Internal Validation of the ASFV MONODOSE dtec-qPCR Kit for African Swine Fever Virus Detection under the UNE-EN ISO/IEC 17025:2005 Criteria
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. DNA Extraction/Purification
2.2. qPCR Protocol
2.3. Analytical Specificity
2.4. Analytical Sensitivity
2.5. Diagnostic Performance
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Alonso, C.; Borca, M.; Dixon, L.; Revilla, Y.; Rodriguez, F.; Escribano, J.M.; ICTV Report Consortium. ICTV Virus Taxonomy Profile: Asfarviridae. J. Gen. Virol. 2018, 99, 613–614. [Google Scholar] [CrossRef] [PubMed]
- Borca, M.V.; Ramirez-Medina, E.; Silva, E.; Vuono, E.; Rai, A.; Pruitt, S.; Espinoza, N.; Velazquez-Salinas, L.; Gay, C.G.; Gladue, D.P. ASFV-G-∆I177L as an Effective Oral Nasal Vaccine against the Eurasia Strain of Africa Swine Fever. Viruses 2021, 13, 765. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, E.G.; Pérez-Núñez, D.; Revilla, Y. Development of vaccines against African swine fever virus. Virus Res. 2019, 265, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, C.; Casado, N.; Soler, A.; Djadjovski, I.; Krivko, L.; Madueño, E.; Nieto, R.; Perez, C.; Simon, A.; Ivanova, E.; et al. A multi gene-approach genotyping method identifies 24 genetic clusters within the genotype II-European African swine fever viruses circulating from 2007 to 2022. Front. Vet. Sci. 2023, 10, 1112850. [Google Scholar] [CrossRef] [PubMed]
- Quembo, C.; Jori, F.; Vosloo, W.; Heath, L. Genetic characterization of African swine fever virus isolates from soft ticks at the wildlife/domestic interface in Mozambique and identification of a novel genotype. Transbound. Emerg. Dis. 2018, 65, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Achenbach, J.E.; Gallardo, C.; Nieto-Pelegrín, E.; Rivera-Arroyo, B.; Degefa-Negi, T.; Arias, M.; Jenberie, S.; Mulisa, D.D.; Gizaw, D.; Gelaye, E.; et al. Identification of a New Genotype of African Swine Fever Virus in Domestic Pigs from Ethiopia. Transbound. Emerg. Dis. 2017, 64, 1393–1404. [Google Scholar] [CrossRef]
- Sánchez-Vizcaíno, J.M.; Mur, L.; Gomez-Villamandos, J.C.; Carrasco, L. An Update on the Epidemiology and Pathology of African Swine Fever. J. Comp. Pathol. 2015, 152, 9–21. [Google Scholar] [CrossRef]
- Gaudreault, N.N.; Madden, D.W.; Wilson, W.C.; Trujillo, J.D.; Richt, J.A. African Swine Fever Virus: An Emerging DNA Arbovirus. Front. Vet. Sci. 2020, 7, 215. [Google Scholar] [CrossRef]
- Sánchez-Cordón, P.J.; Nunez, A.; Neimanis, A.; Wikström-Lassa, E.; Montoya, M.; Crooke, H.; Gavier-Widén, D. African Swine Fever: Disease Dynamics in Wild Boar Experimentally Infected with ASFV Isolates Belonging to Genotype I and II. Viruses 2019, 11, 852. [Google Scholar] [CrossRef]
- WOAH—World Organisation for Animal Health. African Swine Fever (ASF)—Situation Report 26 n.d. Available online: https://www.woah.org/en/document/african-swine-fever-asf-situation-report-26/ (accessed on 31 January 2023).
- Gallardo, C.; Fernández-Pinero, J.; Arias, M. African swine fever (ASF) diagnosis, an essential tool in the epidemiological investigation. Virus Res. 2019, 271, 197676. [Google Scholar] [CrossRef]
- Wade, A.; Achenbach, J.E.; Gallardo, C.; Settypalli, T.B.K.; Souley, A.; Djonwe, G.; Loitsch, A.; Dauphin, G.; Ngang, J.J.E.; Boyomo, O.; et al. Genetic characterization of African swine fever virus in Cameroon, 2010–2018. J. Microbiol. 2019, 57, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Bellini, S.; Rutili, D.; Guberti, V. Preventive measures aimed at minimizing the risk of African swine fever virus spread in pig farming systems. Acta Vet. Scand. 2016, 58, 82. [Google Scholar] [CrossRef] [PubMed]
- Belák, S. The molecular diagnosis of porcine viral diseases: A review. Acta Vet. Hung. 2005, 53, 113–124. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sloan, L.M. Real-time PCR in clinical microbiology: Verification, validation, and contamination control. Clin. Microbiol. Newsl. 2007, 29, 87–95. [Google Scholar] [CrossRef]
- WOAH—World Organisation for Animal Health. Terrestrial Manual Online Access n.d. Available online: https://www.woah.org/en/what-we-do/standards/codes-and-manuals/terrestrial-manual-online-access/ (accessed on 1 February 2023).
- WOAH—World Organisation for Animal Health. Chapter 3.8.1 African Swine Fever. OIE Terrestrial Manual; OIE: Paris, France, 2019. p. Chapter 3.8.1 African swine fever. Available online: https://www.woah.org/fileadmin/Home/fr/Health_standards/tahm/3.08.01_ASF.pdf (accessed on 1 July 2023).
- Beltrán-Alcrudo, D.; Arias, M.; Gallardo, C.; Kramer, S.A.; Penrith, M.-L. African Swine Fever: Detection and Diagnosis: A Manual for Veterinarians; Food and Agriculture Organization of the United Nations: Rome, Italy, 2017. [Google Scholar]
- Tignon, M.; Gallardo, C.; Iscaro, C.; Hutet, E.; Van der Stede, Y.; Kolbasov, D.; De Mia, G.M.; Le Potier, M.-F.; Bishop, R.P.; Arias, M.; et al. Development and inter-laboratory validation study of an improved new real-time PCR assay with internal control for detection and laboratory diagnosis of African swine fever virus. J. Virol. Methods 2011, 178, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Agüero, M.; Fernández, J.; Romero, L.; Sánchez Mascaraque, C.; Arias, M.; Sánchez-Vizcaíno, J.M. Highly Sensitive PCR Assay for Routine Diagnosis of African Swine Fever Virus in Clinical Samples. J. Clin. Microbiol. 2003, 41, 4431–4434. [Google Scholar] [CrossRef]
- Oluwole, O.; Omitogun, O. Polymerase Chain Reaction Detection of ASFV Infection in Nigerian Indigenous Pig. Am. J. Mol. Biol. 2014, 4, 159–162. [Google Scholar] [CrossRef][Green Version]
- King, D.P.; Reid, S.M.; Hutchings, G.H.; Grierson, S.S.; Wilkinson, P.J.; Dixon, L.K.; Bastos, A.D.; Drew, T.W. Development of a TaqMan® PCR assay with internal amplification control for the detection of African swine fever virus. J. Virol. Methods 2003, 107, 53–61. [Google Scholar] [CrossRef]
- Zsak, L.; Borca, M.V.; Risatti, G.R.; Zsak, A.; French, R.A.; Lu, Z.; Kutish, G.F.; Neilan, J.G.; Callahan, J.D.; Nelson, W.M.; et al. Preclinical diagnosis of African swine fever in contact-exposed swine by a real-time PCR assay. J. Clin. Microbiol. 2005, 43, 112–119. [Google Scholar] [CrossRef]
- Haines, F.J.; Hofmann, M.A.; King, D.P.; Drew, T.W.; Crooke, H.R. Development and validation of a multiplex, real-time RT PCR assay for the simultaneous detection of classical and African swine fever viruses. PLoS ONE 2013, 8, e71019. [Google Scholar] [CrossRef]
- Grau, F.R.; Schroeder, M.E.; Mulhern, E.L.; McIntosh, M.T.; Bounpheng, M.A. Detection of African swine fever, classical swine fever, and foot-and-mouth disease viruses in swine oral fluids by multiplex reverse transcription real-time polymerase chain reaction. J. Vet. Diagn. Investig. 2015, 27, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Pinero, J.; Gallardo, C.; Elizalde, M.; Robles, A.; Gómez, C.; Bishop, R.; Heath, L.; Couacy-Hymann, E.; Fasina, F.O.; Pelayo, V.; et al. Molecular diagnosis of African Swine Fever by a new real-time PCR using universal probe library. Transbound. Emerg. Dis. 2013, 60, 48–58. [Google Scholar] [CrossRef] [PubMed]
- UNE-EN ISO/IEC 17025. 2005. Conformity Assessment. General Requirements for the Competence of Testing and Calibration Laboratories. Available online: https://www.iso.org/standard/39883.html (accessed on 1 July 2023).
- Martínez-Murcia, A.; Navarro, A.; Bru, G.; Chowdhary, A.; Hagen, F.; Meis, J.F. Internal validation of GPSTM MONODOSE CanAur dtec-qPCR kit following the UNE/EN ISO/IEC 17025, 2005 for detection of the emerging yeast Candida auris. Mycoses 2018, 61, 877–884. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information n.d. Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 30 November 2022).
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11, Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Martínez-Murcia, A.; Bru, G.; Navarro, A.; Ros-Tárraga, P.; García-Sirera, A.; Pérez, L. Comparative in silico design and validation of GPSTM COVID-19 dtec-RT-qPCR test. J. Appl. Microbiol. 2021, 130, 2–13. [Google Scholar] [CrossRef]
- Anderson, E.C.; Hutchings, G.H.; Mukarati, N.; Wilkinson, P.J. African swine fever virus infection of the bushpig (Potamochoerus porcus) and its significance in the epidemiology of the disease. Vet. Microbiol. 1998, 62, 1–15. [Google Scholar] [CrossRef]
- Council Directive 2002/60/EC of 27 June 2002 laying down specific provisions for the control of African swine fever and amending Directive 92/119/EEC as regards Teschen disease and African swine fever (Text with EEA relevance). Off. J. Eur. Community 2002, 192, 27–46.
- De Lorenzi, G.; Borella, L.; Alborali, G.L.; Prodanov-Radulović, J.; Štukelj, M.; Bellini, S. African swine fever: A review of cleaning and disinfection procedures in commercial pig holdings. Res. Vet. Sci. 2020, 132, 262–267. [Google Scholar] [CrossRef]
- Gallardo, C.; Nieto, R.; Soler, A.; Pelayo, V.; Fernández-Pinero, J.; Markowska-Daniel, I.; Pridotkas, G.; Nurmoja, I.; Granta, R.; Simón, A.; et al. Assessment of African Swine Fever Diagnostic Techniques as a Response to the Epidemic Outbreaks in Eastern European Union Countries: How to Improve Surveillance and Control Programs. J. Clin. Microbiol. 2015, 53, 2555–2565. [Google Scholar] [CrossRef]
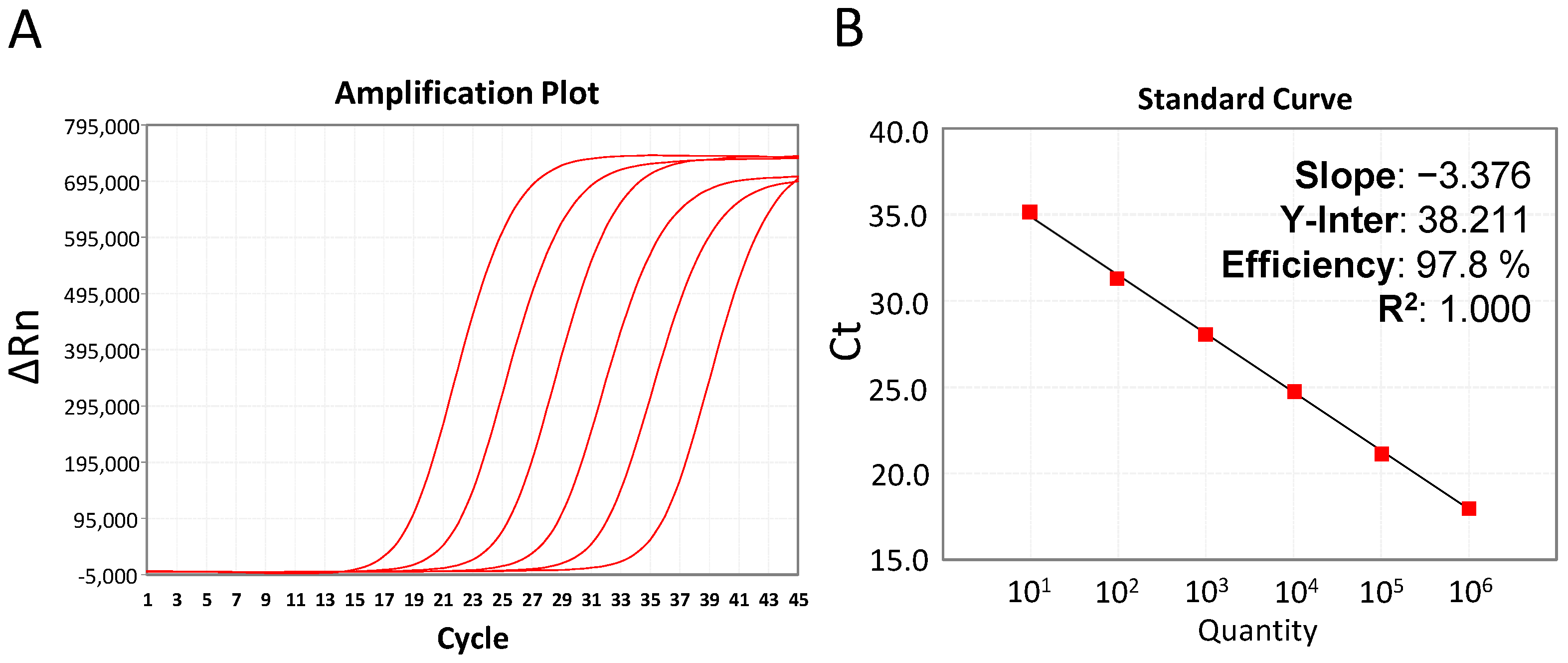
| Term of Validation | Obtained Values | Acceptance Criteria | Conclusion | |
|---|---|---|---|---|
| Specificity | Inclusivity: in silico assays against all available sequences from the target species. Assessed with 21 reference DNAs (INIA-CISA) and 8 reference samples (Poland) positive for ASFV | Inclusivity: positive amplification for all ASFV strains and for all reference samples | ACCEPTED | |
| Exclusivity: in silico studies against all available sequences | Exclusivity: negative amplification for virus other than ASFV | ACCEPTED | ||
| Standard curve n = 10 | Y = −3.369 × X + 36.375 a = −3.369 R2 = 1.000 | −4.114 < a < −2.839 R2 > 0.98 | ACCEPTED | |
| Fassay = 3.261 Ffisher = 5.318 | Fassay < Ffisher | ACCEPTED | ||
| Efficiency (e) = 98.1% | 75% < e < 125% | VALIDATED | ||
| Reliability n = 10 | Repeatability | CV < 10% | REPEATABLE | |
| Conc. | CV (%) | |||
| 106 copies | 1.94% | |||
| 105 copies | 0.65% | |||
| 104 copies | 0.62% | |||
| 103 copies | 0.63% | |||
| 102 copies | 0.90% | |||
| 101 copies | 1.12% | |||
| Reproducibility | CV < 10% | REPRODUCIBLE | ||
| Conc. | CV (%) | |||
| 106 copies | 1.65% | |||
| 105 copies | 0.95% | |||
| 104 copies | 0.97% | |||
| 103 copies | 0.86% | |||
| 102 copies | 1.04% | |||
| 101 copies | 1.34% | |||
| Detection limit (LOD) n = 15 | 10 copies | Posit = 15/15 | Positives ≥ 90% | ACCEPTED |
| Quantification limit (LOQ) n = 15 | 10 copies | t value = 1.161 | t value < tstudent | ACCEPTED |
| tstudent = 2.145 | ||||
| Diagnostic sensitivity | True Positives: 105 False Negatives: 0 SD = 100% | DS > 90% | ACCEPTED | |
| Diagnostic specificity | True Positives: 76 False Positives: 0 ED = 100% | DE > 90% | ACCEPTED | |
| Isolate | Origin Country | Host | Year | Town/Province | Geno Type | Laboratory | GPS™ KIT (Ct) |
|---|---|---|---|---|---|---|---|
| E70 | Spain | Pig | 1970 | Pontevedra | I | INIA-CISA * | 24.73 |
| BF07 OUAGA 2 | Burkina | Pig | 2007 | Ouaguodaga | I | INIA-CISA * | 27.49 |
| SS14/WB-Sassari1 | Italy | Wild boar | 2014 | Sassari | I | INIA-CISA * | 25.82 |
| SS14/DP-Cagliari1 | Italy | Pig | 2014 | Cagliari | I | INIA-CISA * | 29.90 |
| Arm07 | Armenia | Pig | 2007 | Dilijan | II | INIA-CISA * | 24.94 |
| Ukr12/Zapo | Ukraine | Pig | 2012 | Zaporozhye | II | INIA-CISA * | 23.87 |
| Ukr15/DP-Kieve 1 | Ukraine | Pig | 2015 | Kiev | II | INIA-CISA * | 26.73 |
| LT14/1490 | Lithuania | Wild boar | 2014 | Vilnius | II | INIA-CISA * | 24.16 |
| Pol14/Krus | Poland | Wild boar | 2014 | Podlaskie | II | INIA-CISA * | 24.96 |
| Lv14/DP/Robez3 | Latvia | Pig | 2014 | Dienvidlatgale | II | INIA-CISA * | 27.50 |
| Est14/WB-Valga-1 | Estonia | Wild boar | 2014 | Valga | II | INIA-CISA * | 27.59 |
| Est15/WB-Tartu14 | Estonia | Wild boar | 2015 | Tartu | II | INIA-CISA * | 29.16 |
| MOL16/DP-CERNO1 | Moldova | Pig | 2016 | Cernoleuca | II | INIA-CISA * | 28.35 |
| MOL16/DP-MOSA1 | Moldova | Pig | 2016 | Mosana | II | INIA-CISA * | 25.96 |
| Moz64 | Mozambique | Pig | 1964 | NK | V | INIA-CISA * | 24.58 |
| MwLil 20/1 | Malawi | Tick | 1983 | Chalaswa | VIII | Complete genome | 26.34 |
| Ken11/KisP52 | Kenya | Pig | 2011 | Kisumu | IX | INIA-CISA * | 28.42 |
| Ken06.Bus | Kenya | Pig | 2006 | Busia | IX | INIA-CISA * | 30.54 |
| Ken08Tk.2/1 | Kenya | Tick | 2007 | Kapiti | X | INIA-CISA * | 27.40 |
| UG10/Tk3.2 | Uganda | Tick | 2010 | Mburu | X | INIA-CISA * | 32.35 |
| Eth13/1505 | Ethiopia | Pig | 2013 | Bishoftu | XXIII | INIA-CISA * | 26.51 |
| Pol18/Var1 | Poland | Pig | 2018 | Warsaw | - | PIWet ** | 24.73 |
| Pol18/Var2 | Poland | Pig | 2018 | Warsaw | - | PIWet ** | 27.49 |
| Pol18/Var3 | Poland | Wild boar | 2018 | Warsaw | - | PIWet ** | 25.82 |
| Pol18/Var4 | Poland | Wild boar | 2018 | Warsaw | - | PIWet ** | 29.90 |
| Pol18/Var5 | Poland | Wild boar | 2018 | Warsaw | - | PIWet ** | 24.94 |
| Pol18/Var6 | Poland | Wild boar | 2018 | Warsaw | - | PIWet ** | 23.87 |
| Pol18/Var7 | Poland | Pig | 2018 | Warsaw | - | PIWet ** | 26.73 |
| Pol18/Var8 | Poland | Pig | 2018 | Warsaw | - | PIWet ** | 24.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bru, G.; Martínez-Candela, M.; Romero, P.; Navarro, A.; Martínez-Murcia, A. Internal Validation of the ASFV MONODOSE dtec-qPCR Kit for African Swine Fever Virus Detection under the UNE-EN ISO/IEC 17025:2005 Criteria. Vet. Sci. 2023, 10, 564. https://doi.org/10.3390/vetsci10090564
Bru G, Martínez-Candela M, Romero P, Navarro A, Martínez-Murcia A. Internal Validation of the ASFV MONODOSE dtec-qPCR Kit for African Swine Fever Virus Detection under the UNE-EN ISO/IEC 17025:2005 Criteria. Veterinary Sciences. 2023; 10(9):564. https://doi.org/10.3390/vetsci10090564
Chicago/Turabian StyleBru, Gema, Marta Martínez-Candela, Paloma Romero, Aaron Navarro, and Antonio Martínez-Murcia. 2023. "Internal Validation of the ASFV MONODOSE dtec-qPCR Kit for African Swine Fever Virus Detection under the UNE-EN ISO/IEC 17025:2005 Criteria" Veterinary Sciences 10, no. 9: 564. https://doi.org/10.3390/vetsci10090564
APA StyleBru, G., Martínez-Candela, M., Romero, P., Navarro, A., & Martínez-Murcia, A. (2023). Internal Validation of the ASFV MONODOSE dtec-qPCR Kit for African Swine Fever Virus Detection under the UNE-EN ISO/IEC 17025:2005 Criteria. Veterinary Sciences, 10(9), 564. https://doi.org/10.3390/vetsci10090564






