Surgical Outcomes of Laminectomy, Durotomy and a Non-Synthetic Dura Substitute Application in Ten Dogs with a Spinal Subarachnoid Diverticulum
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
3. Results
3.1. History and Clinical Signs
3.2. Preoperative Evaluation
3.2.1. Minimum Data Base
3.2.2. Diagnostic Imaging
3.3. Anaesthesia, Analgesia, and Perioperative Period
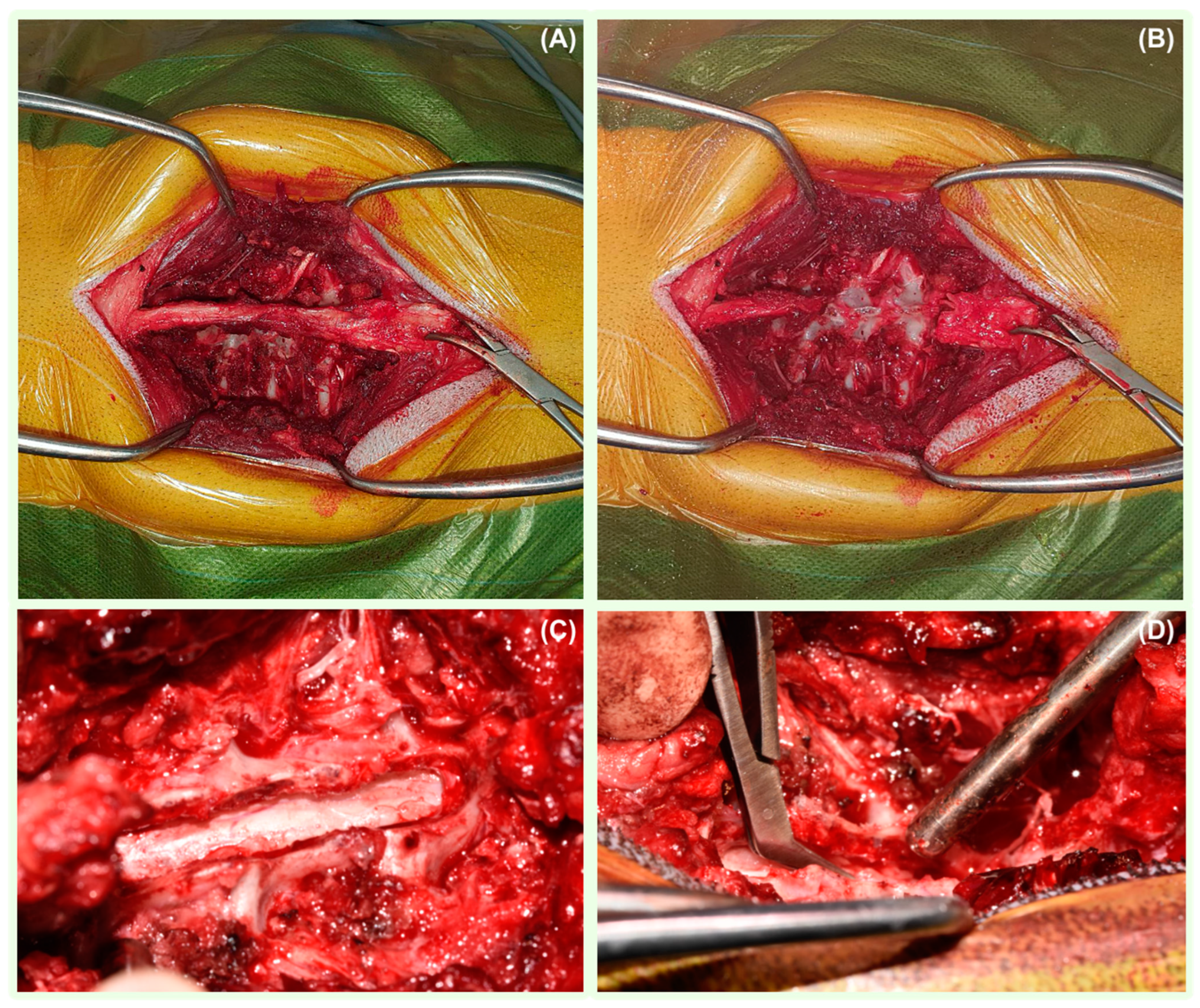
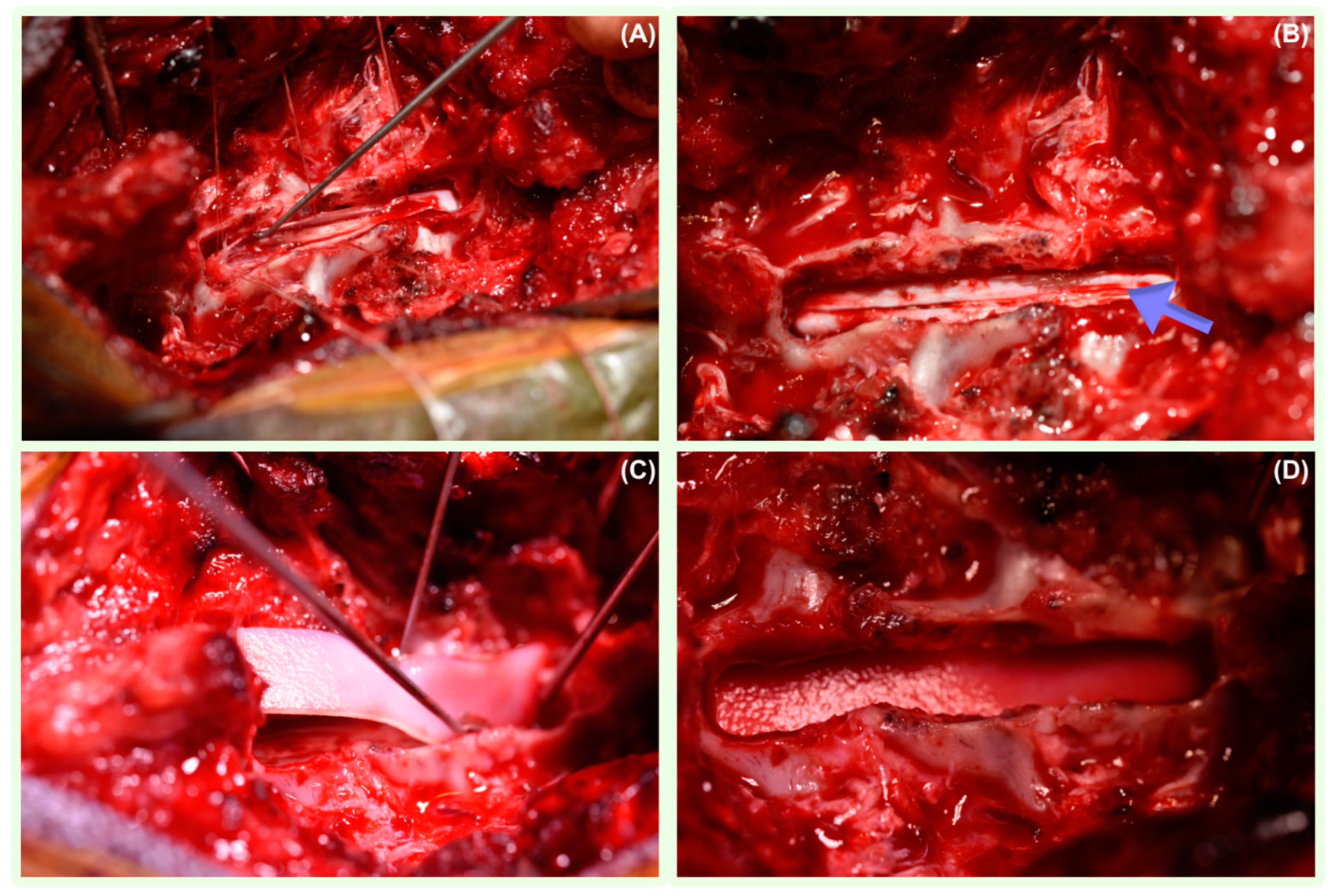
3.4. Surgical Management
3.5. Follow-Up and Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gage, E.D.; Hoerlein, B.F.; Bartels, J.E. Spinal cord compression resulting from a leptomeningeal cyst in the dog. J. Am. Vet. Med. Assoc. 1968, 152, 1664–1670. [Google Scholar]
- Parker, A.J.; Smith, C.W. Meningeal cyst in a dog. J. Am. Anim. Hosp. Assoc. 1974, 10, 595–597. [Google Scholar]
- Hardie, R.J.; Linn, K.A.; Rendano, V.T. Spinal meningeal cyst in a dog: A case report and literature review. J. Am. Anim. Hosp. Assoc. 1996, 32, 477–480. [Google Scholar] [CrossRef]
- Dyce, J.; Herrtage, M.E.; Houlton, E.F.; Palmer, A.C. Canine spinal ‘arachnoid cysts’. J. Small Anim. Pract. 1991, 32, 433–437. [Google Scholar] [CrossRef]
- Jurina, K.; Grevel, V. Spinal arachnoid pseudocysts in 10 rottweilers. J. Small Anim. Pract. 2004, 45, 9–15. [Google Scholar] [CrossRef]
- Rohdin, C.; Nyman, H.T.; Wohlsein, P.; Hultin Jäderlund, K. Cervical spinal intradural arachnoid cysts in related, young pugs. J. Small Anim. Pract. 2014, 55, 229–234. [Google Scholar] [CrossRef]
- Gnirs, K.; Ruel, Y.; Blot, S.; Begon, D.; Rault, D.; Delisle, F.; Boulouha, L.; Colle, M.; Carozzo, C.; Moissonnier, P. Spinal subarachnoid cysts in 13 dogs. Vet. Radiol. Ultrasound 2003, 4, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Garg, K.; Borkar, S.A.; Kale, S.S.; Sharma, B.S. Spinal arachnoid cysts—Our experience and review of literature. Br. J. Neurosurg. 2017, 31, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Mauler, D.; De Decker, S.; De Risio, L.; Volk, H.; Dennis, R.; Gielen, I.; Van der Vekens, E.; Goethals, K.; Van Ham, L. Signalment, Clinical Presentation, and Diagnostic Findings in 122 Dogs with Spinal Arachnoid Diverticula. J. Vet. Intern. Med. 2014, 28, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Skeen, T.M.; Olby, N.J.; Munana, K.R.; Sharp, N.J. Spinal arachnoid cysts in 17 dogs. J. Am. Anim. Hosp. Assoc. 2003, 39, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Guevar, J. Spinal subarachnoid diverticula in dogs: A review. Can. Vet. J. 2020, 61, 1162–1169. [Google Scholar]
- Mauler, D.A.; De Decker, S.; De Risio, L.; Volk, H.; Dennis, R.; Gielen, I.; Van der Vekens, E.; Goethals, K.; Van Ham, L. Spinal Arachnoid Diverticula: Outcome in 96 Medically or Surgically Treated Dogs. J. Vet. Intern. Med. 2017, 31, 849–853. [Google Scholar] [CrossRef]
- Rylander, H.; Lipsitz, D.; Berry, W.L.; Stages, B.K.; Vernau, K.M.; Dickinson, P.J.; Añor, S.A.; Higgins, R.J.; LeCouteur, R.A. Retrospective analysis of spinal arachnoid cysts in 14 dogs. J. Vet. Intern. Med. 2002, 16, 690–696. [Google Scholar] [CrossRef]
- Alisauskaite, N.; Cizinauskas, S.; Jeserevics, J.; Rakauskas, M.; Cherubini, G.B.; Anttila, M.; Steffen, F. Short and long-term outcome and magnetic resonance imaging findings after surgical treatment of thoracolumbar spinal arachnoid diverticula in 25 Pugs. J. Vet. Intern. Med. 2019, 33, 1376–1383. [Google Scholar] [CrossRef]
- Tauro, A.; Rose, J.H.; Rusbridge, C.; Driver, C.J. Surgical Management of Thoracolumbar Myelopathies in Pug Dogs with Concurrent Articular Facet Dysplasia. VCOT Open 2019, 2, 60–72. [Google Scholar] [CrossRef]
- Seiler, G.S.; Robertson, I.D.; Mai, W.; Widmer, W.R.; Suran, J.; Nemanic, S.; Lamb, C.R.; Lang, J.; Johnson, J.L.; Thrall, D.E. Usefulness of a half-fourier acquisition single-shot turbo spin-echo pulse sequence in identifying arachnoid diverticula in dogs. Vet. Radiol. Ultrasound 2012, 53, 157–161. [Google Scholar] [CrossRef]
- Driver, C.J.; Rose, J.; Tauro, A.; Fernandes, R.; Rusbridge, C. Magnetic resonance image findings in pug dogs with thoracolumbar myelopathy and concurrent caudal articular process dysplasia. BMC Vet. Res. 2019, 15, 182. [Google Scholar] [CrossRef]
- McKee, W.M.; Renwick, P.W. Marsupialisation of an arachnoid cyst in a dog. J. Small Anim. Pract. 1994, 35, 108–111. [Google Scholar] [CrossRef]
- Aikawa, T.; Shimatsu, T.; Miyazaki, Y. Hemilaminectomy, diverticular marsupialization, and vertebral stabilization for thoracolumbar spinal arachnoid diverticula in five dogs. J. Am. Anim. Hosp. Assoc. 2019, 55, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Spinillo, S.; Golini, L.; Mariscoli, M.; Motta, L. Retrospective evaluation of surgical outcomes after closure of durotomy in eight dogs affected by spinal subarachnoid diverticulum. Open Vet. J. 2021, 10, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Meren, I.L.; Chavera, J.A.; Alcott, C.J.; Barker, A.K.; Jeffery, N.D. Shunt tube placement for amelioration of cerebrospinal fluid flow obstruction caused by spinal cord subarachnoid fibrosis in dogs. Vet. Surg. 2017, 46, 289–296. [Google Scholar] [CrossRef]
- Alcoverro, E.; McConnell, J.F.; Sanchez-Masian, D.; De Risio, L.; De Decker, S.; Gonçalves, R. Late-onset recurrence of neurological deficits after surgery for spinal arachnoid diverticula. Vet. Rec. 2018, 182, 380. [Google Scholar] [CrossRef]
- Jones, B.; Behr, S.; Shaw, T.; Cappello, R.; Jeffery, N.; Liebel, F.X.; Harcourt-Brown, T. Surgical techniques used in the management of intra-arachnoid diverticula in dogs across four referral centres and their immediate outcome. J. Small Anim. Pract. 2022, 63, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Bismuth, C.; Ferrand, F.X.; Millet, M.; Buttin, P.; Fau, D.; Cachon, T.; Viguier, E.; Escriou, C.; Carozzo, C. Original surgical treatment of thoracolumbar subarachnoid cysts in six chondrodystrophic dogs. Acta Vet. Scand. 2014, 56, 32. [Google Scholar] [CrossRef]
- Frykman, O.F. Spinal arachnoid cyst in four dogs: Diagnosis, surgical treatment and follow-up results. J. Small Anim. Pract. 1999, 40, 544–549. [Google Scholar] [CrossRef]
- Adams, R.J.; Garosi, L.; Matiasek, K.; Lowrie, M. Acquired cervical spinal arachnoid diverticulum in a cat. J. Small Anim. Pract. 2015, 56, 285–288. [Google Scholar] [CrossRef]
- Hoey, C.; Nye, G.; Fadda, A.; Bradshaw, J.; Barker, E.N. Subarachnoid diverticulum associated with feline infectious peritonitis in a Siberian cat. J. Feline Med. Surg. Open Rep. 2020, 6, 2055116920941477. [Google Scholar] [CrossRef]
- Allison, N.; Moeller, R.B., Jr. Spinal ataxia in a horse caused by an arachnoid diverticulum (cyst). J. Vet. Diagn. Investig. 2000, 12, 279–281. [Google Scholar] [CrossRef]
- Abbe, R. Rubber tissue for meningeal adhesions. Trans. Am. Surg. Assoc. 1895, 13, 490–491. [Google Scholar]
- Maurer, P.K.; McDonald, J.V. Vicryl (polyglactin 910) mesh as a dural substitute. J. Neurosurg. 1985, 63, 448–452. [Google Scholar] [CrossRef]
- Parizek, J.; Mericka, P.; Spacek, J.; Nemecek, S.; Elias, P.; Sercl, M. Xenogeneic pericardium as a dural substitute in reconstruction of suboccipital dura mater in children. J. Neurosurg. 1989, 70, 905–909. [Google Scholar] [CrossRef]
- Warren, W.L.; Medary, M.B.; Dureza, C.D.; Bellotte, J.B.; Flannagan, P.P.; Oh, M.Y.; Fukushima, T. Dural repair using acellular human dermis: Experience with 200 cases: Technique assessment. Neurosurgery 2000, 46, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Miyamoto, S.; Takayama, M.; Nagata, I.; Hashimoto, N.; Ikada, Y.; Kikuchi, H. Clinical application of a new bioabsorbable artificial dura mater. J. Neurosurg. 2002, 96, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Berjano, R.; Vinas, F.; Dujovny, M. A review of dural substitutes used in neurosurgery. Crit. Rev. Neurosurg. 1999, 9, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Constantino, P.; Wolpoe, M. Human dural replacement with acellular dermis: Clinical results with a review of literature. Head Neck 2000, 22, 765–771. [Google Scholar] [CrossRef]
- Zerris, V.A.; James, K.S.; Roberts, J.B.; Bell, E.; Heilman, C.B. Repair of the dura mater with processed collagen devices. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 83, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, S.J.; Sharp, N.J.H. Small Animal Spinal Disorders, Diagnosis and Surgery; Mosby-Wolfe Publishers: Maryland Heights, MO, USA, 1994. [Google Scholar]
- Muir, W.W., 3rd; Wiese, A.J.; March, P.A. Effects of morphine, lidocaine, ketamine, and morphine-lidocaine-ketamine drug combination on minimum alveolar concentration in dogs anesthetized with isoflurane. Am. J. Vet. Res. 2003, 64, 1155–1160. [Google Scholar] [CrossRef]
- Dafford, E.E.; Anderson, P.A. Comparison of dural repair techniques. Spine J. 2015, 15, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Anson, J.A.; Marchand, E.P. Bovine pericardium for dural grafts: Clinical results in 35 patients. Neurosurgery 1996, 39, 764–768. [Google Scholar] [CrossRef]
- Baharuddin, A.; Go, B.T.; Firdaus, M.N.; Abdullah, J. Bovine pericardium for dural graft: Clinical results in 22 patients. Clin. Neurol. Neurosurg. 2002, 104, 342–344. [Google Scholar] [CrossRef]
- Fillipi, R.; Schwartz, M.; Voth, D.; Reisch, R.; Grunert, P.; Perneczky, A. Bovine pericardium for duraplasty: Clinical results in 32 patients. Neurosurg. Rev. 2001, 24, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Foy, A.B.; Giannini, C.; Raffel, C. Allergic reaction to a bovine dural substitute following spinal cord untethering Case report. J. Neurosurg. Pediatr. 2008, 1, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Täuber, M.G.; Khayam-Bashi, H.; Sande, M.A. Effects of ampicillin and corticosteroids on brain water content, cerebrospinal fluid pressure, and cerebrospinal fluid lactate levels in experimental pneumococcal meningitis. J. Infect. Dis. 1985, 151, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Girod, M.; Allerton, F.; Gommeren, K.; Tutunaru, A.C.; De Marchin, J.; Van Soens, I.; Ramery, E.; Peeters, D. Evaluation of the effect of oral omeprazole on canine cerebrospinal fluid production: A pilot study. Vet. J. 2016, 209, 119–124. [Google Scholar] [CrossRef]
- Ma, L.; Fei, B. Comprehensive review of surgical microscopes: Technology development and medical applications. J. Biomed. Opt. 2021, 26, 010901. [Google Scholar] [CrossRef]
- Chen, A.V.; Bagley, R.S.; West, C.L.; Gavin, P.R.; Tucker, R.L. Fecal incontinence and spinal cord abnormalities in seven dogs. J. Am. Vet. Med. Assoc. 2005, 227, 1945–1951, 1928. [Google Scholar] [CrossRef]
- Bertram, S.; Ter Haar, G.; De Decker, S. Caudal articular process dysplasia of thoracic vertebrae in neurologically normal French bulldogs, English bulldogs, and Pugs: Prevalence and characteristics. Vet. Radiol. Ultrasound 2018, 59, 396–404. [Google Scholar] [CrossRef] [PubMed]

| Case | Symptoms Duration (Months) | Gait at Presentation | Faecal Incontinence | Urinary Incontinence |
|---|---|---|---|---|
| 1. French Bulldog, 0Y8M M, 12.5 kg | 1 | Moderate paraparesis, spinal PL(s) ataxia | No | No |
| 2. Pug, 6Y3M FN, 6.9 kg | 3 | Moderate paraparesis L-lateralisation, spinal PL(s) ataxia | No | No |
| 3. Pug, 8Y11M FN, 6.5 kg | 2 | Non-ambulatory paraparesis, spinal PL(s) ataxia, faecal incontinence | Yes | No |
| 4. Staffordshire Bull Terrier, 4Y7M F, 14.1 kg | 14 | Moderate paraparesis, spinal PL(s) ataxia | No | No |
| 5. Pug, 8Y1M M, 10.1 kg | 1 | Moderate paraparesis, spinal PL(s) ataxia | No | No |
| 6. French Bulldog, 4Y1M MN, 15.7 kg | 24 | Moderate paraparesis, spinal PL(s) ataxia | No | No |
| 7. French Bulldog, 0Y7M M, 8.6 kg | 2 | Moderate paraparesis, spinal PL(s) ataxia | No | No |
| 8. Rottweiler, 4Y0M M, 35 kg | 1 | Moderate ambulatory tetraparesis, L-hypermetria, general proprioceptive ataxia | No | No |
| 9.West Highland White Terrier, 1Y3M F, 8.2 kg | 2 | Mild paraparesis L-lateralisation, spinal PL(s) ataxia | No | No |
| 10. French Bulldog, 3Y7M, M, 10.9 kg | 2 | Mild paraparesis, spinal PL(s) ataxia | No | No |
| Case | MRI | CT | Surgery | Immediate Postop Results | Outcome |
|---|---|---|---|---|---|
| 1. French Bulldog | Dorsal SAD at the T10–T11. A mild intramedullary T2 hyperintensity at the T11–T12 | No | Dorsal laminectomy T10–T12 | Nonambulatory paraparesis for 3 days, regained ambulation on day 4. | Improved: 30 months postop: no deficits. |
| 2. Pug | Dorsal SAD T13–L1, syrinx T12–T13 Non-compressive T13–L1, L1–L2, L2–L3 disc protrusions | R CAPs T12, T13 atrophy | Dorsal laminectomy T13–L1 | Nonambulatory paraparesis for 5 days | Improved: 16 months of no gait deficits, 26 months of mild spastic ambulatory paraparesis recurrence, 30 months mild spastic ambulatory paraparesis. |
| 3. Pug | Dorsal-L-sided SAD T11–T12, mild T11–T12 disc protrusion | T11, T12 caudal CAPs atrophy, ventral spondylosis deformans T10–T13. | Left-sided T11–T12 hemilaminectomy | Nonambulatory paraparetic during 6 days hospitalisation | Improved: 25 months: mild ambulatory paraparesis, no faecal incontinence. |
| 4. Staffordshire Bull Terrier | Dorsal T12–T13 SAD, cranial syringohydromyelia. | Mild thoracolumbar vertebral spondylosis | Dorsal laminectomy T12–T13 | Ambulatory paraparesis | Improved: 9 months: mild paraparesis, mild PL(s) ataxia, 32 months: moderate paraparesis. |
| 5. Pug | Dorsal SAD T13–L1 | T11, T12, T12, T13, L1 CAPs dysplasia | Dorsal laminectomy T13–L1 | Nonambulatory paraparesis for 4 days | Improved: 22 months no PL(s) weakness or ataxia. |
| 6. French Bulldog | Dorsal R-sided SAD T9–T10, hemivertebrae T10, T13 | No | Dorsal laminectomy T9–T1 | Ambulatory paraparetic | Improved: 9 months no deficits. Euthanised 10 months postop due to acute paraplegia from a traumatic incident |
| 7. French Bulldog | Dorsal SAD T10. T2W-intramedullary hyperintensity T10–T13 secondary syringohydromyelia | Vertebral malformations T7–T12 without spinal cord compression | Dorsal laminectomy T9–T10 | Nonambulatory paraparesis for 6 days | Improved: 40 months no deficits. |
| 8. Rottweiler | Dorsal SAD C2–C3 with marked spinal cord compression | No | Right-sided C2–C3 cervical dorso-lateral laminectomy | Nonambulatory tetraparesis for one day | Improved: 32 months: no deficits. |
| 9.West Highland White Terrier | Dorsal SAD T12–T13 | No | Dorsal laminectomy T11–T13 | Ambulatory paraparetic | Improved: 24 months: no deficits. |
| 10. French Bulldog | Dorsal SAD T7, changes at T8 suggestive of oedema, hemivertebrae T5, T7, non-compressive disc protrusions at C3–C4, C4–C5, T10–T11, T11–T12, L2–L3, L7–S1 | No | Dorsal laminectomy T6–T7 | Ambulatory paraparetic | Improved: 16 months: no deficits. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mól, M.; Fernandes, R.; Wheeler, S.; Mariscoli, M. Surgical Outcomes of Laminectomy, Durotomy and a Non-Synthetic Dura Substitute Application in Ten Dogs with a Spinal Subarachnoid Diverticulum. Vet. Sci. 2024, 11, 128. https://doi.org/10.3390/vetsci11030128
Mól M, Fernandes R, Wheeler S, Mariscoli M. Surgical Outcomes of Laminectomy, Durotomy and a Non-Synthetic Dura Substitute Application in Ten Dogs with a Spinal Subarachnoid Diverticulum. Veterinary Sciences. 2024; 11(3):128. https://doi.org/10.3390/vetsci11030128
Chicago/Turabian StyleMól, Michał, Ricardo Fernandes, Simon Wheeler, and Massimo Mariscoli. 2024. "Surgical Outcomes of Laminectomy, Durotomy and a Non-Synthetic Dura Substitute Application in Ten Dogs with a Spinal Subarachnoid Diverticulum" Veterinary Sciences 11, no. 3: 128. https://doi.org/10.3390/vetsci11030128
APA StyleMól, M., Fernandes, R., Wheeler, S., & Mariscoli, M. (2024). Surgical Outcomes of Laminectomy, Durotomy and a Non-Synthetic Dura Substitute Application in Ten Dogs with a Spinal Subarachnoid Diverticulum. Veterinary Sciences, 11(3), 128. https://doi.org/10.3390/vetsci11030128






