Canine Amniotic Fluid at Birth Holds Information about Neonatal Antibody Titres against Core Vaccine Viruses
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Records
2.2. Maternal Blood Collection
2.3. Amniotic Fluid Collection
2.4. Amniotic Fluid Analysis
2.4.1. Total IgGs
2.4.2. Specific IgG—VacciCheck
2.4.3. Lecithin, Sphingomyelin, Cortisol, SP-A, and PTX3 Detection in Amniotic Fluid
2.5. Statistical Analysis
Experimental Design
3. Results
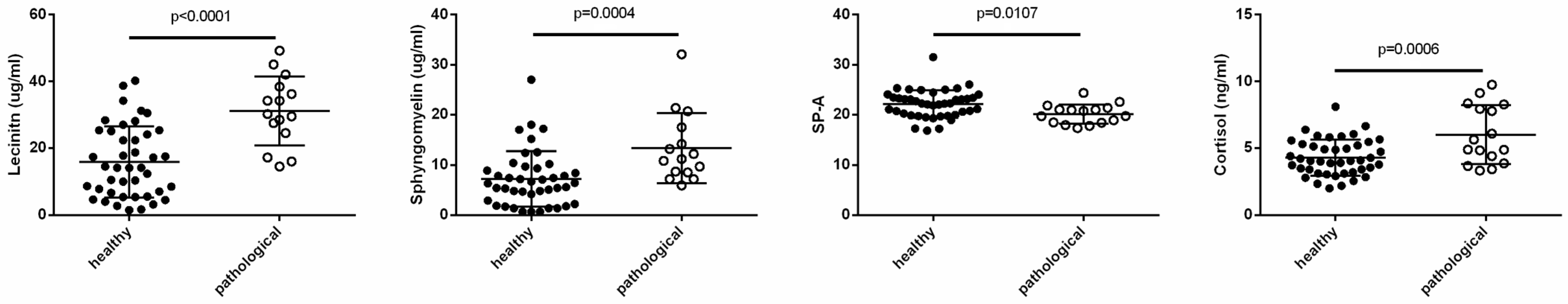
3.1. Clinical Outcomes
3.2. Immunization State
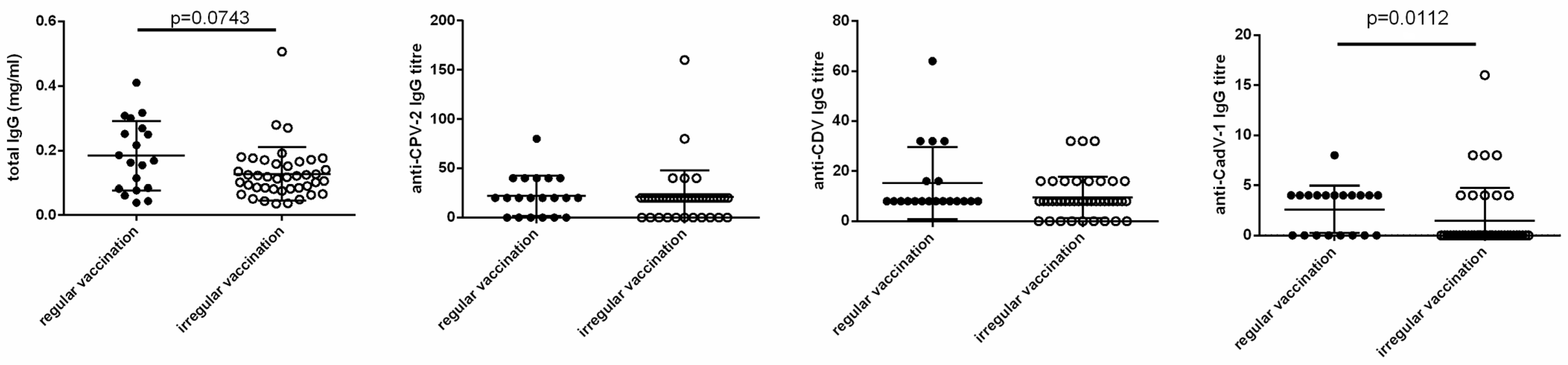
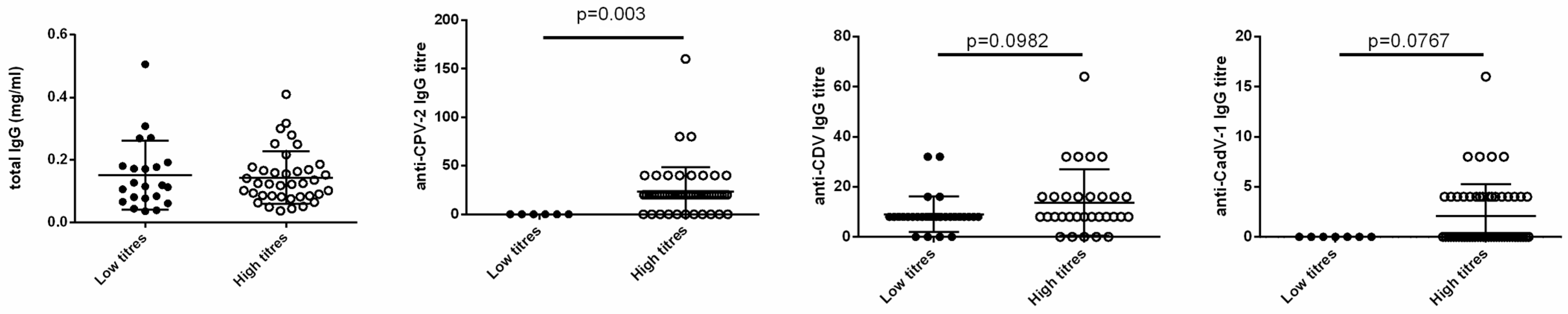
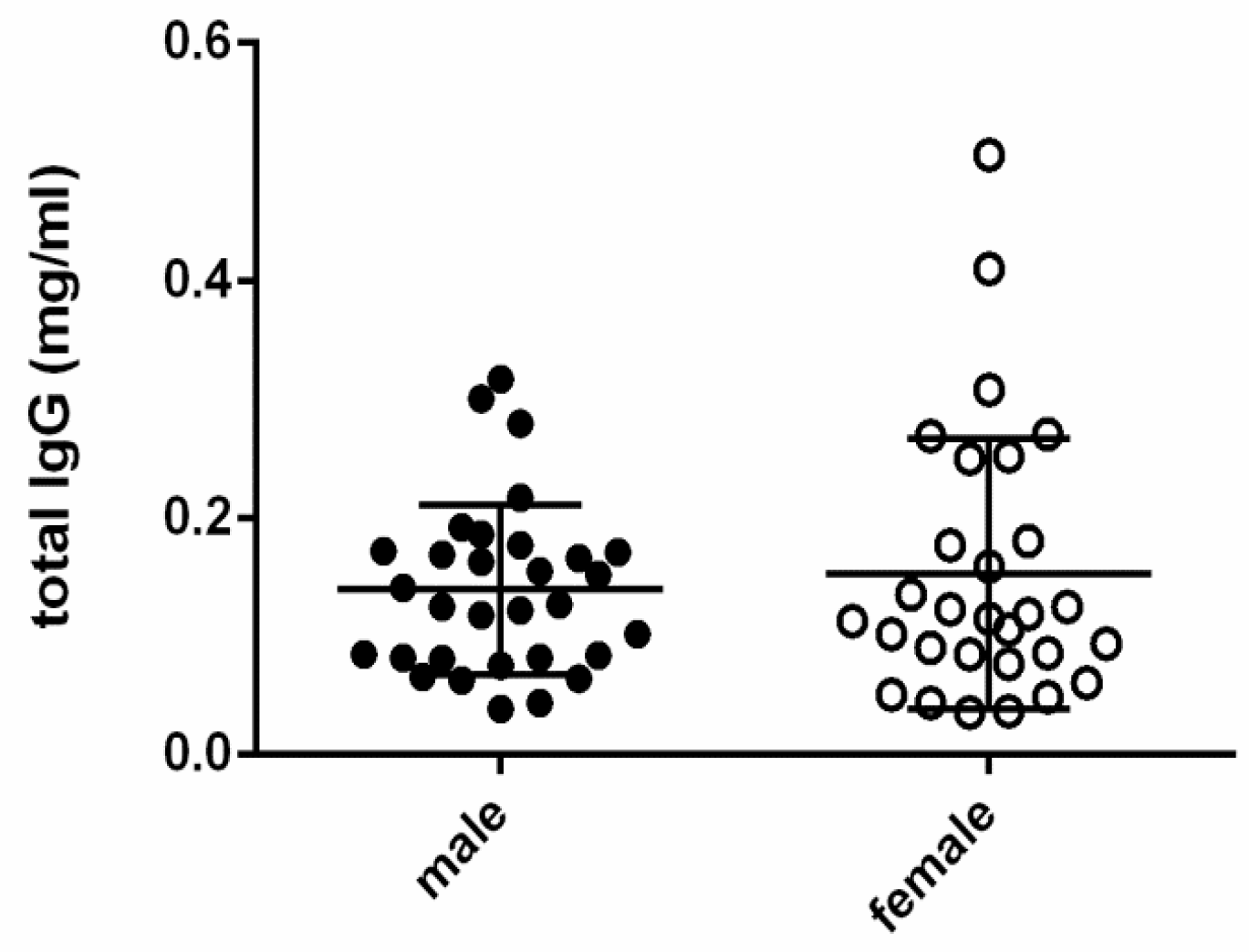
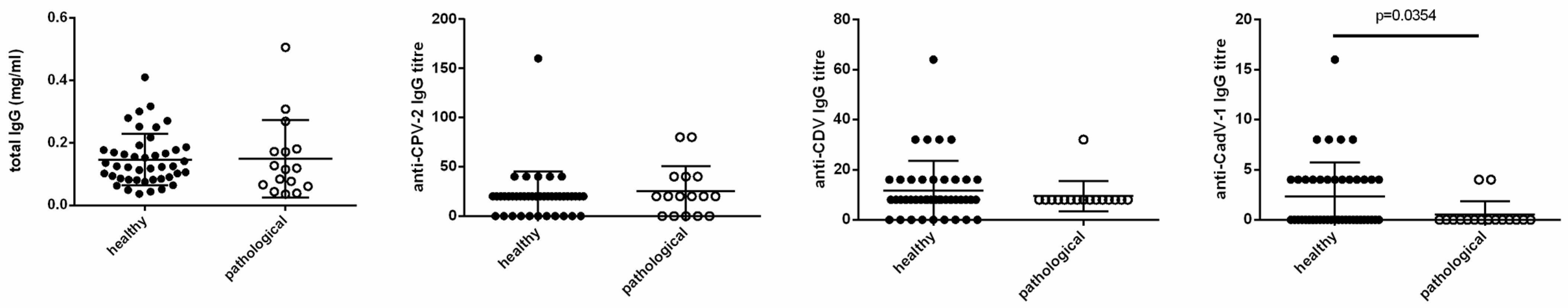
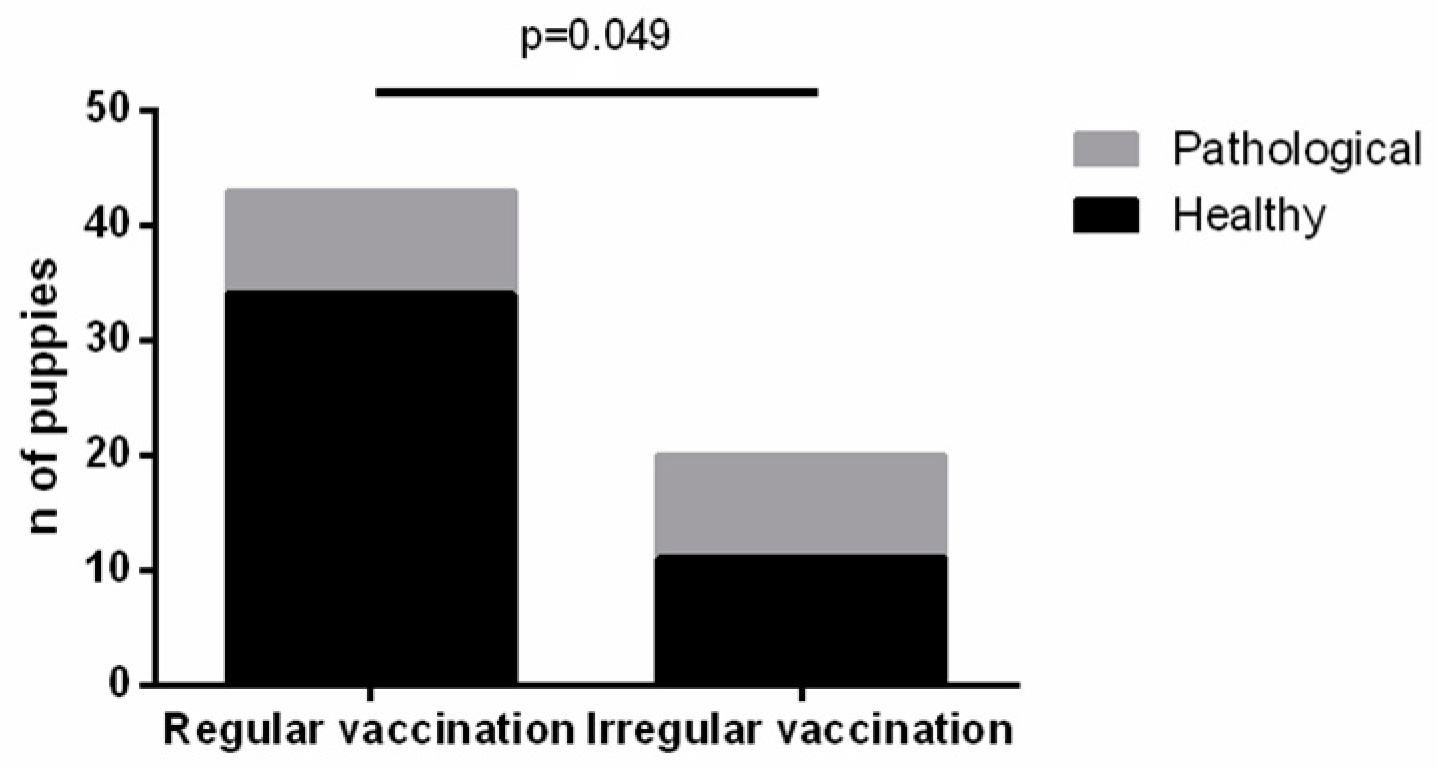
3.3. Statistical Correlations with Clinical Features
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Day, M.J.; Schultz, R.D. Veterinary Immunology: Principles and Practice, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar] [CrossRef]
- Pereira, M.; Valério-Bolas, A.; Saraiva-Marques, C.; Alexandre-Pires, G.; Pereira da Fonseca, I.; Santos-Gomes, G. Development of Dog Immune System: From in Uterus to Elderly. Vet. Sci. 2019, 6, 83. [Google Scholar] [CrossRef]
- Schultz, R.D.; Thiel, B.; Mukhtar, E.; Sharp, P.; Larson, L.J. Age and long-term protective immunity in dogs and cats. J. Comp. Pathol. 2010, 142 (Suppl. 1), S102–S108. [Google Scholar] [CrossRef]
- Nowicki, S.; Goldblum, R.M. Amniotic Fluid and the Fetal Mucosal Immune System, 4th ed.; Mucosal Immunology 2015; Elsevier: Amsterdam, The Netherlands, 2015; Volume 2, Chapter 115; pp. 2251–2268. [Google Scholar]
- Mila, H.; Grellet, A.; Desario, C.; Feugier, A.; Decaro, N.; Buonavoglia, C.; Chastant-Maillard, S. Protection against canine parvovirus type 2 infection in puppies by colostrum-derived antibodies. J. Nutr. Sci. 2014, 3, e54. [Google Scholar] [CrossRef] [PubMed]
- Chastant-Maillard, S.; Aggouni, C.; Albaret, A.; Fournier, A.; Mila, H. Canine and feline colostrum. Reprod. Domest. Anim. 2017, 52 (Suppl. 2), 148–152. [Google Scholar] [CrossRef] [PubMed]
- Day, M.J.; Horzinek, M.C.; Schultz, R.D.; Squires, R.A.; Vaccination Guidelines Group (VGG) of the World Small Animal Veterinary Association (WSAVA). WSAVA Guidelines for the vaccination of dogs and cats. J. Small Anim. Pract. 2016, 57, E1–E45. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.; Marziani, E.; Aziz, C.; Brown, C.M.; Cohn, L.A.; Lea, C.; Moore, G.E.; Taneja, N. 2022 AAHA Canine Vaccination Guidelines. J. Am. Anim. Hosp. Assoc. 2022, 58, 213–230. [Google Scholar] [CrossRef]
- Federation of Veterinarians of Europe (FVE). Joint American Veterinary Medical Association (AVMA)-Federation of Veterinarians of Europe (FVE)-Canadian Veterinary Medical Association (CVMA) Statement on the Benefits of Animal Vaccination Programs in Advancing Animal and Human Health. Available online: https://fve.org/cms/wp-content/uploads/AVMA-CVMA-FVE_vacconation_joint-paper.docx.pdf (accessed on 16 November 2018).
- Dall’Ara, P.; Meloni, T.; Rota, A.; Servida, F.; Filipe, J.; Veronesi, M.C. Immunoglobulins G and lysozyme concentrations in canine fetal fluids at term of pregnancy. Theriogenology 2015, 83, 766–771. [Google Scholar] [CrossRef]
- Dall’Ara, P. Vaccini e Vaccinazioni Degli Animali da Compagnia, 1st ed.; EDRA: Milano, Italy, 2020. [Google Scholar]
- Day, M.J. Immunoglobulin G subclass distribution in canine leishmaniosis: A review and analysis of pitfalls in interpretation. Vet. Parasitol. 2007, 147, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, M.H.; Friess, A.E.; Hartmann, S.H. Ultrastructural evidence of transplacental transport of immunoglobulin G in bitches. J. Reprod. Fertil. 2000, 118, 315–326. [Google Scholar] [CrossRef]
- Melville, J.M.; Moss, T.J. The immune consequences of preterm birth. Front. Neurosci. 2013, 7, 79. [Google Scholar] [CrossRef]
- Gordon, S.M.; O’Connell, A.E. Inborn Errors of Immunity in the Premature Infant: Challenges in Recognition and Diagnosis. Front. Immunol. 2021, 12, 758373. [Google Scholar] [CrossRef] [PubMed]
- Nunez, N.; Réot, L.; Menu, E. Neonatal Immune System Ontogeny: The Role of Maternal Microbiota and Associated Factors. How Might the Non-Human Primate Model Enlighten the Path? Vaccines 2021, 9, 584. [Google Scholar] [CrossRef] [PubMed]
- Nizard, J. Amniocentesis: Technique and education. Curr. Opin. Obstet. Gynecol. 2010, 22, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Tal, S.; Bar-Gal, G.K.; Arlt, S.P. Evaluation of short-term safety of ultrasound-guided foetal fluid sampling in the dog (Canis lupus familiaris). Vet. Rec. 2021, 188, e31. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, C.R. Measurement of pulmonary surfactant in amniotic fluid in the assessment of fetal lung development and of the risk of neonatal respiratory distress. Eur. J. Obstet. Gynecol. Reprod. Biol. 1973, 3, 215–223. [Google Scholar] [CrossRef]
- Shimizu, H.; Hosoda, K.; Mizumoto, M.; Kuroki, Y.; Sato, H.; Kataoka, K.; Hagisawa, M.; Fujimoto, S.; Akino, T. Improved immunoassay for the determination of surfactant protein A (SP-A) in human amniotic fluid. Tohoku J. Exp. Med. 1989, 157, 269–278. [Google Scholar] [CrossRef]
- Bairoch, A. PROSITE: A dictionary of sites and patterns in proteins. Nucleic Acids Res. 1992, 20, 2013–2018. [Google Scholar] [CrossRef] [PubMed]
- Pepe, G.J.; Ballard, P.L.; Albrecht, E.D. Fetal lung maturation in estrogen-deprived baboons. J. Clin. Endocrinol. Metab. 2003, 88, 471–477. [Google Scholar] [CrossRef] [PubMed]
- St Clair, C.; Norwitz, E.R.; Woensdregt, K.; Cackovic, M.; Shaw, J.A.; Malkus, H.; Ehrenkranz, R.A.; Illuzzi, J.L. The probability of neonatal respiratory distress syndrome as a function of gestational age and lecithin/sphingomyelin ratio. Am. J. Perinatol. 2008, 25, 473–480. [Google Scholar] [CrossRef]
- Cruciani, L.; Romero, R.; Vaisbuch, E.; Kusanovic, J.P.; Chaiworapongsa, T.; Mazaki-Tovi, S.; Mittal, P.; Ogge, G.; Gotsch, F.; Erez, O.; et al. Pentraxin 3 in amniotic fluid: A novel association with intra-amniotic infection and inflammation. J. Perinat. Med. 2010, 38, 161–171. [Google Scholar] [CrossRef]
- Larsson, A.; Palm, M.; Helmersson, J.; Axelsson, O. Pentraxin 3 values during normal pregnancy. Inflammation 2011, 34, 448–451. [Google Scholar] [CrossRef]
- Silva, L.G.; Portari, G.V.; Lúcio, C.F.; Rodrigues, J.A.; Veiga, G.L.; Vannucchi, C.I. The influence of the obstetrical condition on canine neonatal pulmonary functional competence. J. Veter.-Emerg. Crit. Care 2015, 25, 725–730. [Google Scholar] [CrossRef]
- Riva, F.; Filipe, J.; Pavlovic, R.; Luciano, A.M.; Dall’ara, P.; Arioli, F.; Pecile, A.; Groppetti, D. Canine amniotic fluid at birth: From a discarded sample to a potential diagnostic of neonatal maturity. Anim. Reprod. Sci. 2023, 248, 107184. [Google Scholar] [CrossRef]
- Martin, L.F.; Moço, N.P.; Ramos, B.R.; Camargo, R.P.; Silva, M.G. Pentraxin-3 concentration in the amniotic fluid of women at term, in spontaneous preterm labor and when not in labor. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 176, 86–89. [Google Scholar] [CrossRef]
- Doni, A.; Stravalaci, M.; Inforzato, A.; Magrini, E.; Mantovani, A.; Garlanda, C.; Bottazzi, B. The Long Pentraxin PTX3 as a Link between Innate Immunity, Tissue Remodeling, and Cancer. Front. Immunol. 2019, 10, 712. [Google Scholar] [CrossRef]
- Giacomini, A.; Ghedini, G.C.; Presta, M.; Ronca, R. Long pentraxin 3, A novel multifaceted player in cancer. Biochim. Biophys. Acta Rev. Cancer 2018, 1869, 53–63. [Google Scholar] [CrossRef]
- Porte, R.; Davoudian, S.; Asgari, F.; Parente, R.; Mantovani, A.; Garlanda, C.; Bottazzi, B. The Long Pentraxin PTX3 as a Humoral Innate Immunity Functional Player and Biomarker of Infections and Sepsis. Front. Immunol. 2019, 10, 794. [Google Scholar] [CrossRef]
- Cetin, I.; Cozzi, V.; Papageorghiou, A.T.; Maina, V.; Montanelli, A.; Garlanda, C.; Thilaganathan, B. First trimester PTX3 levels in women who subsequently develop preeclampsia and fetal growth restriction. Acta Obstet. Gynecol. Scand. 2009, 88, 846–849. [Google Scholar] [CrossRef]
- Ibrahim, M.I.; Ammar, E.M.; Ramy, A.; Ellaithy, M.I.; Abdelrahman, R.M.; Elkabarity, R. The association between pentraxin 3 in maternal circulation and pathological intrauterine fetal growth restriction. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 185, 1–8. [Google Scholar] [CrossRef]
- Ibrahim, M.I.; Harb, H.M.; Ellaithy, M.I.; Elkabarity, R.H.; Abdelgwad, M.H. First trimester assessment of pentraxin-3 levels in women with primary unexplained recurrent pregnancy loss. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 165, 37–41. [Google Scholar] [CrossRef]
- Ge, M.; Wang, M.; Liu, Y.; Yue, H.; Ding, J.; Wang, X.; Yao, T.; Gao, H. Proteomic Analysis of Preeclampsia Amniotic Fluid Based on a Novel Solid-State Preservation Method. Biopreserv Biobank. 2024. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Rovere-Querini, P.; Antonacci, S.; Dellʼantonio, G.; Angeli, A.; Almirante, G.; Cin, E.D.; Valsecchi, L.; Lanzani, C.; Sabbadini, M.G.; Doglioni, C.; et al. Plasma and tissue expression of the long pentraxin 3 during normal pregnancy and preeclampsia. Obstet. Gynecol. 2006, 108, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Groppetti, D.; Di Cesare, F.; Pecile, A.; Cagnardi, P.; Merlanti, R.; D’Urso, E.S.; Gioeni, D.; Boracchi, P.; Ravasio, G. Maternal and neonatal wellbeing during elective C-section induced with a combination of propofol and dexmedetomidine: How effective is the placental barrier in dogs? Theriogenology 2019, 129, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Groppetti, D.; Vegetti, F.; Bronzo, V.; Pecile, A. Breed-specific fetal biometry and factors affecting the prediction of whelping date in the German shepherd dog. Anim. Reprod. Sci. 2015, 152, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Gil, E.M.; Garcia, D.A.; Froes, T.R. In utero development of the fetal intestine: Sonographic evaluation and correlation with gestational age and fetal maturity in dogs. Theriogenology 2015, 84, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Roos, J.; Maenhoudt, C.; Zilberstein, L.; Mir, F.; Borges, P.; Furthner, E.; Niewiadomska, Z.; Nudelmann, N.; Fontbonne, A. Neonatal puppy survival after planned caesarean section in the bitch using aglepristone as a primer: A retrospective study on 74 cases. Reprod. Domest. Anim. 2018, 53 (Suppl. 3), 85–95. [Google Scholar] [CrossRef] [PubMed]
- Peterson, M.E.; Kutzler, M.A. Small Animal Pediatrics, 1st ed.; WB Saunders: Amsterdam, The Netherlands, 2011; pp. 11–19. [Google Scholar]
- Groppetti, D.; Pecile, A.; Del Carro, A.P.; Copley, K.; Minero, M.; Cremonesi, F. Evaluation of newborn canine viability by means of umbilical vein lactate measurement, apgar score and uterine tocodynamometry. Theriogenology 2010, 74, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Schultz, R.A. Field and Experimental Trial to Assess the Performance of the ImmunoComb Canine VacciCheck Antibody Test Kit; BiogalGaled Labs: Kibbutz Galed, Israel, 2015. [Google Scholar]
- Underwood, M.A.; Gilbert, W.M.; Sherman, M.P. Amniotic fluid: Not just fetal urine anymore. J. Perinatol. 2005, 25, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Geer, L.A.; Pycke, B.F.; Sherer, D.M.; Abulafia, O.; Halden, R.U. Use of amniotic fluid for determining pregnancies at risk of preterm birth and for studying diseases of potential environmental etiology. Environ. Res. 2015, 136, 470–481. [Google Scholar] [CrossRef]
- Srivastava, M.; Ahlawat, N.; Srivastava, A. Amniotic Fluid Stem Cells: A New Era in Regenerative Medicine. J. Obstet. Gynaecol. India 2018, 68, 15–19. [Google Scholar] [CrossRef]
- Shah, U.; Dickinson, B.L.; Blumberg, R.S.; Simister, N.E.; Lencer, W.I.; Walker, W.A. Distribution of the IgG Fc receptor, FcRn, in the human fetal intestine. Pediatr. Res. 2003, 53, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.; Siedek, E.; Thomas, A.; King, V.; Stirling, C.; Plevová, E.; Salt, J.; Sture, G. Influence of maternally-derived antibodies in 6-week old dogs for the efficacy of a new vaccine to protect dogs against virulent challenge with canine distemper virus, adenovirus or parvovirus. Trials Vaccinol. 2014, 3, 107–113. [Google Scholar] [CrossRef]
- Mila, H.; Feugier, A.; Grellet, A.; Anne, J.; Gonnier, M.; Martin, M.; Rossig, L.; Chastant-Maillard, S. Inadequate Passive Immune Transfer in Puppies: Definition, Risk Factors and Prevention in a Large Multi-Breed Kennel. Prev. Vet. Med. 2014, 116, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Mila, H.; Feugier, A.; Grellet, A.; Anne, J.; Gonnier, M.; Martin, M.; Rossig, L.; Chastant-Maillard, S.; Immunoglobulin, G. Concentration in Canine Colostrum: Evaluation and Variability. J. Reprod. Immunol. 2015, 112, 24–28. [Google Scholar] [CrossRef]
- Chastant, S.; Mila, H. Passive Immune Transfer in Puppies. Anim. Reprod. Sci. 2019, 207, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Chastant, S. Lactation in domestic carnivores. Anim. Front. 2023, 13, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, A.M.; Dumitru, A.E.; Gica, N.; Botezatu, R.; Peltecu, G.; Panaitescu, A.M. Benefits and Risks of IgG Transplacental Transfer. Diagnostics 2020, 10, 583. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.M. Changes in serum immunity during pregnancy. Am. J. Hum. Biol. 2009, 21, 401–403. [Google Scholar] [CrossRef] [PubMed]
- Larson, L.; Thiel, B.; Santana, V.; Schultz, R. Canine nomograph evaluation improves puppy immunization. Clin. Theriogenology 2020, 12, 216–221. [Google Scholar]
- Dall’Ara, P.; Lauzi, S.; Zambarbieri, J.; Servida, F.; Barbieri, L.; Rosenthal, R.; Turin, L.; Scarparo, E.; Filipe, J. Prevalence of Serum Antibody Titers against Core Vaccine Antigens in Italian Dogs. Life 2023, 13, 587. [Google Scholar] [CrossRef]
- Dodds, W.J. Gender affects immune response to viruses and vaccines. Glob. Vaccines Immunol. 2016, 2, 1–3. [Google Scholar] [CrossRef][Green Version]
- Shah, P.S.; Diambomba, Y.; Acharya, G.; Morris, S.K.; Bitnun, A. Classification system and case definition for SARS-CoV-2 infection in pregnant women, fetuses, and neonates. Acta Obstet. Gynecol. Scand. 2020, 99, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Pollock, R.V.; Carmichael, L.E. Maternally derived immunity to canine parvovirus infection: Transfer, decline, and interference with vaccination. J. Am. Vet. Med. Assoc. 1982, 180, 37–42. [Google Scholar] [PubMed]
- Beijers, R.; Buitelaar, J.K.; de Weerth, C. Mechanisms underlying the effects of prenatal psychosocial stress on child outcomes: Beyond the HPA axis. Eur. Child. Adolesc. Psychiatry 2014, 23, 943–956. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yao, G.; Wang, R.; Zhu, J.; Li, H.; Yang, D.; Ma, S.; Fu, Y.; Liu, C.; Guan, S. Maternal immune activation mediated prenatal chronic stress induces Th17/Treg cell imbalance may relate to the PI3K/Akt/NF-κB signaling pathway in offspring rats. Int. Immunopharmacol. 2024, 126, 111308. [Google Scholar] [CrossRef] [PubMed]
- Melum, E.; Jiang, X.; Baker, K.D.; Macedo, M.F.; Fritsch, J.; Dowds, C.M.; Wang, J.; Pharo, A.; Kaser, A.; Tan, C.; et al. Control of CD1d-restricted antigen presentation and inflammation by sphingomyelin. Nat. Immunol. 2019, 20, 1644–1655. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Betsuyaku, T.; Kuroki, Y.; Nagai, K.; Nasuhara, Y.; Nishimura, M. Effects of Ageing and Smoking on SP-A and SP-D Levels in Bronchoalveolar Lavage Fluid. Eur. Respir. J. 2004, 24, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Ajanović, A.; Sofić, E.; Tahirović, I.; Šapčanin, A.; Uzunović, A.; Krehić, J.; Gojak, R.; Dizdar, M. Changes in lecithin concentrations in human blood with aging. Bull. Chem. Technol. Bosnia Herzeg. 2015, 44, 59–64. [Google Scholar]
- Gewolb, I.H.; Hobbins, J.C.; Tan, S.Y. Amniotic fluid cortisol in high-risk human pregnancies. Obstet. Gynecol. 1977, 49, 466–470. [Google Scholar]
- Swanson, J.M.; Entringer, S.; Buss, C.; Wadhwa, P.D. Developmental Origins of Health and Disease: Environmental Exposures. Semin. Reprod. Med. 2009, 27, 391–402. [Google Scholar] [CrossRef]
- Garcia-Flores, V.; Romero, R.; Furcron, A.-E.; Levenson, D.; Galaz, J.; Zou, C.; Hassan, S.S.; Hsu, C.-D.; Olson, D.; Metz, G.A.S.; et al. Prenatal Maternal Stress Causes Preterm Birth and Affects Neonatal Adaptive Immunity in Mice. Front. Immunol. 2020, 11, 254. [Google Scholar] [CrossRef]
- King, S.D.; Chen, S.Y. Recent progress on surfactant protein A: Cellular function in lung and kidney disease development. American journal of physiology. Cell Physiol. 2020, 319, C316–C320. [Google Scholar] [CrossRef]
- Witkin, S.S.; Skupski, D.; Herway, C.; Rudge, M.V.; Saito, F.; Harris, M. Fatty acid composition of mid-trimester amniotic fluid in women of different ethnicities. J. Matern. Fetal Neonatal Med. 2012, 25, 818–821. [Google Scholar] [CrossRef]
- Peltier, M.R.; Drobek, C.O.; Bhat, G.; Saade, G.; Fortunato, S.J.; Menon, R. Amniotic fluid and maternal race influence responsiveness of fetal membranes to bacteria. J. Reprod. Immunol. 2012, 96, 68–78. [Google Scholar] [CrossRef][Green Version]
- Gough, A.; Thomas, A. Breed Predispositions to Disease in Dogs and Cats; Wiley-Blackwell: Hoboken, NJ, USA, 2004. [Google Scholar]
- Sahraei, H.; Mogheiseh, A.; Nazifi, S.; Divar, M.R.; Iraji, F. Canine and feline foetal fluids: Volume, hormonal and biochemical characterization during pregnancy. Vet. Med. Sci. 2024, 10, e1452. [Google Scholar] [CrossRef]
- Mormède, P.; Foury, A.; Terenina, E.; Knap, P.W. Breeding robustness: The role of cortisol. Animal 2011, 5, 657–661. [Google Scholar] [CrossRef]
- Mansfield, K.L.; Sayers, R.; Fooks, A.R.; Burr, P.D.; Snodgrass, D. Factors Affecting the Serological Response of Dogs and Cats to Rabies Vaccination. Vet. Rec. 2004, 154, 423–426. [Google Scholar] [CrossRef]
- Kennedy, L.J.; Lunt, M.; Barnes, A.; McElhinney, L.; Fooks, A.R.; Baxter, D.N.; Ollier, W.E.R. Factors Influencing the Antibody Response of Dogs Vaccinated against Rabies. Vaccine 2007, 25, 8500–8507. [Google Scholar] [CrossRef]
- Dall’Ara, P.; Lauzi, S.; Turin, L.; Castaldelli, G.; Servida, F.; Filipe, J. Effect of Aging on the Immune Response to Core Vaccines in Senior and Geriatric Dogs. Vet. Sci. 2023, 10, 412. [Google Scholar] [CrossRef]

| ID | Breed | Age (ys) | BW * (kg) | Litter Size |
|---|---|---|---|---|
| 1 | German Shepherd | 7 | 34.5 | 8 |
| 2 | German Shepherd | 5 | 28.3 | 6 |
| 3 | American Bully | 2 | 23.8 | 2 |
| 4 | American Bully | 3 | 23.3 | 4 |
| 5 | American Bully | 2.5 | 38.5 | 11 |
| 6 | Rhodesian Ridgeback | 7 | 40.5 | 11 |
| 7 | American Bully | 2.5 | 28.5 | 5 |
| 8 | French Bouledogue | 3 | 12.5 | 6 |
| 9 | French Bouledogue | 3.5 | 11.4 | 2 |
| 10 | Bernese Mountain Dog | 3 | 56.2 | 8 |
| Mean ± SD | 3.9 ± 1.8 | 29.7 ± 13.4 | 6.3 ± 3.2 |
| Plasma | AF | |||||||
|---|---|---|---|---|---|---|---|---|
| CPV-2 1 | number of dams (n = 10) | number of puppies (n = 61) | ||||||
| min–max | ≤1:20 | 1:160–320 | >1:320 | min–max | Negative | ≤1:20 | >1:20 | |
| 1:20–1:640 | 1 | 4 | 5 | negative—1:40 | 17 | 33 | 11 | |
| CAdV-1 2 | number of dams (n = 10) | number of puppies (n = 61) | ||||||
| min–max | ≤1:4 | ≤1:32 | >1:32 | Negative | ≤1:4 | >1:8 | ||
| 1:4–1:128 | 2 | 2 | 6 | negative—1:4 | 39 | 16 | 6 | |
| number of dams (n = 10) | number of puppies (n = 61) | |||||||
| min–max | ≤1:8 | ≤1:16 | >1:16 | Negative | ≤1:16 | >1:16 | ||
| CDV 3 | 1:8–1:128 | 2 | 3 | 5 | negative—1:16 | 9 | 45 | 7 |
| Protective Threshold (S3) | CPV-2 ≥1:80 * | CAdV-1 ≥1:16 * | CDV ≥1:32 * |
|---|---|---|---|
| Percentage of regularly vaccinated (4) protected bitches | 100 | 100 | 75 |
| Percentage of irregularly vaccinated (6) protected bitches | 83.3 | 66.7 | 33.3 |
| Percentage of protected bitches out of the total (10) | 90 | 80 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Groppetti, D.; Pecile, A.; Filipe, J.; Riva, F.; Inglesi, A.; Kuhn, P.A.; Giussani, E.; Dall’Ara, P. Canine Amniotic Fluid at Birth Holds Information about Neonatal Antibody Titres against Core Vaccine Viruses. Vet. Sci. 2024, 11, 234. https://doi.org/10.3390/vetsci11060234
Groppetti D, Pecile A, Filipe J, Riva F, Inglesi A, Kuhn PA, Giussani E, Dall’Ara P. Canine Amniotic Fluid at Birth Holds Information about Neonatal Antibody Titres against Core Vaccine Viruses. Veterinary Sciences. 2024; 11(6):234. https://doi.org/10.3390/vetsci11060234
Chicago/Turabian StyleGroppetti, Debora, Alessandro Pecile, Joel Filipe, Federica Riva, Alessia Inglesi, Pietro Andrea Kuhn, Elisa Giussani, and Paola Dall’Ara. 2024. "Canine Amniotic Fluid at Birth Holds Information about Neonatal Antibody Titres against Core Vaccine Viruses" Veterinary Sciences 11, no. 6: 234. https://doi.org/10.3390/vetsci11060234
APA StyleGroppetti, D., Pecile, A., Filipe, J., Riva, F., Inglesi, A., Kuhn, P. A., Giussani, E., & Dall’Ara, P. (2024). Canine Amniotic Fluid at Birth Holds Information about Neonatal Antibody Titres against Core Vaccine Viruses. Veterinary Sciences, 11(6), 234. https://doi.org/10.3390/vetsci11060234









