Evaluation of a Manual Cytocentrifuge versus the Standard Automated Cytocentrifuge in the Analysis of Canine Cerebrospinal Fluid: A Case Series of 55 Dogs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Case Selection
2.2. Cell Counts
2.3. Cytocentrifugation
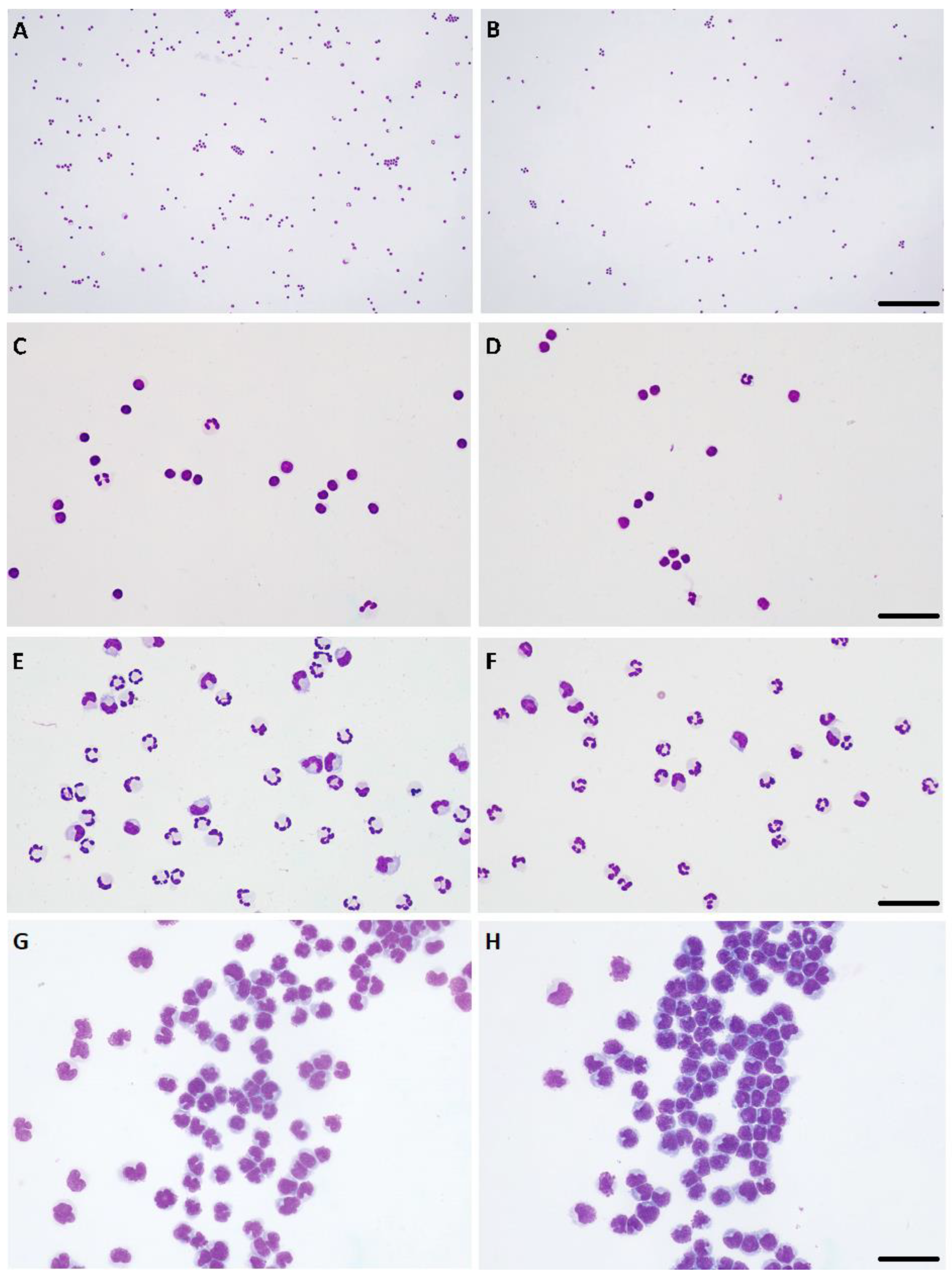
2.4. Microscopic Evaluation
2.5. Pleocytosis Classification
2.6. Presence of Artifacts
2.7. Statistical Analysis
3. Results
3.1. Study Case Series
3.2. Cytocentrifugation Technique
3.3. Comparison of Cell Counts and WBCs Differential
3.4. Presence of Artifacts
3.5. Presence of RBCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Terlizzi, R.; Platt, S. The function, composition and analysis of cerebrospinal fluid in companion animals: Part I—Function and composition. Vet. J. 2006, 172, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Vandevelde, M.; Spano, J.S. Cerebrospinal Fluid Cytology in Canine Neurologic Disease. Am. J. Vet. Res. 1977, 38, 1827–1832. [Google Scholar]
- Raskin, R.E.; Meyer, D.J. The Central Nervous System. In Canine and Feline Cytology. A Color Atlas and Interpretation Guide, 3rd ed.; Elsevier Health Sciences: St. Louis, MO, USA, 2016; pp. 369–407. [Google Scholar]
- Levine, G.; Cook, J. Cerebrospinal Fluid and Central Nervous System Cytology. In Cowell and Tyler’s Diagnostic Cytology and Hematology of the Dog and Cat, 5th ed.; Elsevier Health Sciences: St. Louis, MO, USA, 2020; pp. 210–228. [Google Scholar]
- Di Terlizzi, R.; Platt, S.R. The function, composition and analysis of cerebrospinal fluid in companion animals: Part II—Analysis. Vet. J. 2009, 180, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.; Bauer, N.; Moritz, A. Automated flow cytometric cell count and differentiation of canine cerebrospinal fluid cells using ADVIA 2120. Vet. Clin. Pathol. 2008, 37, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Rusbridge, C. Collection and interpretation of cerebrospinal fluid in cats and dogs. Practice 1997, 19, 322–331. [Google Scholar] [CrossRef]
- Ruotsalo, K.; Poma, R.; da Costa, R.C.; Bienzle, D. Evaluation of the ADVIA 120 for analysis of canine cerebrospinal fluid. Vet. Clin. Pathol. 2008, 37, 242–248. [Google Scholar] [CrossRef]
- Cook, J.R.; DeNicola, D.B. Cerebrospinal Fluid. Vet. Clin. N. Am. Small Anim. Pract. 1988, 18, 475–499. [Google Scholar] [CrossRef]
- Mayhew, I.G.; Beal, C.R. Techinques of analysis of cerebrospinal fluid. Vet. Clin. N. Am. Small Anim. Pract. 1980, 10, 155–176. [Google Scholar] [CrossRef]
- Garma-Aviña, A. An inexpensive sedimentation chamber for the preparation of cytologic specimens of cerebrospinal fluid. J. Vet. Diagn. Investig. 2004, 16, 585–587. [Google Scholar] [CrossRef]
- Hare, C.; Sanchini, L.; Worrall, C.; Van Poucke, S.; Alves, L.; Restif, O.; Freeman, P. Rapid in-house method of CSF analysis utilising sedimentation direct from the spinal needle. J. Small Anim. Pract. 2019, 60, 486–492. [Google Scholar] [CrossRef]
- Newton, P.L.; Fry, D.R.; Best, M.P. Comparison of direct in-house cerebrospinal fluid cytology with commercial pathology results in dogs. J. Small Anim. Pract. 2017, 58, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Marcos, R.; Santos, M.; Marinhas, C.; Correia-Gomes, C.; Caniatti, M. Cytocentrifuge preparation in veterinary cytology: A quick, simple, and affordable manual method to concentrate low cellularity fluids. Vet. Clin. Pathol. 2016, 45, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Duque, C.; Parent, J.; Bienzle, D. The immunophenotype of blood and cerebrospinal fluid mononuclear cells in dogs. J. Vet. Intern. Med. 2002, 16, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Byagathvalli, G.; Challita, E.J.; Bhamla, M.S. Frugal Science Powered by Curiosity. Ind. Eng. Chem. Res. 2021, 60, 15874–15884. [Google Scholar] [CrossRef] [PubMed]
- Doyle, C.; Solano-Gallego, L. Cytologic interpretation of canine cerebrospinal fluid samples with low total nucleated cell concentration, with and without blood contamination. Vet. Clin. Pathol. 2009, 38, 392–396. [Google Scholar] [CrossRef]
- Revelle, W. Procedures for Psychological, Psychometric, and Personality Research [R Package Psych Version 2.3.6]. Available online: https://personality-project.org/r/psych-manual.pdf (accessed on 3 June 2023).
- Christopher, M.M.; Perman, V.; Hardy, R.M. Reassessment of cytologic values in canine cerebrospinal fluid by use of cytocentrifugation. J. Am. Vet. Med. Assoc. 1988, 192, 1726–1729. [Google Scholar]
- Dyken, P.R.; Shirley, S.; Trefz, J.; El Gammal, T. Comparison of Cytocentrifugation and Sedimentation Techniques for CSF Cytomorphology. Acta Cytol. 1980, 24, 167–170. [Google Scholar]
- Fry, M.M.; Vernau, W.; Kass, P.H.; Vernau, K.M. Effects of time, initial composition, and stabilizing agents on the results of canine cerebrospinal fluid analysis. Vet. Clin. Pathol. 2006, 35, 72–77. [Google Scholar] [CrossRef]
- Jensen, A.L.; Kjelgaard-Hansen, M. Method comparison in the clinical laboratory. Vet. Clin. Pathol. 2006, 35, 276–286. [Google Scholar] [CrossRef]
- Hansen, H.H.; Bender, R.A.; Shelton, B.J. The Cyto-centrifuge and Cerebrospinal Fluid Cytology. Acta Cytol. 1974, 18, 259–262. [Google Scholar]
- Talarico, L.R.; Schatzberg, S.J. Idiopathic granulomatous and necrotising inflammatory disorders of the canine central nervous system: A review and future perspectives. J. Small Anim. Pract. 2010, 51, 138–149. [Google Scholar] [CrossRef] [PubMed]

| Prospective Cases | Retrospective Cases | TOTAL | |
|---|---|---|---|
| No pleocytosis | 33 | 5 | 38 |
| Pleocytosis | 15 | 2 | 17 |
| Mild | 6 | 2 | 8 |
| Moderate | 5 | 0 | 5 |
| Severe | 4 | 0 | 4 |
| TOTAL | 48 | 7 | 55 |
| Pleocytosis | Automatic | Manual |
|---|---|---|
| Mild | ||
| Case n. 08 | Mixed-cell | Mixed-cell, predominantly lymphocytic |
| Case n. 10 | Monocytoid | Monocytoid |
| Case n. 12 | Mixed-cell, predominantly lymphocytic | Mixed-cell, predominantly lymphocytic |
| Case n. 13 | Mixed-cell, predominantly neutrophilic | Mixed-cell, predominantly neutrophilic |
| Case n. 21 | Neutrophilic | Neutrophilic |
| Case n. 27 | Lymphocytic | Lymphocytic |
| Case n. 30 | Mixed-cell | Mixed cell |
| Case n. 44 | Lymphocytic | Lymphocytic |
| Moderate | ||
| Case n. 05 | Neutrophilic | Neutrophilic |
| Case n. 15 | Lymphocytic | Lymphocytic |
| Case n. 32 | Mixed-cell, predominantly monocytoid | Mixed-cell |
| Case n. 41 | Mixed-cell, predominantly lymphocytic | Mixed-cell, predominantly lymphocytic |
| Case n. 55 | Lymphocytic | Lymphocytic |
| Severe | ||
| Case n. 03 | Lymphocytic | Lymphocytic |
| Case n. 24 | Neutrophilic | Neutrophilic |
| Case n. 47 | Lymphocytic | Lymphocytic |
| Case n. 54 | Mixed-cell, predominantly monocytoid | Mixed-cell, predominantly monocytoid |
| MANUAL | AUTOMATIC | |||
| Presence | Total | |||
| Presence | 31 | Presence | 36 | |
| Absence | 10 | Absence | 11 | |
| Total | 41 | Total | 47 | |
| MANUAL | AUTOMATIC | |||
| Presence | Absence | Total | ||
| Presence | 17 | 4 | 21 | |
| Absence | 6 | 28 | 34 | |
| Total | 23 | 32 | 55 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fonte-Oliveira, L.; Pereira, A.; Gregório, H.; Ribeiro, J.; Correia-Gomes, C.; Marcos, R.; Santos, M. Evaluation of a Manual Cytocentrifuge versus the Standard Automated Cytocentrifuge in the Analysis of Canine Cerebrospinal Fluid: A Case Series of 55 Dogs. Vet. Sci. 2023, 10, 631. https://doi.org/10.3390/vetsci10110631
Fonte-Oliveira L, Pereira A, Gregório H, Ribeiro J, Correia-Gomes C, Marcos R, Santos M. Evaluation of a Manual Cytocentrifuge versus the Standard Automated Cytocentrifuge in the Analysis of Canine Cerebrospinal Fluid: A Case Series of 55 Dogs. Veterinary Sciences. 2023; 10(11):631. https://doi.org/10.3390/vetsci10110631
Chicago/Turabian StyleFonte-Oliveira, Luísa, André Pereira, Hugo Gregório, João Ribeiro, Carla Correia-Gomes, Ricardo Marcos, and Marta Santos. 2023. "Evaluation of a Manual Cytocentrifuge versus the Standard Automated Cytocentrifuge in the Analysis of Canine Cerebrospinal Fluid: A Case Series of 55 Dogs" Veterinary Sciences 10, no. 11: 631. https://doi.org/10.3390/vetsci10110631
APA StyleFonte-Oliveira, L., Pereira, A., Gregório, H., Ribeiro, J., Correia-Gomes, C., Marcos, R., & Santos, M. (2023). Evaluation of a Manual Cytocentrifuge versus the Standard Automated Cytocentrifuge in the Analysis of Canine Cerebrospinal Fluid: A Case Series of 55 Dogs. Veterinary Sciences, 10(11), 631. https://doi.org/10.3390/vetsci10110631






