Carvacrol and Thymol Enhance the Quality of Beni Arouss Buck Semen Stored at 4 °C Thanks to Their Antimicrobial Properties
Abstract
Simple Summary
Abstract
1. Introduction
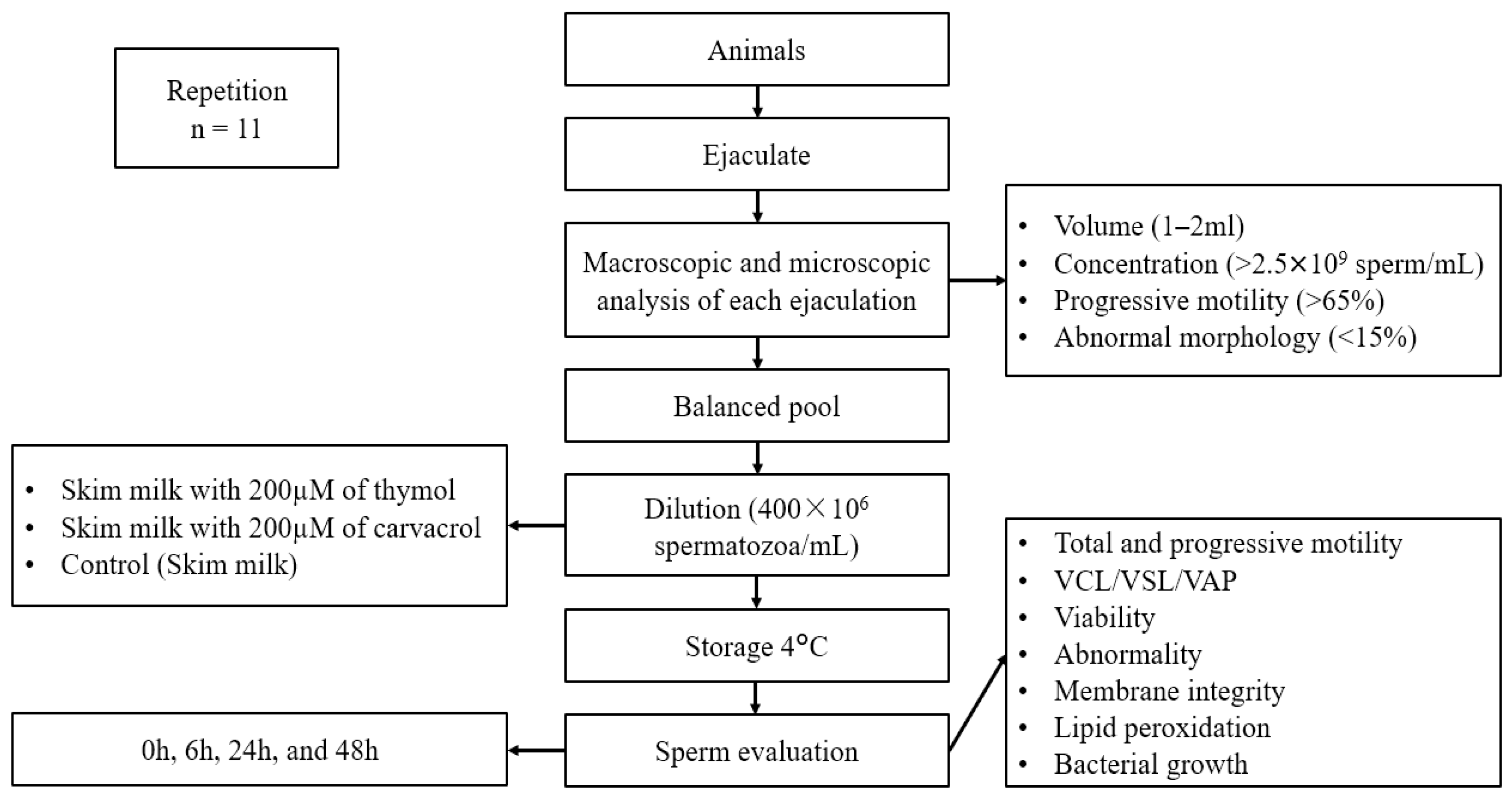
2. Materials and Methods
2.1. Antioxidant Activity Assessment
2.2. Semen Collection and Evaluation
2.3. Sperm Motility Evaluation
2.4. Viability and Abnormality
2.5. Membrane Integrity
2.6. Lipid Peroxidation
2.7. Bacterial Growth Assessment
2.8. Statistical Analysis
3. Results
3.1. Antioxidant Activity
3.2. Sperm Motility
3.3. Viability and Abnormalities
3.4. Membrane Integrity and Lipid Peroxidation
3.5. Bacterial Growth
3.6. Correlation between Sperm Quality Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atigui, M.; Chniter, M. Ovine Artificial Insemination in the Maghreb Region: Present Status and Future Prospects. In Sheep Farming: Herds Husbandry, Management System, Reproduction and Improvement of Animal Health; IntechOpen: Rijeka, Croatia, 2022; pp. 113–114. [Google Scholar] [CrossRef]
- Rizkallah, N.; Chambers, C.G.; de Graaf, S.P.; Rickard, J.P. Factors affecting the survival of ram spermatozoa during liquid storage and options for improvement. Animals 2022, 12, 244. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, K.; Yao, Y.; Li, J.; Deng, S. Bacterial infections affect male fertility: A focus on the oxidative stress-autophagy axis. Front. Cell Dev. Biol. 2021, 9, 727812. [Google Scholar] [CrossRef]
- Kuster, C.E.; Althouse, G.C. The impact of bacteriospermia on boar sperm storage and reproductive performance. Theriogenology 2016, 85, 21–26. [Google Scholar] [CrossRef]
- Gangwar, C.; Mishra, A.K.; Gururaj, K.; Kumar, A.; Kharche, S.D.; Saraswat, S.R.; Kumar, N.; Ramachandran, N. Semen Quality and Total Microbial Load: An Association Study in Important Indian Goat Breeds during Different Seasons. Andrologia 2021, 53, e13995. [Google Scholar] [CrossRef] [PubMed]
- Qamar, A.Y.; Naveed, M.I.; Raza, S.; Fang, X.; Roy, P.K.; Bang, S.; Tanga, B.M.; Saadeldin, I.M.; Lee, S.; Cho, J. Role of antioxidants in fertility preservation of sperm—A narrative review. Anim. Biosci. 2023, 36, 385. [Google Scholar] [CrossRef]
- Pereira, B.A.; Chaves, B.R.; Teles, M.C.; Pontelo, T.P.; Oliveira, C.R.; de Souza, R.V.; Rodríguez-Gil, J.E.; Zangeronimo, M.G. Chlorogenic acid improves the quality of boar semen subjected to cooled storage at 15 °C. Andrologia 2018, 50, e12978. [Google Scholar] [CrossRef] [PubMed]
- Bahmyari, R.; Zare, M.; Sharma, R.; Agarwal, A.; Halvaei, I. The efficacy of antioxidants in sperm parameters and production of reactive oxygen species levels during the freeze-thaw process: A systematic review and meta-analysis. Andrologia 2020, 52, e13514. [Google Scholar] [CrossRef]
- Pei, Y.; Yang, L.; Wu, L.; He, H.; Geng, G.; Xu, D.; Chen, H.; Li, Q. Combined effect of apigenin and ferulic acid on frozen-thawed boar sperm quality. Anim. Sci. J. 2018, 89, 956–965. [Google Scholar] [CrossRef]
- Kchikich, A.; Kirschvink, N.; El Kadili, S.; Raes, M.; El Otmani, S.; Chebli, Y.; Bister, J.L.; El Amiri, B.; Barrijal, S.; Chentouf, M. Effects of Origanum majorana essential oil and antibiotics on the quality of frozen thawed Beni Arouss buck semen. Reprod. Domest. Anim. 2022, 58, 288–297. [Google Scholar] [CrossRef]
- Nguyen, V.V.; Ponchunchoovong, S.; Kupittayanant, S.; Kupittayanant, P. The potential of using Ocimum gratissimum leaf essential oils as a supplement in extender to improve chilled canine sperm quality by assessing its antioxidant effects. Adv. Anim. Vet. Sci. 2023, 11, 1338–1347. [Google Scholar] [CrossRef]
- Dash, K.T.; Jena, S.; Ray, A.; Sahoo, A.; Kar, S.K.; Sahoo, R.K.; Subudhi, E.; Panda, P.C.; Nayak, S. Chemical composition of carvacrol rich leaf essential oil of Thymus vulgaris from India: Assessment of antimicrobial, antioxidant and cytotoxic potential. J. Essent. Oil-Bear. Plants 2021, 24, 1134–1145. [Google Scholar] [CrossRef]
- Nascimento, L.D.D.; Silva, S.G.; Cascaes, M.M.; Costa, K.S.D.; Figueiredo, P.L.B.; Costa, C.M.L.; Andrade, E.H.A.; de Faria, L.J.G. Drying effects on chemical composition and antioxidant activity of Lippia thymoides essential oil, a natural source of thymol. Molecules 2021, 26, 2621. [Google Scholar] [CrossRef] [PubMed]
- Tisserand, R.; Young, R. Essential Oil Safety: A Guide for Health Care Professionals; Churchill Livingstone, Elsevier: London, UK, 2014. [Google Scholar]
- Chebli, Y.; El Otmani, S.; Hilal, B.; Cabaraux, J.F.; Chentouf, M. Pastoral and milk production in an extensive farming in northern Morocco. Opt. Méditerr. 2016, 115, 649–654. [Google Scholar]
- Kchikich, A.; Kirschvink, N.; El Kadili, S.; Raes, M.; El Otmani, S.; Bister, J.L.; El Amiri, B.; Barrijal, S.; Chentouf, M. Thymus satureioides and Origanum majorana essential oils improve the quality of Beni Arouss buck semen during storage at 4 °C. Reprod. Domest. Anim. 2021, 56, 1572–1581. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi Pirbalouti, A.; Izadi, A.; Malek Poor, F.; Hamedi, B. Chemical composition, antioxidant and antibacterial activities of essential oils from Ferulago angulata. Pharm. Biol. 2016, 54, 2515–2520. [Google Scholar] [CrossRef]
- Hilal, B.; El Otmani, S.; Chentouf, M.; Boujenane, I. Multivariate analysis for morphological traits of the Hamra goat population in two regions of Morocco. Anim. Genet. Resour. 2016, 59, 55–62. [Google Scholar] [CrossRef]
- El Kadili, S.; Raes, M.; Bister, J.L.; Archa, B.; Chentouf, M.; Kirschvink, N. Effect of season on sexual behavior, testicular measurements and seminal characteristics in “Beni Arouss” north Moroccan bucks. Anim. Reprod. Sci. 2019, 201, 41–54. [Google Scholar] [CrossRef]
- Evans, G.; Maxwell, W.C. Salamon’s Artificial Insemination of Sheep and Goats, 2nd ed.; Butterworths: Sydney, Australia, 1987. [Google Scholar]
- Kleshchev, M.; Osadchuk, L.; Osadchuk, A. Age-Related Changes in Sperm Morphology and Analysis of Multiple Sperm Defects. Front. Biosci.-Schol. 2023, 15, 12. [Google Scholar] [CrossRef]
- Revell, S.G.; Mrode, R.A. An osmotic resistance test for bovine semen. Anim. Reprod. Sci. 1994, 36, 77–86. [Google Scholar] [CrossRef]
- Buckett, W.M.; Farquharson, R.G.; Luckas, M.J.M.; Kingsland, C.R.; Aird, I.A.; Lewis-Jones, D.I. The hypo-osmotic swelling test in recurrent miscarriage. Fertil. Steril. 1997, 68, 506–509. [Google Scholar] [CrossRef]
- Allai, L.; Druart, X.; Contell, J.; Louanjli, N.; Ben Moula, A.; Badi, A.; Essamadi, A.; Nasser, B.; El Amiri, B. Effect of argan oil on liquid storage of ram semen in Tris or skim milk based extenders. Anim. Reprod. Sci. 2015, 160, 57–67. [Google Scholar] [CrossRef]
- Bussalleu, E.; Sancho, S.; Briz, M.D.; Yeste, M.; Bonet, S. Do antimicrobial peptides PR-39, PMAP-36 and PMAP-37 have any effect on bacterial growth and quality of liquid stored boar semen? Theriogenology 2017, 89, 235–243. [Google Scholar] [CrossRef]
- Frydrychová, S.; Lustyková, A.; Václavková, E.; Lipenský, J.; Rozkot, M.; Opletal, L. Effect of natural substances as a potential substitute for antibiotics in boar semen extender on semen survival time. Res. Pig Breed. 2012, 6, 20–23. [Google Scholar]
- Restrepo, G.; Zapata, K.; Colorado, P.; Rojano, B. Cooling of porcine semen in an extender supplemented with carvacrol. Reprod. Domest. Anim. 2023, 58, 860–866. [Google Scholar] [CrossRef]
- Usuga, A.; Gutiérrez, V.; López, M.E.; Pérez, L.F.; Jaramillo, L.; Rojano, B.; Restrepo, G. Evaluation of the effect of conventional and natural antifungals on motility and kinetics of cooled stallion semen. Reprod. Domest. Anim. 2022, 57, 701–710. [Google Scholar] [CrossRef]
- Chikhoune, A.; Stouvenel, L.; Iguer-Ouada, M.; Hazzit, M.; Schmitt, A.; Lores, P.; Wolf, J.P.; Aissat, K.; Auger, J.; Vaiman, D.; et al. In vitro effects of Thymus munbyanus essential oil and thymol on human sperm motility and function. Reprod. Biomed. Online 2015, 31, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Güvenç, M.; Cellat, M.; Gökçek, İ.; Yavaş, İ.; Yurdagül Özsoy, Ş. Effects of thymol and carvacrol on sperm quality and oxidant/antioxidant balance in rats. Arch. Physiol. Biochem. 2019, 125, 396–403. [Google Scholar] [CrossRef]
- Tijani, A.S.; Daba, T.M.; Ubong, I.A.; Olufunke, O.; Ani, E.J.; Farombi, E.O. Co-administration of thymol and sulfoxaflor impedes the expression of reproductive toxicity in male rats. Drug Chem. Toxicol. 2023, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rúa, J.; Del Valle, P.; de Arriaga, D.; Fernández-Álvarez, L.; García-Armesto, M.R. Combination of carvacrol and thymol: Antimicrobial activity against Staphylococcus aureus and antioxidant activity. Foodborne Pathog. Dis. 2019, 16, 622–629. [Google Scholar] [CrossRef]
- Al-Mansori, B.; El-Ageeli, W.H.; Alsagheer, S.H.; Ben-Khayal, F.A. Antioxidant Activity-Synergistic Effects of Thymol and Carvacrol. Al-Mukhtar J. Sci. 2020, 35, 185–194. [Google Scholar] [CrossRef]
- David, M. Le Thymol: Sources, Propriétés et Applications. Doctor of. Pharmacy Thesis, Limoges University, Limoges, France, 2019. [Google Scholar]
- Gavaric, N.; Mozina, S.S.; Kladar, N.; Bozin, B. Chemical profile, antioxidant and antibacterial activity of thyme and oregano essential oils, thymol and carvacrol and their possible synergism. J. Essent. Oil-Bear. Plants 2015, 18, 1013–1021. [Google Scholar] [CrossRef]
- Mazurova, J.; Kukla, R.; Rozkot, M.; Lustykova, A.; Slehova, E.; Sleha, R.; Lipensky, J.; Opletal, L. Use of natural substances for boar semen decontamination. Vet. Med. 2015, 60, 235–247. [Google Scholar] [CrossRef]
- Kou, Z.; Wang, C.; Gao, L.; Chu, G.; Yang, G.; Pang, W. Icariin improves pig sperm quality through antioxidant and antibacterial effects during liquid storage at 17 °C. Livest. Sci. 2022, 256, 104827. [Google Scholar] [CrossRef]
- Khalaf, Y.; Farrag, A.; Shaeer, E.K. Effect of Female Genital Candidiasis on Semen Parameters Female Genital Candidiasis and Semen. Kasr Al Ainy Med. J. 2022, 28, 32–37. [Google Scholar] [CrossRef]
- Kommisrud, E.; Myromslien, F.D.; Stenseth, E.B.; Zeremichael, T.T.; Hofman, N.; Grevle, I.; Sunde, J. Viability, motility, ATP content and fertilizing potential of sperm from Atlantic salmon (Salmo salar L.) in milt stored before cryopreservation. Theriogenology 2020, 151, 58–65. [Google Scholar] [CrossRef]
- Oghbaei, H.; Rastgar Rezaei, Y.; Nikanfar, S.; Zarezadeh, R.; Sadegi, M.; Latifi, Z.; Nouri, M.; Fattahi, A.; Ahmadi, Y.; Bleisinger, N. Effects of bacteria on male fertility: Spermatogenesis and sperm function. Life Sci. 2020, 256, 117891. [Google Scholar] [CrossRef] [PubMed]
- Khanmohammad, K.R.; Khalili, M.B.; Sadeh, M.; Talebi, A.R.; Astani, A.; Shams, A.; Zare, F. The effect of lipopolysaccharide from uropathogenic Escherichia coli on the immune system, testis tissue, and spermatozoa of BALB/c mice. Clin. Exp. Reprod. Med. 2021, 48, 105. [Google Scholar] [CrossRef] [PubMed]
- Tvrdá, E.; Benko, F.; Ďuračka, M. Oxidative stress as an underlying mechanism of bacteria-inflicted damage to male gametes. Oxygen 2022, 2, 547–569. [Google Scholar] [CrossRef]
- Kaur, K.; Prabha, V. Sperm impairment by sperm agglutinating factor isolated from Escherichia coli: Receptor specific interactions. BioMed Res. Int. 2013, 2013, 548497. [Google Scholar] [CrossRef]
- Ďuračka, M.; Belić, L.; Tokárová, K.; Žiarovská, J.; Kačániová, M.; Lukáč, N.; Tvrdá, E. Bacterial communities in bovine ejaculates and their impact on the semen quality. Syst. Biol. Reprod. Med. 2021, 67, 438–449. [Google Scholar] [CrossRef]
- Farsimadan, M.; Motamedifar, M. Bacterial infection of the male reproductive system causing infertility. J. Reprod. Immunol. 2020, 142, 103183. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, S.; Singh, G.; Gupta, N.P.; Kumar, R.; Deecaraman, M.; Dada, R. Correlation of sperm morphology and oxidative stress in infertile men. Iran. J. Reprod. Med. 2009, 7, 29–34. [Google Scholar]
- Oehninger, S.; Kruger, T.F. Sperm morphology and its disorders in the context of infertility. F S Rev. 2021, 2, 75–92. [Google Scholar] [CrossRef]
| Parameters | Treatments | Storage Duration | Interaction Storage Duration and Treatments | |||
|---|---|---|---|---|---|---|
| 0 h | 6 h | 24 h | 48 h | |||
| Total motility | Control | 95 ± 1 aA | 91 ± 2 bA | 85 ± 2 cA | 72 ± 2 dB | p < 0.0013 |
| Thymol | 94 ± 2 aA | 92 ± 3 aA | 84 ± 3 bA | 77 ± 3 cA | ||
| Carvacrol | 94 ± 2 aA | 91 ± 2 bA | 84 ± 3 cA | 77 ± 3 dA | ||
| Progressive motility | Control | 73 ± 3 aA | 67 ± 3 bA | 51 ± 3 cA | 45 ± 2 dB | p < 0.1906 |
| Thymol | 74 ± 3 aA | 67 ± 2 bA | 54 ± 4 cA | 48 ± 2 dAB | ||
| Carvacrol | 74 ± 4 aA | 69 ± 2 bA | 54 ± 4 cA | 49 ± 2 dA | ||
| Parameters | Treatments | Storage Duration | Interaction Storage Duration and Treatments | |||
|---|---|---|---|---|---|---|
| 0 h | 6 h | 24 h | 48 h | |||
| VCL | Control | 121 ± 6 aA | 116 ± 6 aB | 109 ± 7 bB | 100 ± 5 cB | p < 0.1767 |
| Thymol | 123 ± 6 aA | 121 ± 5 aA | 119 ± 12 aA | 110 ± 8 bA | ||
| Carvacrol | 128 ± 11 aA | 126 ± 4 abA | 120 ± 3 bcA | 114 ± 7 cA | ||
| VSL | Control | 88 ± 9 aA | 83 ± 9 abB | 81 ± 3 bB | 74 ± 6 cC | p < 0.4311 |
| Thymol | 90 ± 5 aA | 85 ± 5 bAB | 84 ± 8 bAB | 78 ± 3 cB | ||
| Carvacrol | 93 ± 11 aA | 90 ± 4 abA | 87 ± 3 bcA | 83 ± 3 cA | ||
| VAP | Control | 109 ± 7 aA | 102 ± 5 bB | 100 ± 6 bB | 88 ± 6 cB | p < 0.1806 |
| Thymol | 110 ± 7 aA | 107 ± 5 aA | 105 ± 11 aAB | 96 ± 9 bA | ||
| Carvacrol | 113 ± 9 aA | 111 ± 4 aA | 108 ± 3 aA | 98 ± 8 bA | ||
| Parameters | Treatments | Storage Duration | Interaction Storage Duration and Treatments | |||
|---|---|---|---|---|---|---|
| 0 h | 6 h | 24 h | 48 h | |||
| Viability | Control | 96 ± 1 aA | 94 ± 2 bA | 89 ± 4 cA | 76 ± 3 dB | p < 0.0091 |
| Thymol | 98 ± 2 aA | 95 ± 2 bA | 87 ± 3 cA | 79 ± 3 dA | ||
| Carvacrol | 97 ± 2 aA | 94 ± 3 bA | 88 ± 3 cA | 81 ± 4 dA | ||
| Abnormality | Control | 7 ± 2 cA | 8 ± 2 cA | 17 ± 1 bA | 23 ± 4 aA | p < 0.4130 |
| Thymol | 7 ± 3 cA | 9 ± 2 cA | 16 ± 3 bA | 20 ± 5 aA | ||
| Carvacrol | 7 ± 3 cA | 9 ± 3 cA | 15 ± 3 bA | 21 ± 4 aA | ||
| Parameters | Treatments | Storage Duration | Interaction Storage Duration and Treatments | |||
|---|---|---|---|---|---|---|
| 0 h | 6 h | 24 h | 48 h | |||
| Membrane integrity | Control | 92 ± 2 aA | 84 ± 3 bA | 74 ± 3 cA | 65 ± 2 dA | p < 0.5888 |
| Thymol | 92 ± 1 aA | 86 ± 4 bA | 74 ± 3 cA | 65 ± 2 dA | ||
| Carvacrol | 92 ± 2 aA | 86 ± 3 bA | 75 ± 3 cA | 67 ± 2 dA | ||
| Lipid peroxidation | Control | 0.4 ± 0.1 dA | 0.6 ± 0.1 cA | 1.1 ± 0.1 bA | 1.8 ± 0.1 aA | p < 0.0001 |
| Thymol | 0.4 ± 0.1 dA | 0.6 ± 0.1 cA | 0.8 ± 0.1 bB | 1.4 ± 0.1 aB | ||
| Carvacrol | 0.4 ± 0.1 dA | 0.5 ± 0.1 cA | 0.7 ± 0.1 bC | 1.2 ± 0.1 aC | ||
| Parameters | Treatments | Storage Duration | Interaction Storage Duration and Treatments | |||
|---|---|---|---|---|---|---|
| 0 h | 6 h | 24 h | 48 h | |||
| Bacterial growth | Control | 930 ± 243 dA | 2762 ± 203 cA | 14,313 ± 861 bA | 24,097 ± 688 aA | p < 0.0001 |
| Thymol | 394 ± 66 dB | 1550 ± 291 cB | 9744 ± 892 bB | 22,930 ± 1003 aB | ||
| Carvacrol | 320 ± 123 dB | 1013 ± 110 cC | 7883 ± 492 bC | 20,086 ± 1241 aC | ||
| Total Motility | Progressive Motility | VCL | VSL | VAP | Viability | Abnormality | Membrane Integrity | Lipid Peroxidation | Bacterial Growth | |
|---|---|---|---|---|---|---|---|---|---|---|
| Total motility | 1 | 0.88 *** | 0.65 *** | 0.61 *** | 0.72 *** | 0.98 *** | −0.53 *** | 0.76 *** | NS | −0.84 *** |
| Progressive motility | 1 | 0.63 *** | 0.61 *** | 0.67 *** | 0.90 *** | −0.51 *** | 0.71 *** | NS | −0.78 *** | |
| VCL | 1 | 0.79 *** | 0.82 *** | 0.67 *** | −0.32 *** | 0.49 *** | NS | −0.59 *** | ||
| VSL | 1 | 0.78 *** | 0.63 *** | −0.32 *** | 0.50 *** | NS | −0.49 *** | |||
| VAP | 1 | 0.73 *** | −0.37 *** | 0.57 *** | NS | −0.60 *** | ||||
| Viability | 1 | −0.51 *** | 0.76 *** | NS | −0.82 *** | |||||
| Abnormality | 1 | −0.89 *** | 0.58 *** | 0.44 *** | ||||||
| Membrane integrity | 1 | −0.42 *** | −0.61 *** | |||||||
| Lipid peroxidation | 1 | NS | ||||||||
| Bacterial growth | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kchikich, A.; Kirschvink, N.; Raes, M.; El Otmani, S.; Chebli, Y.; Bister, J.-L.; El Amiri, B.; Barrijal, S.; Chentouf, M. Carvacrol and Thymol Enhance the Quality of Beni Arouss Buck Semen Stored at 4 °C Thanks to Their Antimicrobial Properties. Vet. Sci. 2024, 11, 406. https://doi.org/10.3390/vetsci11090406
Kchikich A, Kirschvink N, Raes M, El Otmani S, Chebli Y, Bister J-L, El Amiri B, Barrijal S, Chentouf M. Carvacrol and Thymol Enhance the Quality of Beni Arouss Buck Semen Stored at 4 °C Thanks to Their Antimicrobial Properties. Veterinary Sciences. 2024; 11(9):406. https://doi.org/10.3390/vetsci11090406
Chicago/Turabian StyleKchikich, Amr, Nathalie Kirschvink, Marianne Raes, Samira El Otmani, Youssef Chebli, Jean-Loup Bister, Bouchra El Amiri, Said Barrijal, and Mouad Chentouf. 2024. "Carvacrol and Thymol Enhance the Quality of Beni Arouss Buck Semen Stored at 4 °C Thanks to Their Antimicrobial Properties" Veterinary Sciences 11, no. 9: 406. https://doi.org/10.3390/vetsci11090406
APA StyleKchikich, A., Kirschvink, N., Raes, M., El Otmani, S., Chebli, Y., Bister, J.-L., El Amiri, B., Barrijal, S., & Chentouf, M. (2024). Carvacrol and Thymol Enhance the Quality of Beni Arouss Buck Semen Stored at 4 °C Thanks to Their Antimicrobial Properties. Veterinary Sciences, 11(9), 406. https://doi.org/10.3390/vetsci11090406








