Plasma γ-Aminobutyric Acid (GABA) Concentrations in Lactating Holstein Cows during Thermoneutral and Heat Stress Conditions and Their Relationships with Circulating Glucose, Insulin and Progesterone Levels
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. IACUC Statement
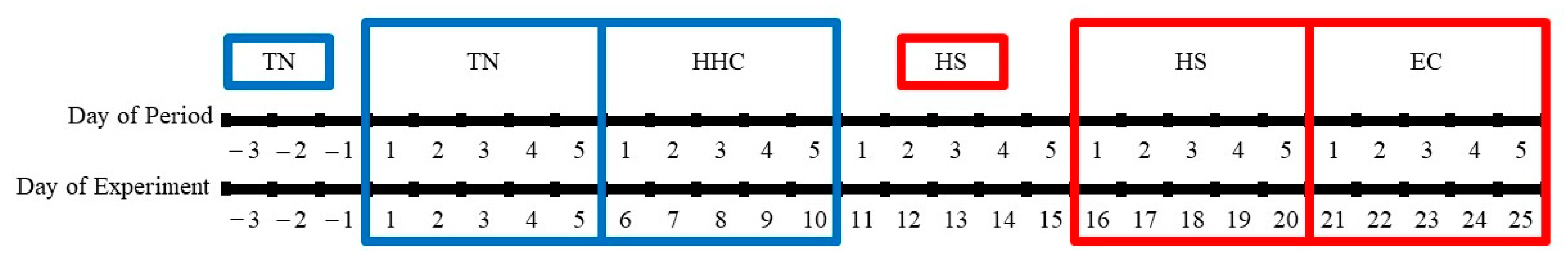
2.2. Animals and Experimental Design
2.3. Thermoneutral and Hyperinsulinemic–Hypoglycemic Clamp Periods
2.4. Heat Stress and Euglycemic Clamp
2.5. Blood Sampling
2.6. Hormone and Metabolite Analysis
2.7. Statistical Analyses
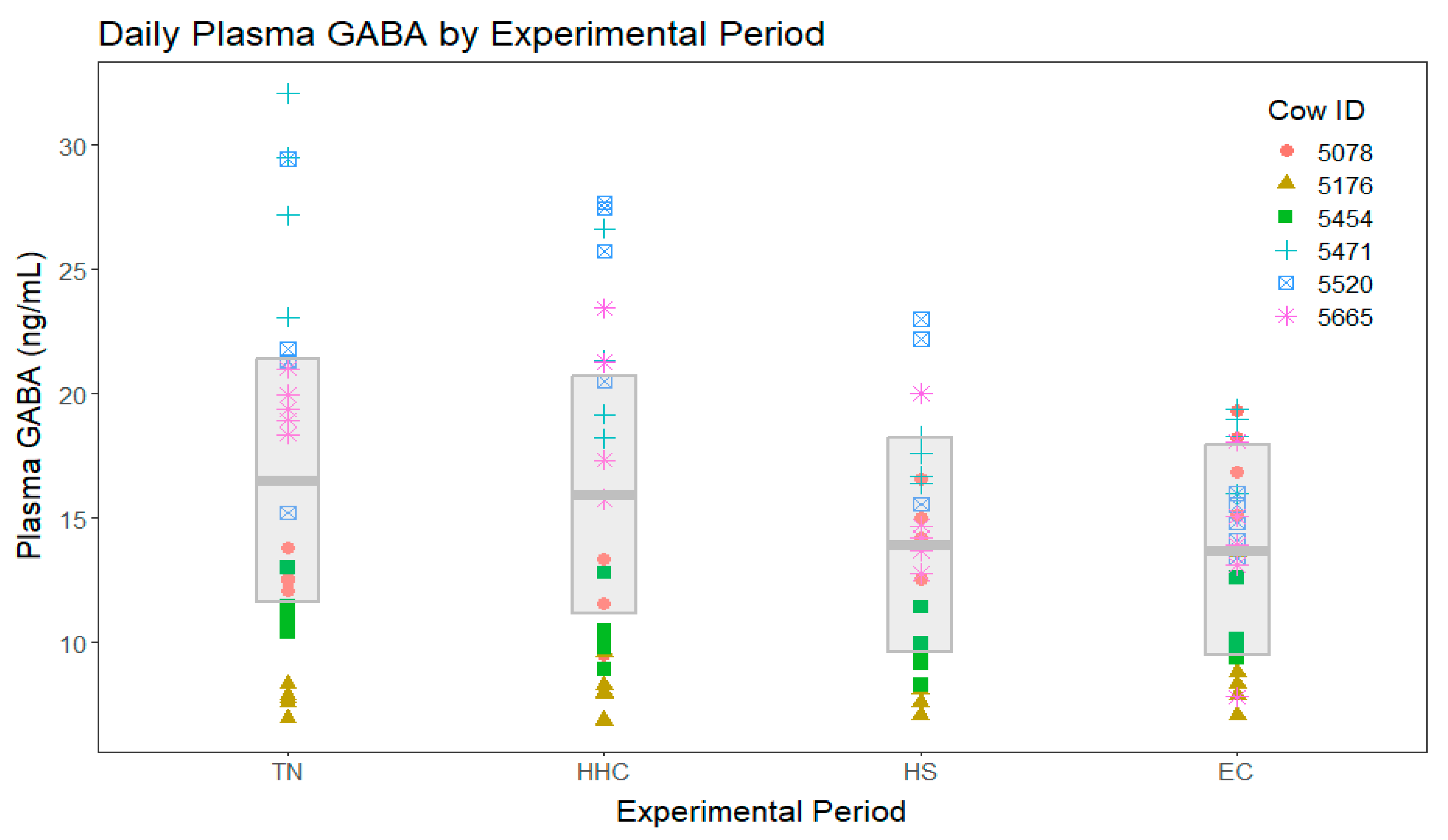
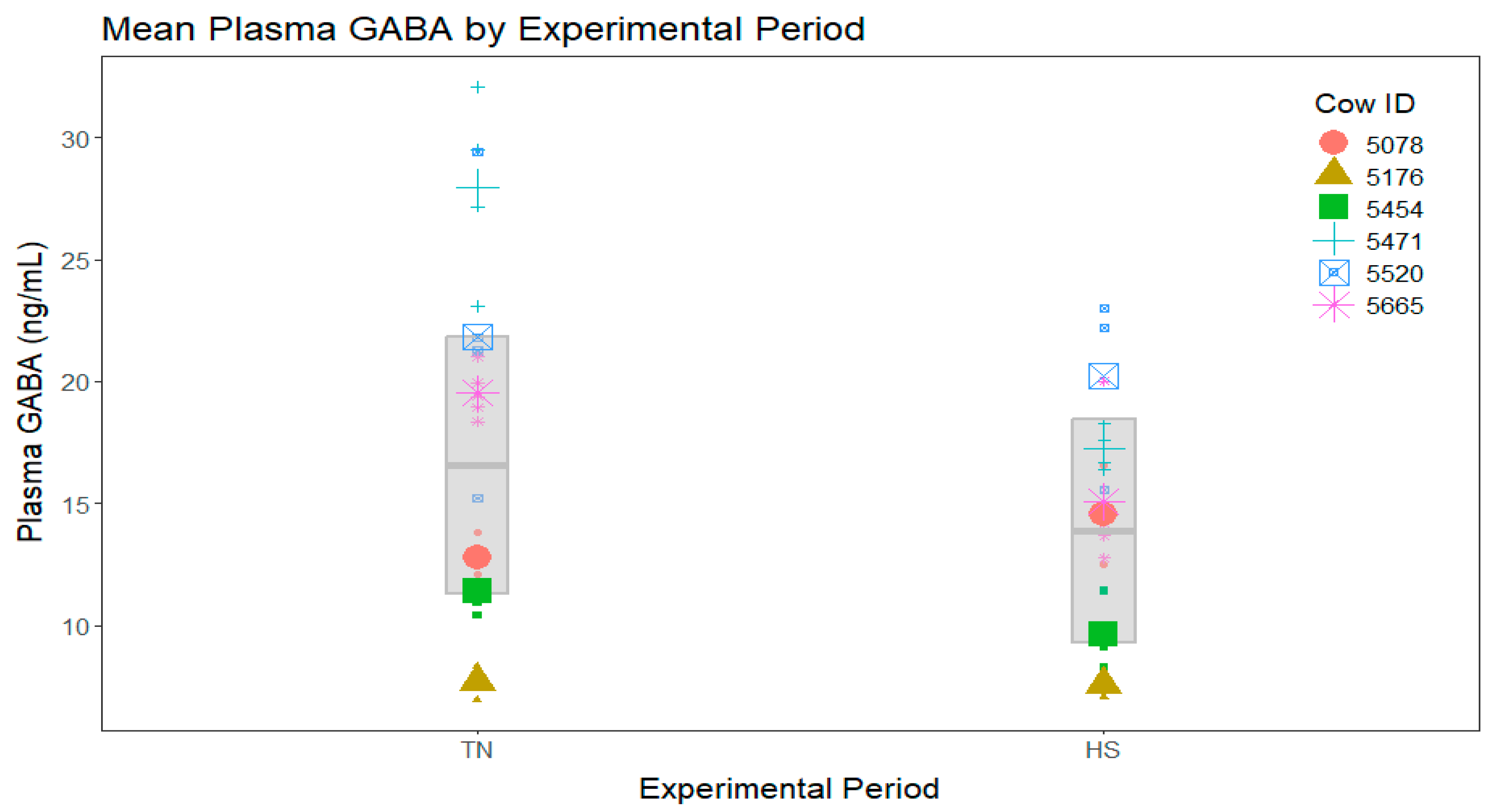
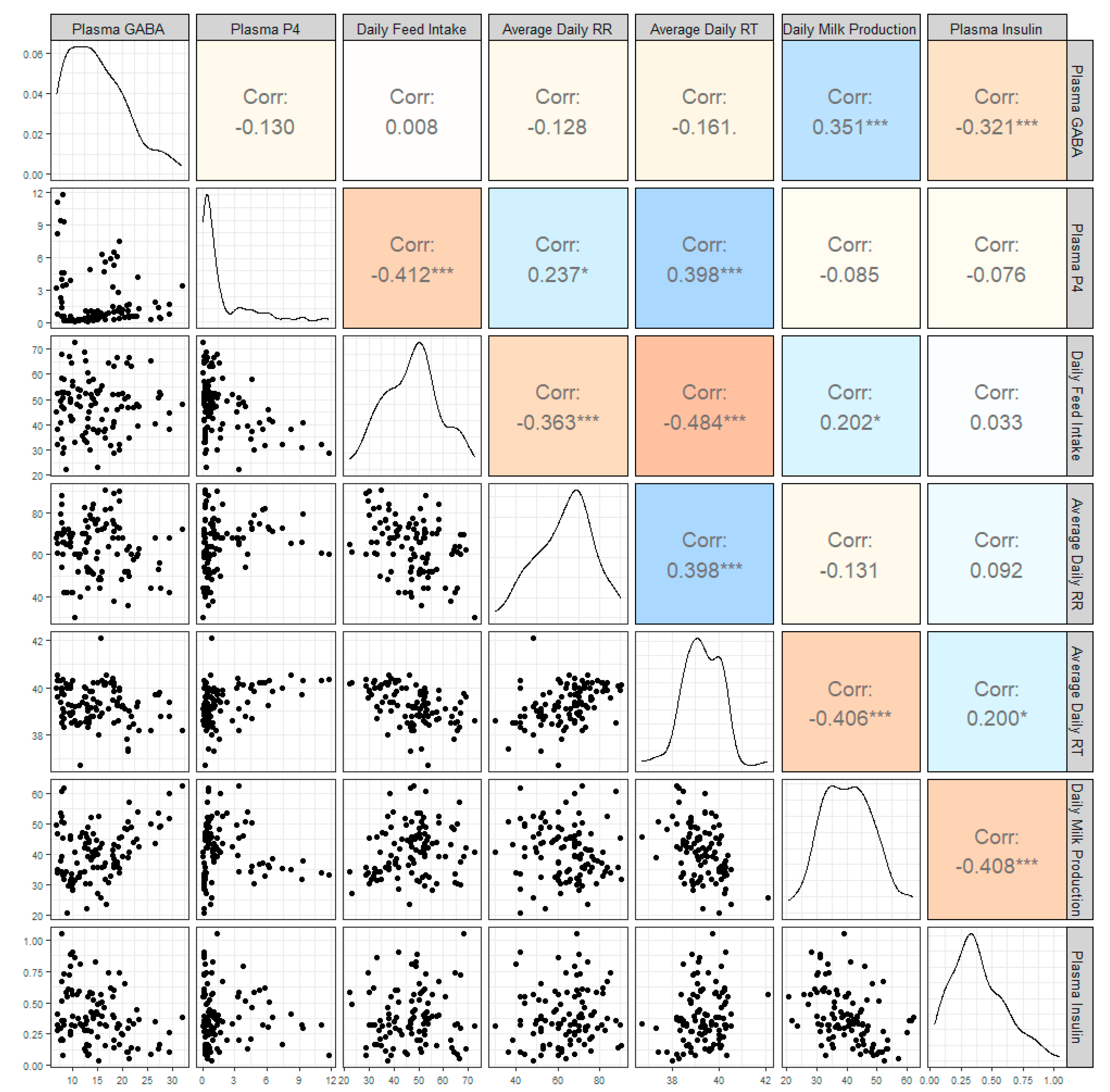
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuriyama, K.; Sze, P.Y. Blood-brain barrier to H3-gamma-aminobutyric acid in normal and amino oxyacetic acid-treated animals. Neuropharmacology 1971, 10, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Erdö, S.L.; Wolff, J.R. γ-Aminobutyric Acid Outside the Mammalian Brain. J. Neurochem. 1990, 54, 363–372. [Google Scholar] [CrossRef] [PubMed]
- del Rio, R.M.; Caballero, A.L. Presence of γ-Aminobutyric Acid in Rat Ovary. J. Neurochem. 1980, 34, 1584–1586. [Google Scholar] [CrossRef]
- Erdö, S.L.; Rosdy, B.; Szporny, L. Higher GABA Concentrations in Fallopian Tube Than in Brain of the Rat. J. Neurochem. 1982, 38, 1174–1176. [Google Scholar] [CrossRef]
- Kawai, K.; Unger, R.H. Effects of γ-Aminobutyric Acid on Insulin, Glucagon, and Somatostatin Release from Isolated Perfused Dog Pancreas. Endocrinology 1983, 113, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.; Wendt, A.; Birnir, B.; Broman, J.; Eliasson, L.; Galvanovskis, J.; Gromada, J.; Mulder, H.; Rorsman, P. Regulated Exocytosis of GABA-containing Synaptic-like Microvesicles in Pancreatic β-cells. J. Gen. Physiol. 2004, 123, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Kursan, S.; McMillen, T.S.; Beesetty, P.; Dias-Junior, E.; Almutairi, M.M.; Sajib, A.A.; Kozak, J.A.; Aguilar-Bryan, L.; di Fulvio, M. The neuronal K+Cl− co-transporter 2 (Slc12a5) modulates insulin secretion. Sci. Rep. 2017, 7, 1732. [Google Scholar] [CrossRef]
- Wendt, A.; Birnir, B.; Buschard, K.; Gromada, J.; Salehi, A.; Sewing, S.; Rorsman, P.; Braun, M. Glucose Inhibition of Glucagon Secretion from Rat α-Cells Is Mediated by GABA Released from Neighboring β-Cells. Diabetes 2004, 53, 1038–1045. [Google Scholar] [CrossRef]
- Xu, E.; Kumar, M.; Zhang, Y.; Ju, W.; Obata, T.; Zhang, N.; Liu, S.; Wendt, A.; Deng, S.; Ebina, Y.; et al. Intra-islet insulin suppresses glucagon release via GABA-GABAA receptor system. Cell Metab. 2006, 3, 47–58. [Google Scholar] [CrossRef]
- Schaeffer, J.M.; Hsueh, A.J.W. Identification of gamma-aminobutyric acid and its binding sites in the rat ovary. Life Sci. 1982, 30, 1599–1604. [Google Scholar] [CrossRef]
- Danzy, L.; Hou, L.; Yalcinkaya, T.M. Bone morphogenetic proteins (BMP)-2 and -6 are potential targets of gaba-A receptor activation in human luteinized granulosa cells (GC). Fertil. Steril. 2011, 96, S127. [Google Scholar] [CrossRef]
- Yoshino, O.; Shi, J.; Osuga, Y.; Harada, M.; Nishii, O.; Yano, T.; Taketani, Y. The function of bone morphogenetic proteins in the human ovary. Reprod. Med. Biol. 2011, 10, 1–7. [Google Scholar] [CrossRef]
- Peluso, J.J.; Pappalardo, A. Progesterone Mediates its Anti-Mitogenic and Anti-Apoptotic Actions in Rat Granulosa Cells Through a Progesterone-Binding Protein with Gamma Aminobutyric AcidA Receptor-Like Features. Biol. Reprod. 1998, 58, 1131–1137. [Google Scholar] [CrossRef]
- Ray, D.E.; Halbach, T.J.; Armstrong, D.V. Season and Lactation Number Effects on Milk Production and Reproduction of Dairy Cattle in Arizona. J. Dairy Sci. 1992, 75, 2976–2983. [Google Scholar] [CrossRef]
- De Rensis, F.; Scaramuzzi, R.J. Heat stress and seasonal effects on reproduction in the dairy cow—A review. Theriogenology 2003, 60, 1139–1151. [Google Scholar] [CrossRef]
- Rhoads, M.L. Review: Reproductive consequences of whole-body adaptations of dairy cattle to heat stress. Animal 2023, 17, 100847. [Google Scholar] [CrossRef] [PubMed]
- Wolfenson, D.; Thatcher, W.W.; Badinga, L.; Savio, J.D.; Meidan, R.; Lew, B.J.; Braw-Tal, R.; Berman, A. Effect of Heat Stress on Follicular Development during the Estrous Cycle in Lactating Dairy Cattle’. Biol. Reprod. 1995, 52, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.J.; Marion, R.S.; Spain, J.N.; Spiers, D.E.; Keisler, D.H.; Lucy, M.C. Effects of Controlled Heat Stress on Ovarian Function of Dairy Cattle. 1. Lactating Cows. J. Dairy Sci. 1998, 81, 2124–2131. [Google Scholar] [CrossRef] [PubMed]
- Roth, Z.; Meidan, R.; Braw-Tal, R.; Wolfenson, D. Immediate and delayed effects of heat stress on follicular development and its association with plasma FSH and inhibin concentration in cows. J. Reprod. Fertil. 2000, 120, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Wheelock, J.B.; Rhoads, R.P.; VanBaale, M.J.; Sanders, S.R.; Baumgard, L.H. Effects of heat stress on energetic metabolism in lactating Holstein cows. J. Dairy Sci. 2010, 93, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, M.L.; Rhoads, R.P.; VanBaale, M.J.; Collier, R.J.; Sanders, S.R.; Weber, W.J.; Crooker, B.A.; Baumgard, L.H. Effects of heat stress and plane of nutrition on lactating Holstein cows: I. Production, metabolism, and aspects of circulating somatotropin. J. Dairy Sci. 2009, 92, 1986–1997. [Google Scholar] [CrossRef]
- Stewart, J.W.; Arneson, A.G.; Byrd, M.K.H.; Negron-Perez, V.M.; Newberne, H.M.; White, R.R.; El-Kadi, S.W.; Ealy, A.D.; Rhoads, R.P.; Rhoads, M.L. Comparison of production-related responses to hyperinsulinemia and hypoglycemia induced by clamp procedures or heat stress of lactating dairy cattle. J. Dairy Sci. 2022, 105, 8439–8453. [Google Scholar] [CrossRef]
- Engel, C.L.; Patterson, H.H.; Perry, G.A. Effect of dried corn distillers grains plus solubles compared with soybean hulls, in late gestation heifer diets, on animal and reproductive performance. J. Anim. Sci. 2008, 86, 1697–1708. [Google Scholar] [CrossRef]
- Gong, J.G.; Lee, W.J.; Garnsworthy, P.C.; Webb, R. Effect of dietary-induced increases in circulating insulin concentrations during the early postpartum period on reproductive function in dairy cows. Reproduction 2002, 123, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Garnsworthy, P.C.; Fouladi-Nashta, A.A.; Mann, G.E.; Sinclair, K.D.; Webb, R. Effect of dietary-induced changes in plasma insulin concentrations during the early post partum period on pregnancy rate in dairy cows. Reproduction 2009, 137, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Adamiak, S.J.; Mackie, K.; Watt, R.G.; Webb, R.; Sinclair, K.D. Impact of nutrition on oocyte quality: Cumulative effects of body composition and diet leading to hyperinsulinemia in cattle. Biol. Reprod. 2005, 73, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Fouladi-Nashta, A.A.; Gutierrez, C.G.; Garnsworthy, P.C.; Webb, R. Effects of dietary carbohydrate source on oocyte/embryo quality and development in high-yielding, lactating dairy cattle. Biol. Reprod. 2005, 135–136. [Google Scholar]
- Adamiak, S.J.; Powell, K.; Rooke, J.A.; Webb, R.; Sinclair, K.D. Body composition, dietary carbohydrates and fatty acids determine post-fertilisation development of bovine oocytes in vitro. Reproduction 2006, 131, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, E.; Pavone, M.E.; Hirshfeld-Cytron, J.E. Metabolic syndrome and oocyte quality. Trends Endocrinol. Metab. 2011, 22, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Feng, H.L. Extra- and intra-ovarian factors in polycystic ovary syndrome: Impact on oocyte maturation and embryo developmental competence. Hum. Reprod. Update 2011, 17, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.H.; Li, S.; Wang, Z.B.; Li, M.; Quan, S.; Xing, F.; Guo, L.; Chao, S.B.; Chen, Z.; Liang, X.W.; et al. Maternal insulin resistance causes oxidative stress and mitochondrial dysfunction in mouse oocytes. Hum. Reprod. 2012, 27, 2130–2145. [Google Scholar] [CrossRef]
- Cheng, J.B.; Bu, D.P.; Wang, J.Q.; Sun, X.Z.; Pan, L.; Zhou, L.Y.; Liu, W. Effects of rumen-protected γ-aminobutyric acid on performance and nutrient digestibility in heat-stressed dairy cows. J. Dairy Sci. 2014, 97, 5599–5607. [Google Scholar] [CrossRef]
- Cheng, J.; Zheng, N.; Sun, X.; Li, S.; Wang, J.; Zhang, Y. Feeding rumen-protected gamma-aminobutyric acid enhances the immune response and antioxidant status of heat-stressed lactating dairy cows. J. Therm. Biol. 2016, 60, 103–108. [Google Scholar] [CrossRef]
- Wang, D.M.; Wang, C.; Liu, H.Y.; Liu, J.X.; Ferguson, J.D. Effects of rumen-protected γ-aminobutyric acid on feed intake, lactation performance, and antioxidative status in early lactating dairy cows. J. Dairy Sci. 2013, 96, 3222–3227. [Google Scholar] [CrossRef]
- Caicedo, A. Paracrine and autocrine interactions in the human islet: More than meets the eye. Semin. Cell Dev. Biol. 2013, 24, 11–21. [Google Scholar] [CrossRef]
- Dong, H.; Kumar, M.; Zhang, Y.; Gyulkhandanyan, A.; Xiang, Y.Y.; Ye, B.; Perrella, J.; Hyder, A.; Zhang, N.; Wheeler, M.; et al. Gamma-aminobutyric acid up- and downregulates insulin secretion from beta cells in concert with changes in glucose concentration. Diabetologia 2006, 49, 697–705. [Google Scholar] [CrossRef]
- Pizarro-Delgado, J.; Braun, M.; Hernández-Fisac, I.; Martín-Del-Río, R.; Tamarit-Rodriguez, J. Glucose promotion of GABA metabolism contributes to the stimulation of insulin secretion in β-cells. Biochem. J. 2010, 431, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Bansal, P.; Wang, S.; Liu, S.; Xiang, Y.Y.; Lu, W.Y.; Wang, Q. GABA Coordinates with Insulin in Regulating Secretory Function in Pancreatic INS-1 β-Cells. PLoS ONE 2011, 6, e26225. [Google Scholar] [CrossRef] [PubMed]
- Feng, A.L.; Xiang, Y.Y.; Gui, L.; Kaltsidis, G.; Feng, Q.; Lu, W.Y. Paracrine GABA and insulin regulate pancreatic alpha cell proliferation in a mouse model of type 1 diabetes. Diabetologia 2017, 60, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Purwana, I.; Zheng, J.; Li, X.; Deurloo, M.; Son, D.O.; Zhang, Z.; Liang, C.; Shen, E.; Tadkase, A.; Feng, Z.P.; et al. GABA promotes human β-cell proliferation and modulates glucose homeostasis. Diabetes 2014, 63, 4197–4205. [Google Scholar] [CrossRef]
- Untereiner, A.; Abdo, S.; Bhattacharjee, A.; Gohil, H.; Pourasgari, F.; Ibeh, N.; Lai, M.; Batchuluun, B.; Wong, A.; Khuu, N.; et al. GABA promotes β-cell proliferation, but does not overcome impaired glucose homeostasis associated with diet-induced obesity. FASEB J. 2019, 33, 3968–3984. [Google Scholar] [CrossRef]
- Rorsman, P.; Berggren, P.O.; Bokvist, K.; Ericson, H.; Möhler, H.; Östenson, C.G.; Smith, P.A. Glucose-inhibition of glucagon secretion involves activation of GABAA-receptor chloride channels. Nature 1989, 341, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.E.; Kendrick, K.M. Inhibition of Luteinizing Hormone Secretion in the Ewe by Progesterone: Associated Changes in the Release of Gamma-Aminobutyric Acid and Noradrenaline in the Preoptic Area as Measured by Intracranial Microdialysis. J. Neuroendocrinol. 1992, 4, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, X.; Shen, X.; Ivanova, D.; Lass, G.; He, W.; Chen, Q.; Yu, S.; Wang, Y.; Long, H.; et al. Dynorphin and GABAA Receptor Signaling Contribute to Progesterone’s Inhibition of the LH Surge in Female Mice. Endocrinology 2020, 161, bqaa036. [Google Scholar] [CrossRef] [PubMed]
- Kon, H.; Takei, G.L.; Fujinoki, M.; Shinoda, M. Suppression of Progesterone-enhanced Hyperactivation in Hamster Spermatozoa by γ-aminobutyric Acid. J. Reprod. Dev. 2014, 60, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Stiernman, L.; Dubol, M.; Comasco, E.; Sundström-Poromaa, I.; Boraxbekk, C.-J.; Johansson, M.; Bixo, M. Emotion-induced brain activation across the menstrual cycle in individuals with premenstrual dysphoric disorder and associations to serum levels of progesterone-derived neurosteroids. Transl. Psychiatry 2023, 13, 124. [Google Scholar] [CrossRef]
- Deligiannidis, K.M.; Kroll-Desrosiers, A.R.; Mo, S.; Nguyen, H.P.; Svenson, A.; Jaitly, N.; Hall, J.E.; Barton, B.A.; Rothschild, A.J.; Shaffer, S.A. Peripartum neuroactive steroid and γ-aminobutyric acid profiles in women at-risk for postpartum depression. Psychoneuroendocrinology 2016, 70, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Kapur, J. Neurosteroid regulation of GABAA receptors: A role in catamenial epilepsy. Brain Res. 2019, 1703, 31–40. [Google Scholar] [CrossRef]
- Hedström, H.; Bäckström, T.; Bixo, M.; Nyberg, S.; Wang, M.; Gideonsson, I.; Turkmen, S. Women with polycystic ovary syndrome have elevated serum concentrations of and altered GABAA receptor sensitivity to allopregnanolone. Clin. Endocrinol. 2015, 83, 643–650. [Google Scholar] [CrossRef]
- Battista, P.J.; Condon, W.A. Serotonin-induced stimulation of progesterone production by cow luteal cells in vitro. Reproduction 1986, 76, 231–238. [Google Scholar] [CrossRef]
- Zhang, H.; Ni, J.; Zhang, W.; Tian, S.J. GABA inhibits progesterone production of luteal cells of rat ovary. Acta Physiol. Sin. 2000, 52, 185–187. [Google Scholar]
- Jung, C.; Fernandez-Dueñas, V.; Plata, C.; Garcia-Elias, A.; Ciruela, F.; Fernandez-Fernandez, J.M.; Valverder, M.A. Functional coupling of GABAA/B receptors and the channel TRPV4 mediates rapid progesterone signaling in the oviduct. Sci. Signal. 2018, 11. [Google Scholar] [CrossRef]
- Opolski, A.; Mazurkiewicz, M.; Wietrzyk, J.; Kleinrok, Z.; Radzikowski, C. The role of GABA-ergic system in human mammary gland pathology and in growth of transplantable murine mammary cancer. J. Exp. Clin. Cancer Res. 2000, 19, 383–390. [Google Scholar] [PubMed]
- Sizemore, G.M.; Sizemore, S.T.; Seachrist, D.D.; Keri, R.A. GABA(A) Receptor Pi (GABRP) Stimulates Basal-like Breast Cancer Cell Migration through Activation of Extracellular-regulated Kinase 1/2 (ERK1/2). Mol. Bases Dis. 2014, 289, 24102–24113. [Google Scholar] [CrossRef] [PubMed]
- Lamberts, S.W.J.; MacLeod, R.M. Studies on the Mechanism of the GABA-Mediated Inhibition of Prolactin Secretion. Exp. Biol. Med. 1978, 158, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Mansky, T.; Mestres-Ventura, P.; Wuttke, W. Involvement of GABA in the feedback action of estradiol on gonadotropin and prolactin release: Hypothalamic GABA and catecholamine turnover rates. Brain Res. 1982, 231, 353–364. [Google Scholar] [CrossRef]
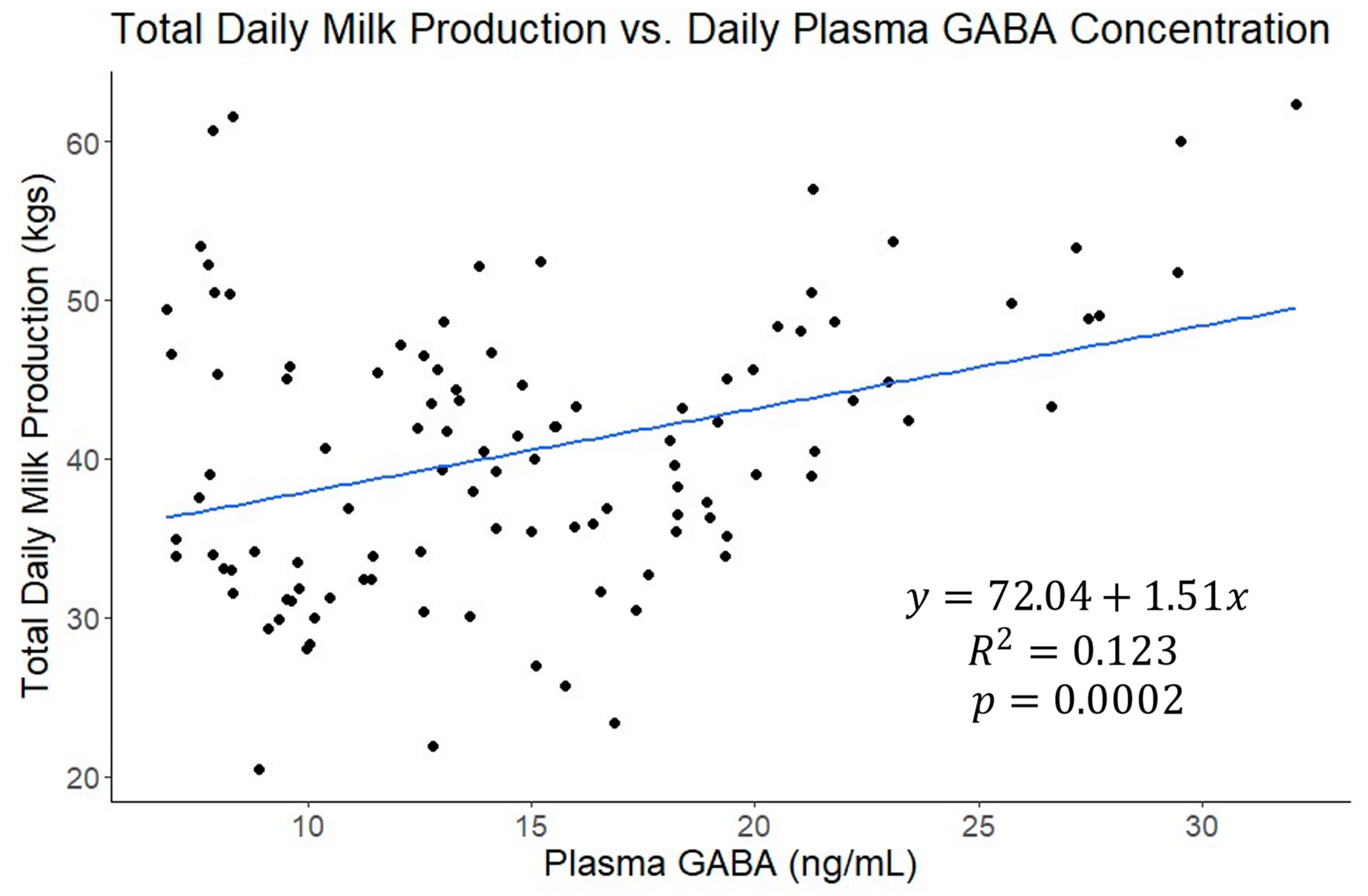

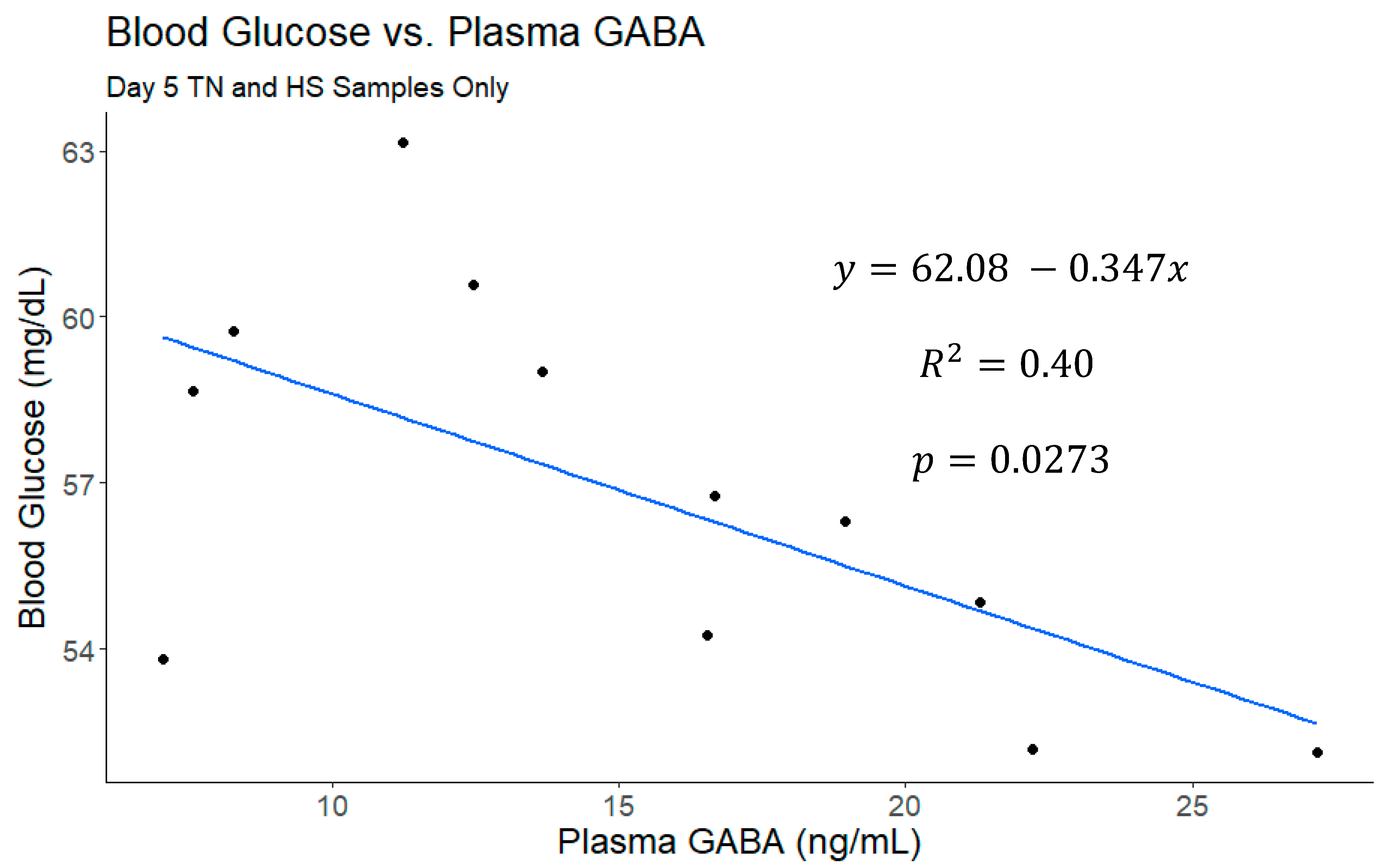
| Means from Analysis with All Periods | |||
|---|---|---|---|
| Plasma Insulin (µg/L) | Standard Error | p-value | |
| TN 1 | 0.27 | 0.05 | 0.0131 |
| HHC 1,2 | 0.42 | 0.06 | |
| HS 2 | 0.43 | 0.06 | |
| EC 2 | 0.43 | 0.06 | |
| Means from analysis with all TN and HS periods only | |||
| Plasma Insulin (µg/L) | Standard Error | p-value | |
| TN 1 | 0.27 | 0.06 | 0.0026 |
| HS 2 | 0.43 | 0.06 | |
| Means from Analysis with All Periods | |||
|---|---|---|---|
| Plasma Progesterone (ng/mL) | Standard Error | p-value | |
| TN | 0.78 | 0.44 | 0.1151 |
| HHC | 0.76 | 0.43 | |
| HS | 1.05 | 0.60 | |
| EC | 1.17 | 0.66 | |
| Means from analysis with all TN and HS periods only | |||
| Plasma Progesterone (ng/mL) | Standard Error | p-value | |
| TN | 0.73 | 0.40 | 0.2336 |
| HS | 1.11 | 0.62 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arneson, A.G.; Stewart, J.W.; Byrd, M.H.; Perry, G.A.; Rhoads, M.L. Plasma γ-Aminobutyric Acid (GABA) Concentrations in Lactating Holstein Cows during Thermoneutral and Heat Stress Conditions and Their Relationships with Circulating Glucose, Insulin and Progesterone Levels. Vet. Sci. 2024, 11, 137. https://doi.org/10.3390/vetsci11030137
Arneson AG, Stewart JW, Byrd MH, Perry GA, Rhoads ML. Plasma γ-Aminobutyric Acid (GABA) Concentrations in Lactating Holstein Cows during Thermoneutral and Heat Stress Conditions and Their Relationships with Circulating Glucose, Insulin and Progesterone Levels. Veterinary Sciences. 2024; 11(3):137. https://doi.org/10.3390/vetsci11030137
Chicago/Turabian StyleArneson, Alicia G., Jacob W. Stewart, MaryKate H. Byrd, George A. Perry, and Michelle L. Rhoads. 2024. "Plasma γ-Aminobutyric Acid (GABA) Concentrations in Lactating Holstein Cows during Thermoneutral and Heat Stress Conditions and Their Relationships with Circulating Glucose, Insulin and Progesterone Levels" Veterinary Sciences 11, no. 3: 137. https://doi.org/10.3390/vetsci11030137
APA StyleArneson, A. G., Stewart, J. W., Byrd, M. H., Perry, G. A., & Rhoads, M. L. (2024). Plasma γ-Aminobutyric Acid (GABA) Concentrations in Lactating Holstein Cows during Thermoneutral and Heat Stress Conditions and Their Relationships with Circulating Glucose, Insulin and Progesterone Levels. Veterinary Sciences, 11(3), 137. https://doi.org/10.3390/vetsci11030137






