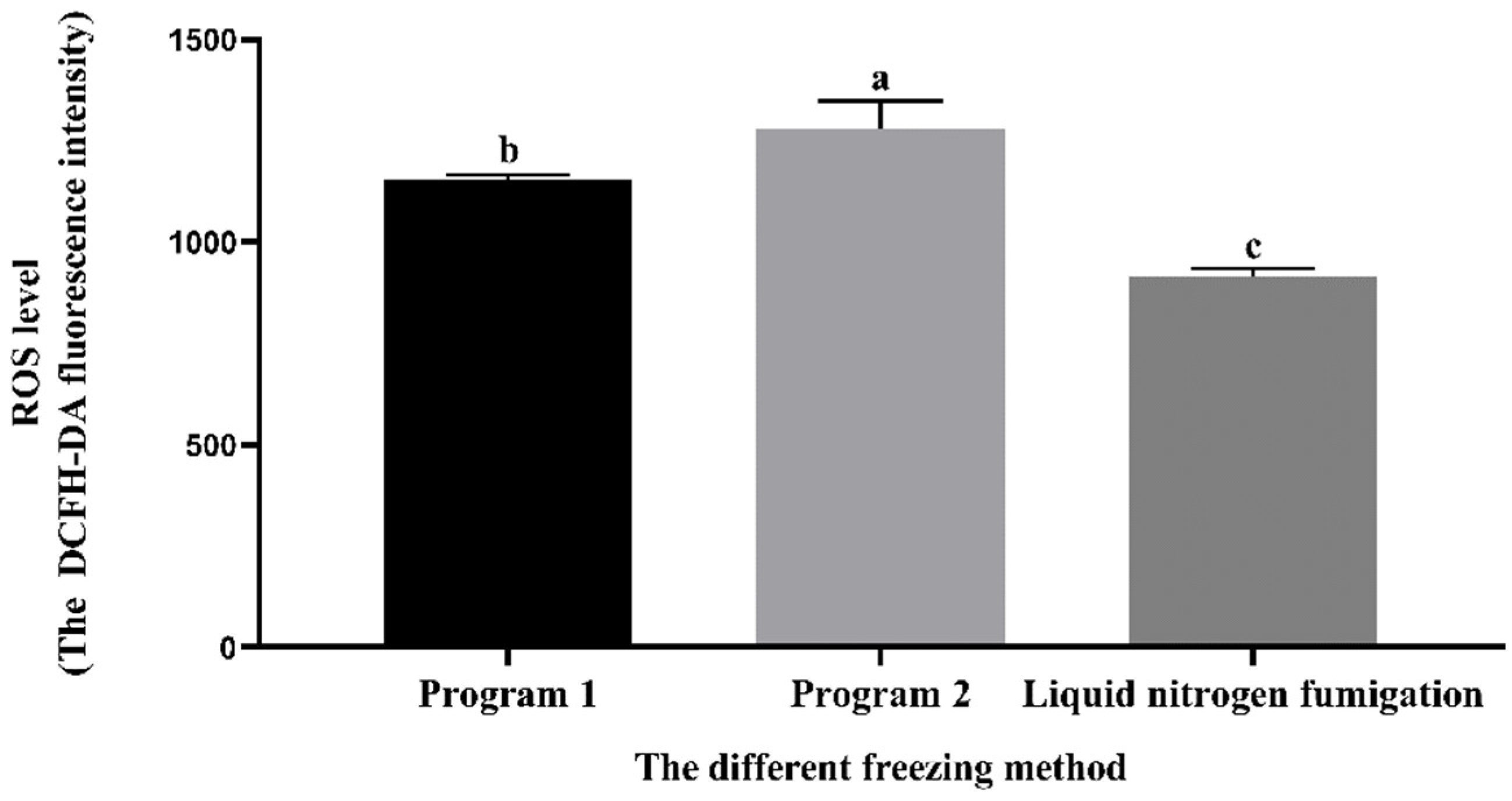
3.7. Effect of Different Freezing Methods on the Sperm ROS Level during Cryopreservation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Chen, Z.; Fang, Y.; Cao, C.; Zhang, Z.; Pan, Y.; Wang, Q. Runs of Homozygosity Revealed Reproductive Traits of Hu Sheep. Genes 2022, 13, 1848. [Google Scholar] [CrossRef]
- Chen, T.; Wang, L.; Li, Q.; Long, Y.; Lin, Y.; Yin, J.; Zeng, Y.; Huang, L.; Yao, T.; Abbasi, M.N.; et al. Functional Probiotics of Lactic Acid Bacteria from Hu Sheep Milk. BMC Microbiol. 2020, 20, 228. [Google Scholar] [CrossRef]
- Li, J.; Tang, C.; Yang, Y.; Hu, Y.; Zhao, Q.; Ma, Q.; Yue, X.; Li, F.; Zhang, J. Characterization of Meat Quality Traits, Fatty Acids and Volatile Compounds in Hu and Tan Sheep. Front. Nutr. 2023, 10, 1072159. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Q.; Wang, Y.; Bao, M.; Sun, X.; Li, Y. Changes in Meat of Hu Sheep during Postmortem Aging Based on ACQUITY UPLC I-Class Plus/VION IMS QTof. Foods 2024, 13, 174. [Google Scholar] [CrossRef] [PubMed]
- Skidmore, J.A.; Malo, C.M.; Crichton, E.G.; Morrell, J.M.; Pukazhenthi, B.S. An Update on Semen Collection, Preservation and Artificial Insemination in the Dromedary Camel (Camelus dromedarius). Anim. Reprod. Sci. 2018, 194, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Oldenhof, H.; Wolkers, W.F.; Sieme, H. Cryopreservation of Semen from Domestic Livestock: Bovine, Equine, and Porcine Sperm. Methods. Mol. Biol. 2021, 2180, 365–377. [Google Scholar] [CrossRef]
- Kalwar, Q.; Chu, M.; Korejo, R.A.; Soomro, H.; Yan, P. Cryopreservation of Yak Semen: A Comprehensive Review. Animals 2022, 12, 3451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, X.; Sohail, T.; Jiang, C.; Sun, Y.; Wang, J.; Sun, X.; Li, Y. Punicalagin Protects Ram Sperm from Oxidative Stress by Enhancing Antioxidant Capacity and Mitochondrial Potential During Liquid Storage at 4 °C. Animals 2024, 14, 318. [Google Scholar] [CrossRef]
- Zou, J.; Wei, L.; Li, D.; Zhang, Y.; Wang, G.; Zhang, L.; Cao, P.; Yang, S.; Li, G. Effect of Glutathione on Sperm Quality in Guanzhong Dairy Goat Sperm During Cryopreservation. Front. Vet. Sci. 2021, 8, 771440. [Google Scholar] [CrossRef]
- Pontbriand, D.; Howard, J.G.; Schiewe, M.C.; Stuart, L.D.; Wildt, D.E. Effect of Cryoprotective Diluent and Method of Freeze-thawing on Survival and Acrosomal Integrity of Ram Sperm. Cryobiology 1989, 26, 341–354. [Google Scholar] [CrossRef]
- Tekin, N. Effects of Different taurine Doses and Freezing Rate on Freezing of Row Semen. Ankara. Üniv. Vet. Fak. 2006, 53, 179–184. [Google Scholar]
- Fernandes, M.; Hernández, P.R.; Simões, J.; Barbas, J.P. Effects of Three Semen Extenders, Breeding Season Month and Freezing-thawing Cycle on Spermatozoa Preservation of Portuguese Merino sheep. Animals 2021, 11, 2619. [Google Scholar] [CrossRef] [PubMed]
- Rakha, B.A.; Ansari, M.S.; Akhter, S.; Hussain, I.; Blesbois, E. Cryopreservation of Indian Red Jungle Fowl (Gallus Gallus Murghi) Semen. Anim. Reprod. Sci. 2016, 174, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Vichas, L.; Tsakmakidis, I.A.; Vafiadis, D.; Tsousis, G.; Malama, E.; Boscos, C.M. The Effect of Antioxidant Agents’ Addition and Freezing Method on Quality Parameters of Frozen Thawed Ram Semen. Cell Tissue. Bank. 2018, 19, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Maziero, R.R.D.; Guaitolini, C.R.F.; Guasti, P.N.; Monteiro, G.A.; Martin, I.; Silva, J.P.M.D.; Crespilho, A.M.; Papa, F.O. Effect of Using Two Cryopreservation Methods on Viability and Fertility of Frozen Stallion Sperm. J. Equine. Vet. Sci. 2019, 72, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Macedo, S.; Bliebernicht, M.; Carvalheira, J.; Costa, A.; Ribeiro, F.; Rocha, A. Effects of Two Freezing Methods and Two Cryopreservation Media on Post-thaw Quality of Stallion Spermatozoa. Reprod. Domest. Anim. 2018, 53, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Falchi, L.; Pau, S.; Pivato, I.; Bogliolo, L.; Zedda, M.T. Resveratrol Supplementation and Cryopreservation of Buck Semen. Cryobiology 2020, 95, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Igbokwe, A.A.; Iyasere, O.S.; Sobayo, R.A.; Iyasere, S.; Animashaun, R.I.; Balogun, F.A.; Aganran, Z.O.; Fasola, M.O.; Adedokun, A.D.; Lakehinde, O.A.; et al. Comparative Effect of Slow and Rapid Freezing on Sperm Functional Attributes and Oxidative Stress Parameters of Goat Spermatozoa Cryopreserved with Tiger Nut Milk Extender. Reprod. Domest. Anim. 2019, 54, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Nanç, M.E.; Güngör, Ş.; Avdatek, F.; Yeni, D.; Gülhan, M.F.; Olğaç, K.T.; Denk, B.; Taşdemir, U. Thymoquinone Improves Motility, Plasma Membrane Integrity and DNA Integrity of Frozen-thawed Ram Semen. Andrologia 2022, 54, e14547. [Google Scholar] [CrossRef]
- Pradiee, J.; Esteso, M.C.; Castaño, C.; Toledano-Díaz, A.; Lopez-Sebastián, A.; Guerra, R.; Santiago-Moreno, J. Conventional Slow Freezing Cryopreserves Mouflon Spermatozoa Better than Vitrification. Andrologia 2017, 49, 12629. [Google Scholar] [CrossRef]
- Vozaf, J.; Makarevich, A.V.; Balazi, A.; Vasicek, J.; Svoradova, A.; Olexikova, L.; Chrenek, P. Cryopreservation of Ram Semen: Manual Versus Programmable Freezing and Different Lengths of Equilibration. Anim. Sci. J. 2021, 92, e13670. [Google Scholar] [CrossRef] [PubMed]
- Prochowska, S.; Niżański, W.; Fontbonne, A. Hypo-Osmotic Swelling Test (HOST) for Feline Spermatozoa: The Simplified Procedure and the Aspect of Sperm Morphology. Animals 2022, 12, 903. [Google Scholar] [CrossRef] [PubMed]
- Aoki, Y.; Tsujimura, A.; Nagashima, Y.; Hiramatsu, I.; Uesaka, Y.; Nozaki, T.; Ogishima, T.; Shirai, M.; Shoyama, Y.; Tanaka, H.; et al. Effect of Lepidium Meyenii on in Vitro Fertilization Via Improvement in Acrosome Reaction and Motility of Mouse and Human Sperm. Reprod. Med. Biol. 2018, 18, 57–64. [Google Scholar] [CrossRef] [PubMed]
- González-Garzón, A.C.; Ramón-Ugalde, J.P.; Ambríz-García, D.A.; Vazquez-Avendaño, J.R.; Hernández-Pichardo, J.E.; Rodríguez-Suastegui, J.L.; Cortez-Romero, C.; Navarro-Maldonado, M.D.C. Resveratrol Reduces ROS by Increasing GSH in Vitrified Sheep Embryos. Animals 2023, 13, 3602. [Google Scholar] [CrossRef] [PubMed]
- Woelders, H. Cryopreservation of Avian semen. Methods Mol. Biol. 2021, 2180, 379–399. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, M.; Rusco, G.; Iampietro, R.; Maiuro, L.; Schiavone, A.; Cerolini, S.; Iaffaldano, N. Validation of the Turkey Semen Cryopreservation by Evaluating the Effect of Two Diluents and the Inseminating Doses. Animals 2020, 10, 1329. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.E.; Negus, C.; Johinke, D.; Bathgate, R. Adjusting Cryodiluent Composition for Improved Post-thaw Quality of Rabbit Spermatozoa. PLoS ONE 2017, 12, e0175965. [Google Scholar] [CrossRef] [PubMed]
- Bravo, P.W.; Alarcon, V.; Baca, L.; Cuba, Y.; Ordoñez, C.; Salinas, J.; Tito, F. Semen Preservation and Artificial Insemination in Domesticated South American Camelids. Anim. Reprod. Sci. 2013, 136, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Graham, E.F.; Crabo, B.G.; Brown, K.I. Effect of Some Zwitter Ion Buffers on the Freezing and Storage of Spermatozoa I. Bull. J. Dairy. Sci. 1972, 55, 372–378. [Google Scholar] [CrossRef]
- Liu, C.H.; Dong, H.B.; Ma, D.L.; Li, Y.W.; Han, D.; Luo, M.J.; Chang, Z.L.; Tan, J.H. Effects of pH During Liquid Storage of Goat Semen on Sperm Viability and Fertilizing Potential. Anim. Reprod. Sci. 2016, 164, 47–56. [Google Scholar] [CrossRef]
- Alamaary, M.S.; Haron, A.W.; Ali, M.; Hiew, M.W.H.; Adamu, L.; Peter, I.D. Effects of Four Extenders on the Quality of Frozen Semen in Arabian Stallions. Vet. World. 2019, 12, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Najafi, A.; Zhandi, M.; Towhidi, A.; Sharafi, M.; Akbari Sharif, A.; Khodaei Motlagh, M.; Martinez-Pastor, F. Trehalose and Glycerol Have a Dose-dependent Synergistic Effect on the Post-thawing Quality of Ram Semen Cryopreserved in a Soybean Lecithin-based Extender. Cryobiology 2013, 66, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Wang, Y.; Zhang, L.; Wang, J.; Wu, X.; Li, Y.; Sun, X. Effect of Different Diluents on Cryopreservation of Hu Sheep Semen. Chin. J. Anim. Sci. 2022, 58, 183–188. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Sun, X.; Kang, Y.; Sohail, T.; Wang, J.; Li, Y. Effects of Different Diluents on Semen Quality of Hu Ram Stored at 4 °C. Animals 2023, 13, 2823. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Zhang, S.; Dai, J.; Sun, L.; Lei, G.; Liang, F.; Zhang, D. Effects of Diluents and Thawing Conditions on the Quality of Hu Rams Semen Freezing. Acta Agric. Shanghai 2019, 35, 63–66. [Google Scholar] [CrossRef]
- Gangwar, C.; Kharche, S.D.; Mishra, A.K.; Saraswat, S.; Kumar, N.; Sikarwar, A.K. Effect of Diluent Sugars on Capacitation Status and Acrosome Reaction of Spermatozoa in Buck Semen at Refrigerated Temperature. Trop. Anim. Health. Prod. 2020, 52, 3409–3415. [Google Scholar] [CrossRef] [PubMed]
- Franklin, A.D.; Waddell, W.T.; Goodrowe, K.L. Red Wolf (Canis rufus) Sperm Quality and Quantity is Affected by Semen Collection Method, Extender Components, and Post-thaw Holding Temperature. Theriogenology 2018, 116, 41–48. [Google Scholar] [CrossRef]
- Avdatek, F.; İnanç, M.E.; Gülhan, M.F.; Güngör, Ş.; Yeni, D.; Olğaç, K.T.; Denk, B.; Taşdemir, U. Investigation of the Effects of Syringic Acid Supplemented to Tris Semen Diluent on Ram Semen Freezability. Reprod. Domest. Anim. 2023, 58, 997–1004. [Google Scholar] [CrossRef]
- Bucak, M.N.; Keskin, N.; Taşpınar, M.; Çoyan, K.; Başpınar, N.; Cenariu, M.C.; Bilgili, A.; Öztürk, C.; Kurşunlu, A.N. Raffinose and Hypotaurine Improve the Post-thawed Merino Ram Sperm Parameters. Cryobiology 2013, 67, 34–39. [Google Scholar] [CrossRef]
- Naer, A. Study on Cryopreservation of Mongolian Stallion Semen. Ph.D. Thesis, Inner Mongolia Agricultural University, Huhehaote, China, 2020. [Google Scholar]
- Demyda-Peyrás, S.; Bottrel, M.; Acha, D.; Ortiz, I.; Hidalgo, M.; Carrasco, J.J.; Gómez-Arrones, V.; Gósalvez, J.; Dorado, J. Effect of Cooling rate on Sperm Quality of Cryopreserved Andalusian Donkey Spermatozoa. Anim. Reprod. Sci. 2018, 193, 201–208. [Google Scholar] [CrossRef]
- Neild, D.M.; Brouwers, J.F.; Colenbrander, B.; Agüero, A.; Gadella, B.M. Lipid Peroxide Formation in Relation to Membrane Stability of Fresh and Frozen Thawed Stallion Spermatozoa. Mol. Reprod. Dev. 2005, 72, 230–238. [Google Scholar] [CrossRef]
- Yeste, M. Sperm Cryopreservation Update: Cryodamage, Markers, and Factors Affecting the Sperm Freezability in Pigs. Theriogenology 2016, 85, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Wainer, R.; Albert, M.; Dorion, A.; Bailly, M.; Bergère, M.; Lombroso, R.; Gombault, M.; Selva, J. Influence of the Number of Motile Spermatozoa Inseminated and of Their Morphology on the Success of Intrauterine Insemination. Hum. Reprod. 2004, 19, 2060–2065. [Google Scholar] [CrossRef] [PubMed]
- Alyethodi, R.R.; Sirohi, A.S.; Karthik, S.; Tyagi, S.; Perumal, P.; Singh, U.; Sharma, A.; Kundu, A. Role of Seminal MDA, ROS, and Antioxidants in Cryopreservation and Their Kinetics Under the Influence of Ejaculatory Abstinence in Bovine Semen. Cryobiology 2021, 98, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Henkel, R.; Kierspel, E.; Hajimohammad, M.; Stalf, T.; Hoogendijk, C.; Mehnert, C.; Menkveld, R.; Schill, W.B.; Kruger, T.F. DNA Fragmentation of Spermatozoa and Assisted Reproduction Technology. Reprod. Biomed. Online 2003, 7, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Stuart, C.C.; Vaughan, J.L.; Kershaw, C.M.; de Graaf, S.P.; Bathgate, R. Effect of Diluent type, Cryoprotectant Concentration, Storage Method and Freeze/thaw Rates on the Post-thaw Quality and Fertility of Cryopreserved Alpaca Spermatozoa. Sci. Rep. 2019, 9, 12826. [Google Scholar] [CrossRef] [PubMed]
- Galarza, D.A.; López-Sebastián, A.; Woelders, H.; Blesbois, E.; Santiago-Moreno, J. Two-step Accelerating Freezing Protocol Yields a Better Motility, Membranes and DNA Integrities of Thawed Ram Sperm than Three-steps Freezing Protocols. Cryobiology 2019, 91, 84–89. [Google Scholar] [CrossRef]
- Esteso, M.C.; Toledano-Díaz, A.; Castaño, C.; Pradiee, J.; Lopez-Sebastián, A.; Santiago-Moreno, J. Effect of Two Cooling Protocols on the Post-thaw Characteristics of Iberian Ibex Sperms. Cryobiology 2018, 80, 12–17. [Google Scholar] [CrossRef]

| Constituent | A | B | C | D | E(PBS) | F(NS) |
|---|---|---|---|---|---|---|
| Tris (Sangon Biotech, Shanghai, China) | 1.82 g | - | 1.82 g | 0.28 g | - | - |
| Glucose (Sangon Biotech, Shanghai, China) | 0.25 g | - | - | 0.25 g | - | - |
| Fructose (Sangon Biotech, Shanghai, China) | - | 0.25 g | 0.25 g | - | - | - |
| Citric acid (Sangon Biotech, Shanghai, China) | 0.91 g | - | 0.91 g | - | - | - |
| Sodium citrate (Sangon Biotech, Shanghai, China) | - | 1.20 g | - | 0.35 g | - | - |
| Sodium bicarbonate (Sangon Biotech, Shanghai, China) | - | - | - | 0.05 g | - | - |
| Sodium chloride (Sangon Biotech, Shanghai, China) | - | - | - | - | 400.33 mg | 0.45 g |
| Potassium chloride (Sangon Biotech, Shanghai, China) | - | - | - | - | 10.06 mg | - |
| Dibasic Sodium Phosphate (Sangon Biotech, Shanghai, China) | - | - | - | - | 56.78 mg | - |
| Potassium dihydrogen phosphate (Sangon Biotech, Shanghai, China) | - | - | - | - | 12.25 mg | - |
| Pen Strep (Thermo, Waltham, MA, USA) | 10,000 IU | 10,000 IU | 10,000 IU | 10,000 IU | 10,000 IU | 10,000 IU |
| Total volume | 50 mL | 50 mL | 50 mL | 50 mL | 50 mL | 50 mL |
| Different Diluents | TM (%) | PM (%) | VSL (µm/s) | VCL (µm/s) | VAP (µm/s) | ALH (µm) | WOB (%) | MAD (°/s) |
|---|---|---|---|---|---|---|---|---|
| A | 74.82 ± 1.22 a | 60.32 ± 1.02 b | 40.98 ± 0.93 b | 60.33 ± 1.73 a | 42.66 ± 1.22 a | 17.67 ± 0.51 a | 0.53 ± 0.02 a | 39.10 ± 2.60 a |
| B | 54.09 ± 0.44 d | 39.56 ± 1.01 d | 41.12 ± 1.24 b | 53.77 ± 0.62 b | 38.02 ± 0.44 b | 15.75 ± 0.18 b | 0.39 ± 0.04 bc | 20.75 ± 0.82 c |
| C | 76.81 ± 1.04 a | 64.42 ± 0.84 a | 41.18 ± 1.41 b | 62.05 ± 0.52 a | 43.88 ± 0.37 a | 18.18 ± 0.15 a | 0.58 ± 0.06 a | 43.54 ± 4.79 a |
| D | 3.81 ± 0.14 g | 2.57 ± 0.21 g | 29.14 ± 2.46 c | 43.71 ± 1.14 c | 30.90 ± 0.81 c | 12.80 ± 0.34 c | 0.36 ± 0.02 c | 2.78 ± 0.27 e |
| E | 39.12 ± 0.49 e | 24.98 ± 0.92 e | 40.54 ± 1.90 b | 53.20 ± 0.99 b | 37.62 ± 0.70 b | 15.58 ± 0.29 b | 0.36 ± 0.01 c | 11.44 ± 0.27 d |
| F | 31.47 ± 1.79 f | 19.38 ± 0.90 f | 40.88 ± 1.00 b | 53.44 ± 0.95 b | 37.79 ± 0.68 b | 15.66 ± 0.28 b | 0.40 ± 0.04 bc | 9.60 ± 0.14 de |
| G | 66.37 ± 2.78 c | 51.57 ± 0.88 c | 47.65 ± 0.30 a | 60.43 ± 0.51 a | 42.73 ± 0.36 a | 17.70 ± 0.15 a | 0.36 ± 0.02 c | 28.21 ± 2.44 b |
| H | 70.68 ± 0.82 b | 57.92 ± 1.37 b | 43.88 ± 0.19 b | 60.39 ± 0.75 a | 42.70 ± 0.53 a | 17.69 ± 0.22 a | 0.48 ± 0.03 ab | 29.61 ± 2.21 b |
| Different Freezing Method | TM (%) | PM (%) | VSL (µm/s) | VCL (µm/s) | VAP (µm/s) | ALH (µm) | WOB (%) | MAD (°/s) |
|---|---|---|---|---|---|---|---|---|
| Program 1 | 66.72 ± 1.36 | 54.62 ± 0.98 a | 40.00 ± 0.46 | 60.10 ± 0.81 | 42.50 ± 0.57 | 17.60 ± 0.24 | 0.53 ± 0.01 | 32.51 ± 3.57 |
| Program 2 | 64.09 ± 2.42 | 50.58 ± 0.89 b | 39.60 ± 0.51 | 58.81 ± 0.64 | 41.58 ± 0.45 | 17.23 ± 0.19 | 0.52 ± 0.03 | 36.89 ± 5.85 |
| Liquid nitrogen fumigation | 69.24 ± 0.58 | 55.52 ± 0.55 a | 40.27 ± 0.76 | 60.56 ± 1.24 | 42.82 ± 0.87 | 17.74 ± 0.36 | 0.56 ± 0.01 | 36.77 ± 1.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Wang, X.; Jiang, C.; Sohail, T.; Sun, Y.; Sun, X.; Wang, J.; Li, Y. Effects of Different Diluents and Freezing Methods on Cryopreservation of Hu Ram Semen. Vet. Sci. 2024, 11, 251. https://doi.org/10.3390/vetsci11060251
Zhang L, Wang X, Jiang C, Sohail T, Sun Y, Sun X, Wang J, Li Y. Effects of Different Diluents and Freezing Methods on Cryopreservation of Hu Ram Semen. Veterinary Sciences. 2024; 11(6):251. https://doi.org/10.3390/vetsci11060251
Chicago/Turabian StyleZhang, Liuming, Xuyang Wang, Caiyu Jiang, Tariq Sohail, Yuxuan Sun, Xiaomei Sun, Jian Wang, and Yongjun Li. 2024. "Effects of Different Diluents and Freezing Methods on Cryopreservation of Hu Ram Semen" Veterinary Sciences 11, no. 6: 251. https://doi.org/10.3390/vetsci11060251
APA StyleZhang, L., Wang, X., Jiang, C., Sohail, T., Sun, Y., Sun, X., Wang, J., & Li, Y. (2024). Effects of Different Diluents and Freezing Methods on Cryopreservation of Hu Ram Semen. Veterinary Sciences, 11(6), 251. https://doi.org/10.3390/vetsci11060251







