Myxomatous Mitral Valve Disease in Large Breed Dogs: Survival Characteristics and Prognostic Variables
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
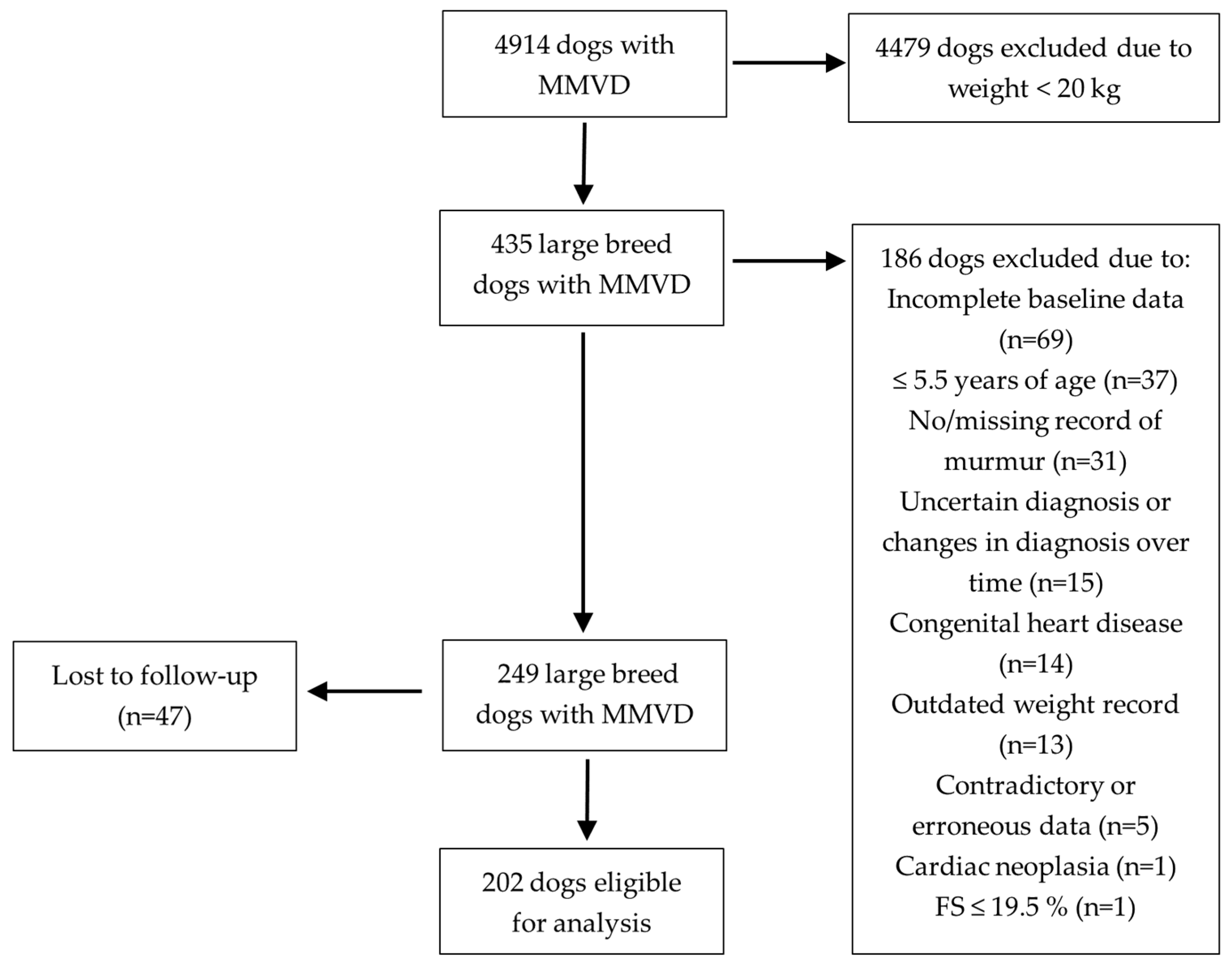
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Additional Data
2.5. Follow-Up
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Echocardiographic Measurements at the Time of Inclusion
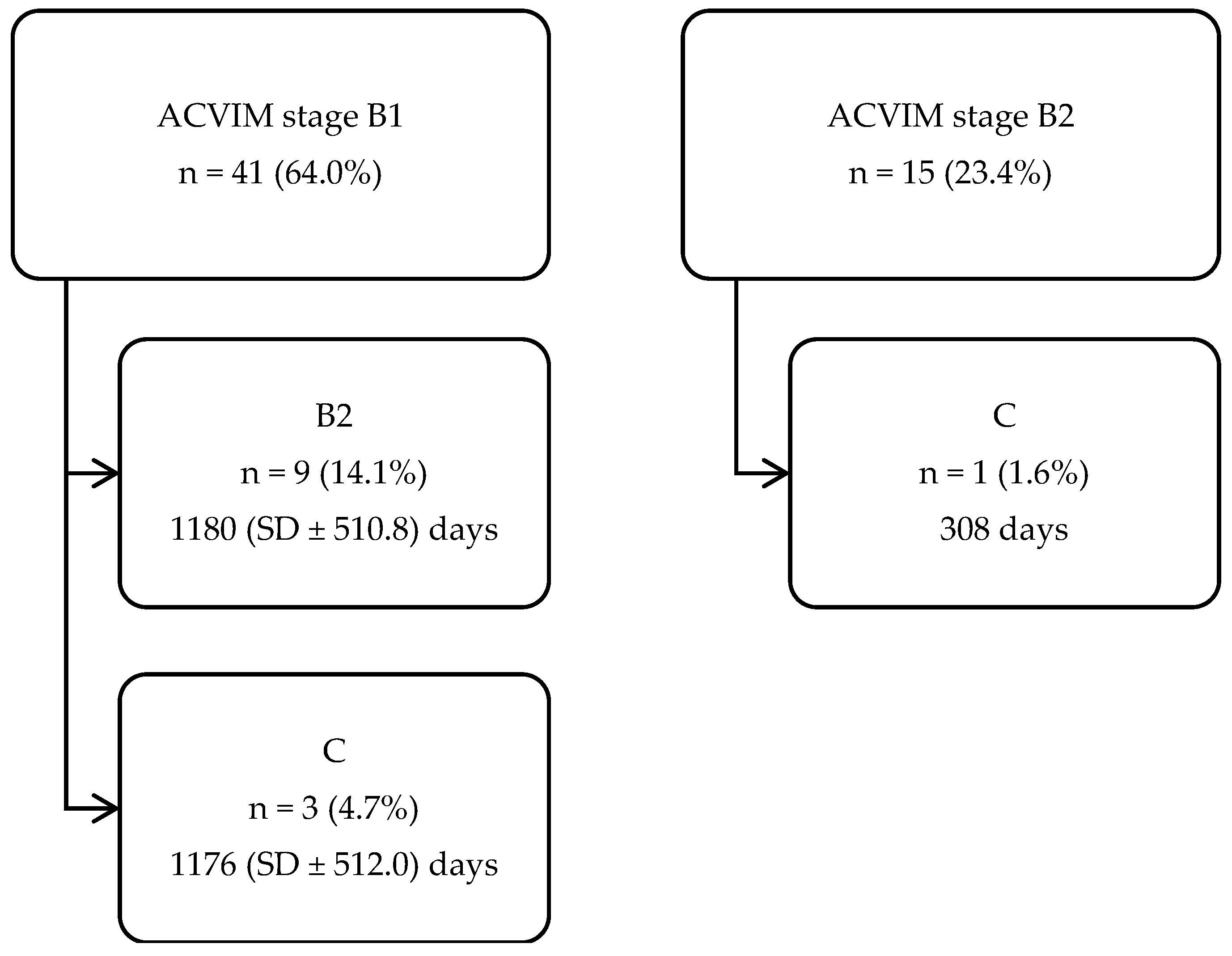
3.3. Disease Progression (from One ACVIM Stage to the Next)
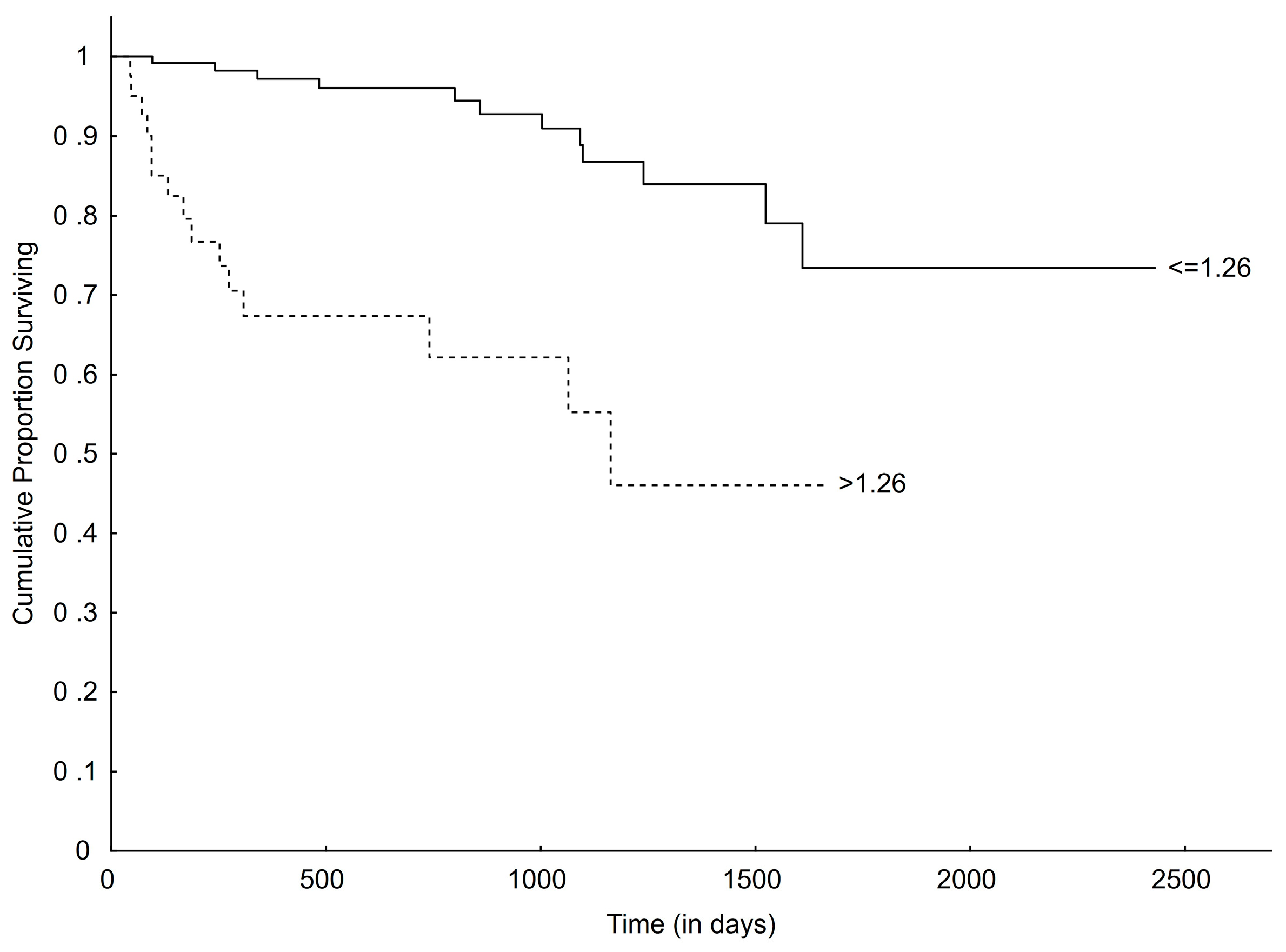
3.4. Survival
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Egenvall, A.; Bonnett, B.N.; Häggström, J. Heart Disease as a Cause of Death in Insured Swedish Dogs Younger Than 10 Years of Age. J. Vet. Intern. Med. 2006, 20, 894–903. [Google Scholar] [CrossRef]
- Häggström, J.; Höglund, K.; Borgarelli, M. An Update on Treatment and Prognostic Indicators in Canine Myxomatous Mitral Valve Disease. J. Small Anim. Pract. 2009, 50, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Borgarelli, M.; Haggstrom, J. Canine Degenerative Myxomatous Mitral Valve Disease: Natural History, Clinical Presentation and Therapy. Vet. Clin. N. Am. Small Anim. Pract. 2010, 40, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Fox, P.R. Pathology of Myxomatous Mitral Valve Disease in the Dog. J. Vet. Cardiol. 2012, 14, 103–126. [Google Scholar] [CrossRef]
- Mattin, M.J.; Boswood, A.; Church, D.B.; López-Alvarez, J.; McGreevy, P.D.; O’Neill, D.G.; Thomson, P.C.; Brodbelt, D.C. Prevalence of and Risk Factors for Degenerative Mitral Valve Disease in Dogs Attending Primary-care Veterinary Practices in England. J. Vet. Intern. Med. 2015, 29, 847–854. [Google Scholar] [CrossRef]
- Keene, B.W.; Atkins, C.E.; Bonagura, J.D.; Fox, P.R.; Häggström, J.; Fuentes, V.L.; Oyama, M.A.; Rush, J.E.; Stepien, R.; Uechi, M. ACVIM Consensus Guidelines for the Diagnosis and Treatment of Myxomatous Mitral Valve Disease in Dogs. J. Vet. Intern. Med. 2019, 33, 1127–1140. [Google Scholar] [CrossRef]
- Borgarelli, M.; Savarino, P.; Crosara, S.; Santilli, R.A.; Chiavegato, D.; Poggi, M.; Bellino, C.; La Rosa, G.; Zanatta, R.; Haggstrom, J.; et al. Survival Characteristics and Prognostic Variables of Dogs with Mitral Regurgitation Attributable to Myxomatous Valve Disease. J. Vet. Intern. Med. 2008, 22, 120–128. [Google Scholar] [CrossRef]
- Boswood, A.; Häggström, J.; Gordon, S.G.; Wess, G.; Stepien, R.L.; Oyama, M.A.; Keene, B.W.; Bonagura, J.; MacDonald, K.A.; Patteson, M.; et al. Effect of Pimobendan in Dogs with Preclinical Myxomatous Mitral Valve Disease and Cardiomegaly: The EPIC Study—A Randomized Clinical Trial. J. Vet. Intern. Med. 2016, 30, 1765–1779. [Google Scholar] [CrossRef] [PubMed]
- Häggström, J.; Boswood, A.; O’Grady, M.; Jöns, O.; Smith, S.; Swift, S.; Borgarelli, M.; Gavaghan, B.; Kresken, J.-G.; Patteson, M.; et al. Effect of Pimobendan or Benazepril Hydrochloride on Survival Times in Dogs with Congestive Heart Failure Caused by Naturally Occurring Myxomatous Mitral Valve Disease: The QUEST Study. J. Vet. Intern. Med. 2008, 22, 1124–1135. [Google Scholar] [CrossRef]
- Swenson, L.; Häggström, J.; Kvart, C.; Juneja, R.K. Relationship between Parental Cardiac Status in Cavalier King Charles Spaniels and Prevalence and Severity of Chronic Valvular Disease in Offspring. J. Am. Vet. Med. Assoc. 1996, 208, 2009–2012. [Google Scholar] [CrossRef]
- Olsen, L.H.; Fredholm, M.; Pedersen, H.D. Epidemiology and Inheritance of Mitral Valve Prolapse in Dachshunds. J. Vet. Intern. Med. 1999, 13, 448–456. [Google Scholar] [CrossRef]
- Lewis, T.; Swift, S.; Woolliams, J.A.; Blott, S. Heritability of Premature Mitral Valve Disease in Cavalier King Charles Spaniels. Vet. J. 2011, 188, 73–76. [Google Scholar] [CrossRef]
- Birkegård, A.C.; Reimann, M.J.; Martinussen, T.; Häggström, J.; Pedersen, H.D.; Olsen, L.H. Breeding Restrictions Decrease the Prevalence of Myxomatous Mitral Valve Disease in Cavalier King Charles Spaniels over an 8- to 10-Year Period. J. Vet. Intern. Med. 2016, 30, 63–68. [Google Scholar] [CrossRef]
- Borgarelli, M.; Zini, E.; D’Agnolo, G.; Tarducci, A.; Santilli, R.A.; Chiavegato, D.; Tursi, M.; Prunotto, M.; Häggström, J. Comparison of Primary Mitral Valve Disease in German Shepherd Dogs and in Small Breeds. J. Vet. Cardiol. 2004, 6, 27–34. [Google Scholar] [CrossRef]
- Borgarelli, M.; Crosara, S.; Lamb, K.; Savarino, P.; La Rosa, G.; Tarducci, A.; Haggstrom, J. Survival Characteristics and Prognostic Variables of Dogs with Preclinical Chronic Degenerative Mitral Valve Disease Attributable to Myxomatous Degeneration. J. Vet. Intern. Med. 2012, 26, 69–75. [Google Scholar] [CrossRef]
- Tidholm, A.; Häggström, J. Prognostic Value of Selected One-, Two- and Three-Dimensional and Doppler Echocardiographic Methods to Assess Severity in Dogs with Myxomatous Mitral Valve Disease. J. Vet. Cardiol. 2022, 39, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Kvart, C.; Häggström, J.; Pedersen, H.D.; Hansson, K.; Eriksson, A.; Järvinen, A.; Tidholm, A.; Bsenko, K.; Ahlgren, E.; Lives, M.; et al. Efficacy of Enalapril for Prevention of Congestive Heart Failure in Dogs with Myxomatous Valve Disease and Asymptomatic Mitral Regurgitation. J. Vet. Intern. Med. 2002, 16, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Atkins, C.E.; Keene, B.W.; Brown, W.A.; Coats, J.R.; Crawford, M.A.; DeFrancesco, T.C.; Edwards, N.J.; Fox, P.R.; Lehmkuhl, L.B.; Luethy, M.W.; et al. Results of the Veterinary Enalapril Trial to Prove Reduction in Onset of Heart Failure in Dogs Chronically Treated with Enalapril Alone for Compensated, Naturally Occurring Mitral Valve Insufficiency. J. Am. Vet. Med. Assoc. 2007, 231, 1061–1069. [Google Scholar] [CrossRef]
- Sargent, J.; Muzzi, R.; Mukherjee, R.; Somarathne, S.; Schranz, K.; Stephenson, H.; Connolly, D.; Brodbelt, D.; Fuentes, V.L. Echocardiographic Predictors of Survival in Dogs with Myxomatous Mitral Valve Disease. J. Vet. Cardiol. 2015, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Borgarelli, M.; Ferasin, L.; Lamb, K.; Bussadori, C.; Chiavegato, D.; D’Agnolo, G.; Migliorini, F.; Poggi, M.; Santilli, R.A.; Guillot, E.; et al. DELay of Appearance of sYmptoms of Canine Degenerative Mitral Valve Disease Treated with Spironolactone and Benazepril: The DELAY Study. J. Vet. Cardiol. 2020, 27, 34–53. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Vezzosi, T.; Domenech, O.; Tognetti, R. Prognostic Relevance of Left Cardiac Enlargement in Dogs with Preclinical Myxomatous Mitral Valve Disease. J. Vet. Cardiol. 2023, 45, 50–58. [Google Scholar] [CrossRef]
- Hansson, K.; Häggström, J.; Kvart, C.; Lord, P. Left atrial to aortic root indices using two-dimensional and m-mode echocardiography in cavalier king charles spaniels with and without left atrial enlargement. Vet. Radiol. Ultrasound 2002, 43, 568–575. [Google Scholar] [CrossRef]
- Ljungvall, I.; Rishniw, M.; Porciello, F.; Ferasin, L.; Ohad, D.G. Murmur Intensity in Small-breed Dogs with Myxomatous Mitral Valve Disease Reflects Disease Severity. J. Small Anim. Pract. 2014, 55, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Levine, S.A. The systolic murmur: Its clinical significance. J. Am. Med. Assoc. 1933, 101, 436–438. [Google Scholar] [CrossRef]
- Häggström, J.; Kvart, C.; Hansson, K. Heart Sounds and Murmurs: Changes Related to Severity of Chronic Valvular Disease in the Cavalier King Charles Spaniel. J. Vet. Intern. Med. 1995, 9, 75–85. [Google Scholar] [CrossRef]
- Cornell, C.C.; Kittleson, M.D.; Torre, P.D.; Häggström, J.; Lombard, C.W.; Pedersen, H.D.; Vollmar, A.; Wey, A. Allometric Scaling of M-Mode Cardiac Measurements in Normal Adult Dogs. J. Vet. Intern. Med. 2004, 18, 311–321. [Google Scholar] [CrossRef]
- Payne, J.R.; Borgeat, K.; Brodbelt, D.C.; Connolly, D.J.; Luis Fuentes, V. Risk Factors Associated with Sudden Death vs. Congestive Heart Failure or Arterial Thromboembolism in Cats with Hypertrophic Cardiomyopathy. J. Vet. Cardiol. 2015, 17, S318–S3282015. [Google Scholar] [CrossRef]
- Borgeat, K.; Pack, M.; Harris, J.; Laver, A.; Seo, J.; Belachsen, O.; Hannabuss, J.; Todd, J.; Ferasin, L.; Payne, J.R. Prevalence of Sudden Cardiac Death in Dogs with Atrial Fibrillation. J. Vet. Intern. Med. 2021, 35, 2588–2595. [Google Scholar] [CrossRef] [PubMed]
- Pouchelon, J.-L.; Jamet, N.; Gouni, V.; Tissier, R.; Serres, F.; Carlos Sampedrano, C.; Castaignet, M.; Lefebvre, H.P.; Chetboul, V. Effect of Benazepril on Survival and Cardiac Events in Dogs with Asymptomatic Mitral Valve Disease: A Retrospective Study of 141 Cases. J. Vet. Intern. Med. 2008, 22, 905–914. [Google Scholar] [CrossRef]
- Baron Toaldo, M.; Romito, G.; Guglielmini, C.; Diana, A.; Pelle, N.G.; Contiero, B.; Cipone, M. Prognostic Value of Echocardiographic Indices of Left Atrial Morphology and Function in Dogs with Myxomatous Mitral Valve Disease. J. Vet. Intern. Med. 2018, 32, 914–921. [Google Scholar] [CrossRef]
- Boswood, A.; Gordon, S.G.; Häggström, J.; Wess, G.; Stepien, R.L.; Oyama, M.A.; Keene, B.W.; Bonagura, J.; MacDonald, K.A.; Patteson, M.; et al. Longitudinal Analysis of Quality of Life, Clinical, Radiographic, Echocardiographic, and Laboratory Variables in Dogs with Preclinical Myxomatous Mitral Valve Disease Receiving Pimobendan or Placebo: The EPIC Study. J. Vet. Intern. Med. 2018, 32, 72–85. [Google Scholar] [CrossRef]
- Vezzosi, T.; Grosso, G.; Tognetti, R.; Meucci, V.; Patata, V.; Marchesotti, F.; Domenech, O. The Mitral INsufficiency Echocardiographic Score: A Severity Classification of Myxomatous Mitral Valve Disease in Dogs. J. Vet. Intern. Med. 2021, 35, 1238–1244. [Google Scholar] [CrossRef]
- Borgarelli, M.; Tarducci, A.; Zanatta, R.; Haggstrom, J. Decreased Systolic Function and Inadequate Hypertrophy in Large and Small Breed Dogs with Chronic Mitral Valve Insufficiency. J. Vet. Intern. Med. 2007, 21, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Menaut, P.; Bélanger, M.C.; Beauchamp, G.; Ponzio, N.M.; Moïse, N.S. Atrial Fibrillation in Dogs with and without Structural or Functional Cardiac Disease: A Retrospective Study of 109 Cases. J. Vet. Cardiol. 2005, 7, 75–83. [Google Scholar] [CrossRef]
- Jung, S.W.; Sun, W.; Griffiths, L.G.; Kittleson, M.D. Atrial Fibrillation as a Prognostic Indicator in Medium to Large-Sized Dogs with Myxomatous Mitral Valvular Degeneration and Congestive Heart Failure. J. Vet. Intern. Med. 2016, 30, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Pedro, B.; Dukes-McEwan, J.; Oyama, M.A.; Kraus, M.S.; Gelzer, A.R. Retrospective Evaluation of the Effect of Heart Rate on Survival in Dogs with Atrial Fibrillation. J. Vet. Intern. Med. 2018, 32, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Wess, G. Screening for Dilated Cardiomyopathy in Dogs. J. Vet. Cardiol. 2022, 40, 51–68. [Google Scholar] [CrossRef]

| Breed | Number of Dogs | Percent (%) |
|---|---|---|
| Afghan Hound | 2 | 1.0 |
| Airedale Terrier | 7 | 3.5 |
| American Staffordshire Terrier | 2 | 1.0 |
| Australian Kelpie | 5 | 2.5 |
| Australian Shepherd | 5 | 2.5 |
| Australian Stock Dog | 1 | 0.5 |
| Basset Fauve De Bretagne | 1 | 0.5 |
| Basset Hound | 3 | 1.5 |
| Bavarian Mountain Scent Hound | 1 | 0.5 |
| Beagle | 2 | 1.0 |
| Bearded Collie | 1 | 0.5 |
| Beauce Sheepdog | 1 | 0.5 |
| Border Collie | 5 | 2.5 |
| Boxer | 2 | 1.0 |
| Briard | 1 | 0.5 |
| Bull Terrier | 2 | 1.0 |
| Italian Cane Corso | 1 | 0.5 |
| Dalmatian | 9 | 4.5 |
| Doberman | 2 | 1.0 |
| Bulldog | 1 | 0.5 |
| English Springer Spaniel | 9 | 4.5 |
| Field Spaniel | 4 | 2.0 |
| Finnish Lapponian Dog | 1 | 0.5 |
| Flat-coated Retriever | 8 | 4.0 |
| German Shepherd Dog | 4 | 2.0 |
| Giant Schnauzer | 3 | 1.5 |
| Golden Retriever | 5 | 2.5 |
| Belgian Shepherd Dog | 1 | 0.5 |
| Hamiltonstövare | 1 | 0.5 |
| Hovawart | 3 | 1.5 |
| Irish Red Setter | 4 | 2.0 |
| Irish Soft Coated Wheaten Terrier | 1 | 0.5 |
| Labrador Retriever | 9 | 4.5 |
| Romagna Water Dog | 2 | 1.0 |
| Mixed Breed Dog | 43 | 21.3 |
| Novia Scotia Duck Tolling Retriever | 3 | 1.5 |
| Old Danish Pointing Dog | 1 | 0.5 |
| Petit Basset Griffon Vendéen | 1 | 0.5 |
| Pharaoh Dog | 1 | 0.5 |
| Poodle | 7 | 3.5 |
| Rhodesian Ridgeback | 9 | 4.5 |
| Rottweiler | 3 | 1.5 |
| Samoyed | 1 | 0.5 |
| Schnauzer | 3 | 1.5 |
| Kleiner Münsterländer | 2 | 1.0 |
| Spanish Water Dog | 1 | 0.5 |
| Staffordshire Bull Terrier | 2 | 1.0 |
| Thai Ridgeback | 1 | 0.5 |
| Hungarian Short-haired Pointer (Vizsla) | 4 | 2.0 |
| German Pointing Dog | 5 | 2.5 |
| Weimaraner | 2 | 1.0 |
| Welsh Springer Spaniel | 3 | 1.5 |
| Whippet | 1 | 0.5 |
| Total | 202 | 100 |
| ACVIM Stage | Age (Years) | Bodyweight (kg) | LVIDDN | LVIDSN | LA/Ao | FS (%) | MVE (m/s) | |
|---|---|---|---|---|---|---|---|---|
| B1 (n = 170) | n = 79 | |||||||
| Mean | 9.91 | 28.73 | 1.75 | 1.05 | 1.17 | 35.78 | 0.77 | |
| SD | 2.12 | 7.83 | 0.22 | 0.20 | 0.16 | 8.20 | 0.18 | |
| Min | 5.64 | 20.00 | 1.08 | 0.52 | 0.80 | 22.20 | 0.38 | |
| Max | 15.13 | 57.50 | 2.51 | 1.79 | 1.70 | 64.02 | 1.59 | |
| B2 (n = 21) | n = 6 | |||||||
| Mean | 10.67 | 29.26 | 2.31 | 1.47 | 1.91 | 32.00 | 1.20 | |
| SD | 1.09 | 6.28 | 0.37 | 0.31 | 0.35 | 7.38 | 0.41 | |
| Min | 8.32 | 21.40 | 1.90 | 1.13 | 1.60 | 19.65 | 0.75 | |
| Max | 12.25 | 43.00 | 3.34 | 2.44 | 2.80 | 46.15 | 1.72 | |
| C (n = 11) | n = 3 | |||||||
| Mean | 10.12 | 31.64 | 2.28 | 1.40 | 2.06 | 34.34 | 1.28 | |
| SD | 2.00 | 8.25 | 0.24 | 0.23 | 0.28 | 6.66 | 0.10 | |
| Min | 7.80 | 20.50 | 1.94 | 1.00 | 1.80 | 26.59 | 1.18 | |
| Max | 14.93 | 45.20 | 2.70 | 1.82 | 2.60 | 50.00 | 1.37 |
| Unadjusted | Adjusted 1 | |||
|---|---|---|---|---|
| Independent Variable | HR [CI] | p-Value | HR [CI] | p-Value |
| Age | 1.46 [1.34–1.59] | <0.001 * | N/A | N/A |
| Sex | 1.16 [0.84–1.61] | 0.372 | N/A | N/A |
| Bodyweight | 1.01 [0.98–1.03] | 0.651 | N/A | N/A |
| Murmur grade | ||||
| Moderate/loud vs. soft | 1.37 [1.00–1.86] | 0.050 | 1.23 [0.89–1.68] | 0.207 |
| Thrilling vs. soft | 1.40 [0.73–2.71] | 0.312 | 1.29 [0.66–2.52] | 0.449 |
| Thrilling vs. moderate/loud | 1.03 [0.53–2.01] | 0.935 | 1.06 [0.54–2.08] | 0.877 |
| LVIDDN (HR for 0.1-unit increment) | 1.13 [1.07–1.20] | <0.001 * | 1.11 [1.05–1.17] | <0.001 * |
| LVIDSN (HR for 0.1-unit increment) | 1.12 [1.05–1.20] | 0.001 * | 1.15 [1.08–1.23] | <0.001 * |
| LA/Ao (HR for 0.1-unit increment) | 1.13 [1.08–1.18] | <0.001 * | 1.10 [1.05–1.15] | <0.001 * |
| FS (HR for 10% increment) | 1.03 [0.85–1.24] | 0.778 | 0.84 [0.70–1.02] | 0.085 |
| MVE (HR for 0.1-unit increment) | 1.04 [0.95–1.14] | 0.405 | 0.97 [0.88–1.08] | 0.572 |
| ACVIM stage | ||||
| Stage B2 vs. B1 | 2.63 [1.61–4.29] | <0.001 * | 1.73 [1.06–2.83] | 0.029 * |
| Stage C vs. B1 | 3.34 [1.78–6.25] | <0.001 * | 2.19 [1.15–4.19] | 0.017 * |
| Stage C vs. B2 | 1.27 [0.60–2.68] | 0.527 | 1.27 [0.59–2.71] | 0.540 |
| LVIDSN > 1.26 | 1.73 [1.21–2.47] | 0.003 * | 1.93 [1.34–2.77] | <0.001 * |
| Unadjusted | Adjusted 1 | |||
|---|---|---|---|---|
| Independent Variable | HR [CI] | p-Value | HR [CI] | p-Value |
| Age | 1.36 [1.10–1.67] | 0.004 * | N/A | N/A |
| Sex | 2.63 [0.91–7.62] | 0.075 | N/A | N/A |
| Bodyweight | 0.97 [0.91–1.02] | 0.241 | N/A | N/A |
| Murmur grade | ||||
| Moderate/loud vs. soft | 2.02 [0.86–4.73] | 0.105 | 1.92 [0.82–4.51] | 0.136 |
| Thrilling vs. soft | 5.93 [1.98–17.71] | 0.001 * | 4.81 [1.55–14.93] | 0.007 * |
| Thrilling vs. moderate/loud | 2.93 [1.04–8.24] | 0.041 * | 2.51 [0.86–7.33] | 0.092 |
| LVIDDN (HR for 0.1-unit increment) | 1.37 [1.24–1.51] | <0.001 * | 1.32 [1.20–1.46] | <0.001 * |
| LVIDSN (HR for 0.1-unit increment) | 1.40 [1.25–1.56] | <0.001 * | 1.35 [1.21–1.51] | <0.001 * |
| LA/Ao (HR for 0.1-unit increment) | 1.35 [1.25–1.47] | <0.001 * | 1.31 [1.21–1.43] | <0.001 * |
| FS (HR for 10% increment) | 0.77 [0.45–1.29] | 0.317 | 0.64 [0.38–1.10] | 0.107 |
| MVE (HR for 0.1-unit increment) | 1.32 [1.11–1.57] | 0.002 * | 1.29 [1.08–1.54] | 0.005 * |
| ACVIM stage | ||||
| Stage B2 vs. B1 | 14.01 [5.60–35.04] | <0.001 * | 12.20 [4.64–32.08] | <0.001 * |
| Stage C vs. B1 | 17.95 [6.26–51.47] | <0.001 * | 18.25 [5.45–61.10] | <0.001 * |
| Stage C vs. B2 | 1.28 [0.45–3.64] | 0.641 | 1.50 [0.50–4.45] | 0.469 |
| LVIDSN >1.26 | 5.50 [2.56–11.79] | <0.001 * | 5.91 [2.71–12.86] | <0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Svensson, M.; Selling, J.; Dirven, M. Myxomatous Mitral Valve Disease in Large Breed Dogs: Survival Characteristics and Prognostic Variables. Vet. Sci. 2024, 11, 136. https://doi.org/10.3390/vetsci11030136
Svensson M, Selling J, Dirven M. Myxomatous Mitral Valve Disease in Large Breed Dogs: Survival Characteristics and Prognostic Variables. Veterinary Sciences. 2024; 11(3):136. https://doi.org/10.3390/vetsci11030136
Chicago/Turabian StyleSvensson, Mikael, Jonas Selling, and Mark Dirven. 2024. "Myxomatous Mitral Valve Disease in Large Breed Dogs: Survival Characteristics and Prognostic Variables" Veterinary Sciences 11, no. 3: 136. https://doi.org/10.3390/vetsci11030136
APA StyleSvensson, M., Selling, J., & Dirven, M. (2024). Myxomatous Mitral Valve Disease in Large Breed Dogs: Survival Characteristics and Prognostic Variables. Veterinary Sciences, 11(3), 136. https://doi.org/10.3390/vetsci11030136






