An In-House ELISA for Treponema Antibodies in Bulk Milk as Part of a Monitoring Tool for Claw Health in Dairy Herds
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Farms in This Study
2.2. Herd Data Collection
2.3. Samples
2.4. Laboratorial Analysis of the Milk Samples
Treponema Antigen Preparation
2.5. Statistical Analysis
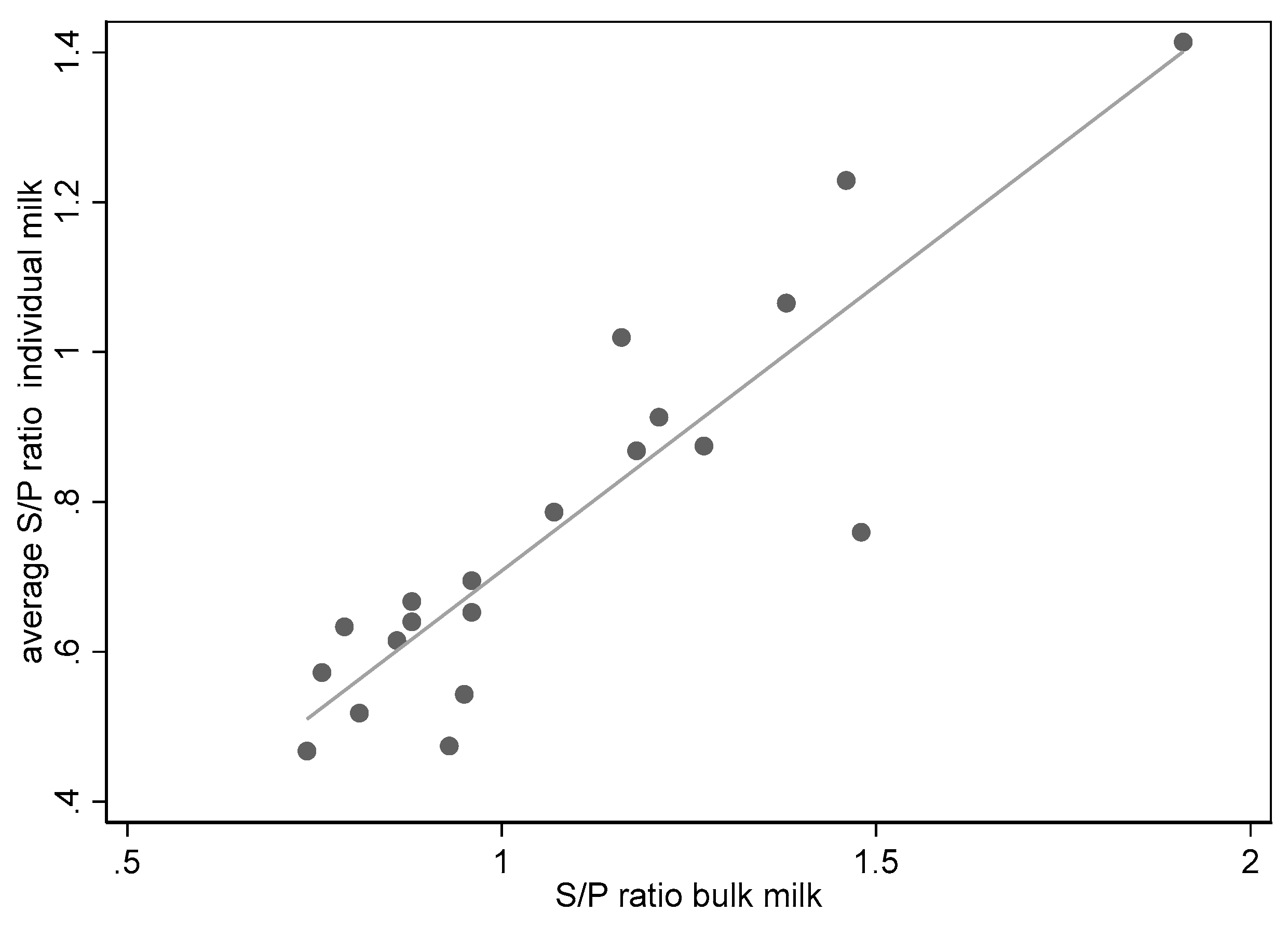
3. Results
Treponema ELISA Bulk Milk Program
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Read, D.H.; Walker, R.L. Papillomatous digital dermatitis (footwarts) in California dairy cattle: Clinical and gross pathologic findings. J. Vet. Diagn. Investing. 1998, 10, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Holzhauer, M.; Hardenberg, C.; Bartels, C.J.M.; Frankena, K. Herd- and cow-level prevalence of digital dermatitis in the Netherlands and associated risk factors. J. Dairy Sci. 2006, 89, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Cheli, R.; Mortellaro, C. Digital dermatitis in cattle. In Proceedings of the 8th International Conference on Diseases of Cattle, Piacenza, Milan, Italy, 3–6 September 1974; Volume 8, pp. 208–213. [Google Scholar]
- Klitgaard, K.; Foix Breto, A.; Boye, M.; Jensen, T.K. Targeting the treponemal microbiome of digital dermatitis infections by high-resolution phylogenetic analyses and comparison with fluorescent in situ hybridization. J. Clin. Microbiol. 2013, 51, 2212–2219. [Google Scholar] [CrossRef] [PubMed]
- Evans, N.J.; Brown, J.M.; Demirkan, I.; Murray, R.D.; Vink, W.D.; Blowey, R.W.; Hart, C.A.; Carter, S.D. Three unique groups of spirochetes isolated from digital dermatitis lesions in UK cattle. Vet. Microbiol. 2008, 130, 141–150. [Google Scholar] [CrossRef]
- Wilson-Welder, J.H.; Elliott, M.K.; Zuerner, R.L.; Bayles, D.O.; Alt, D.P.; Stanton, T.B. Biochemical and molecular characterization of Treponema phagedenis-like spirochetes isolated from a bovine digital dermatitis lesion. BMC Microbiol. 2013, 13, 280. [Google Scholar] [CrossRef]
- Krull, A.C.; Shearer, J.K.; Gorden, P.J.; Cooper, V.L.; Phillips, G.J.; Plummer, P.J. Deep sequencing analysis reveals temporal microbiota changes associated with development of bovine digital dermatitis. Infect. Immun. 2014, 82, 3359–3373. [Google Scholar] [CrossRef]
- Capion, N.; Boye, M.; Ekstrøm, C.T.; Jensen, T.K. Infection dynamics of digital dermatitis in first-lactation Holstein cows in an infected herd. J. Dairy Sci. 2012, 95, 6457–6464. [Google Scholar] [CrossRef]
- Bruijnis, M.R.N.; Hogeveen, H.; Stassen, E.N. Assessing economic consequences of foot disorders in dairy cattle using a dynamic stochastic simulation model. J. Dairy Sci. 2010, 93, 2419–2432. [Google Scholar] [CrossRef]
- Cha, E.; Hertl, J.A.; Bar, D.; Gröhn, Y.T. The cost of different types of lameness in dairy cows calculated by dynamic programming. Prev. Vet. Med. 2010, 97, 1–8. [Google Scholar] [CrossRef]
- Clegg, S.R.; Mansfield, K.G.; Newbrook, K.; Sullivan, L.E.; Blowey, R.W.; Carter, S.D.; Evans, N.J. Isolation of digital dermatitis treponemes from hoof lesions in wild North American elk (Cervus elaphus) in Washington State, USA. J. Clin. Microbiol. 2015, 53, 88–94. [Google Scholar] [CrossRef]
- Evans, N.; Murray, R.; Carter, S. Bovine digital dermatitis: Current concepts from laboratory to farm. Vet. J. 2016, 211, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Somers, J.G.C.J.; Frankena, K.; Noordhuizen-Stassen, E.N.; Metz, J.H.M. Prevalence of claw disorders in Dutch dairy cows exposed to several floor systems. J. Dairy Sci. 2003, 86, 2082–2093. [Google Scholar] [CrossRef] [PubMed]
- Holzhauer, M.; Bartels, C.J.M.; Döpfer, D.; van Schaik, G. Clinical course of digital dermatitis lesions in an endemically infected herd without preventive herd strategies. Vet. J. 2008, 177, 222–230. [Google Scholar] [CrossRef] [PubMed]
- van der Linde, C.; de Jong, G.; Koenen, E.P.C.; Eding, H. Claw health index for Dutch dairy cattle based on claw trimming and conformation data. J. Dairy Sci. 2010, 93, 4883–4891. [Google Scholar] [CrossRef] [PubMed]
- Döpfer, D.; Koopmans, A.; Meijer, F.; Szakall, I.; Schukken, Y.; Klee, W.; Bosma, R.B.; Cornelisse, J.L.; van Asten, A.J.; ter Huurne, A.A. Histological and bacteriological evaluation of digital dermatitis in cattle, with special reference to spirochaetes and Campylobacter faecalis. Vet. Rec. 1997, 140, 620–623. [Google Scholar] [CrossRef]
- Berry, S.L.; Read, D.H.; Famula, T.R.; Mongini, A.; Döpfer, D. Long-term observations on the dynamics of bovine digital dermatitis lesions on a California dairy after topical treatment with lincomycin HCl. Vet. J. 2012, 193, 654–658. [Google Scholar] [CrossRef]
- Greenough, P.R.; Muelling, C.K.W.; Döpfer, D.; Tomlinson, D.J. International atlas of lesions of cattle feet. Nomenclature and atlas update. In Proceedings of the 15th International Symposium & the 7th Conference Lameness Ruminants, Kuopio, Finland, 9–13 June 2008; p. 40. [Google Scholar]
- Thomsen, P.T.; Klaas, I.C.; Bach, K. Short communication: Scoring of digital dermatitis during milking as an alternative to scoring in a hoof trimming chute. J Dairy Sci. 2008, 91, 4679–4682. [Google Scholar] [CrossRef]
- Relun, A.; Guatteo, R.; Roussel, P.; Bareille, N. A simple method to score digital dermatitis in dairy cows in the milking parlor. J. Dairy Sci. 2011, 94, 5424–5434. [Google Scholar] [CrossRef]
- Stokes, J.E.; Leach, K.A.; Main, D.C.; Whay, H.R. The reliability of detecting digital dermatitis in the milking parlor. Vet. J. 2012, 193, 679–684. [Google Scholar] [CrossRef]
- Viera, A.J.; Garrett, J.M. Understanding interobserver agreement: The kappa statistic. Fam. Med. 2005, 37, 360–363. [Google Scholar]
- Alsaaod, M.; Syring, C.; Dietrich, J.; Doherr, M.G.; Gujan, T.; Steiner, A. A field trial of infrared thermography as a non-invasive diagnostic tool for early detection of digital dermatitis in dairy cows. Vet. J. 2014, 199, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Gomez, A.; Anklam, K.S.; Cook, N.B.; Rieman, J.; Dunbar, K.A.; Cooley, K.E.; Socha, M.T.; Döpfer, D. Immune response against Treponema spp. and ELISA detection of digital dermatitis. J. Dairy. Sci. 2014, 97, 4864–4875. [Google Scholar] [CrossRef] [PubMed]
- StataCorp. Stata Statistical Software: Release 17; StataCorp LLC: College Station, TX, USA, 2021. [Google Scholar]
- Frössling, J.; Rosander, A.; Björkman, C.; Näslund, K.; Pringle, M. Detection of Treponema phagedenis-like antibodies in serum and bulk milk from cows with and without digital dermatitis. J. Vet. Diagn. Investig. 2018, 30, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.M.A.; Pereira, R.V.; Caixeta, L.S.; Guard, C.L.; Bicalho, R.C. Microbial diversity in bovine papillomatous digital dermatitis in Holstein dairy cows from upstate New York. FEMS Microbiol. Ecol. 2012, 79, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Aubineau, T.; Relun, A.; Gentin, B.; Guatteo, R. Short communication: Informative value of an ELISA applied to bulk tank milk to assess within-herd prevalence of digital dermatitis in dairy herds. J. Dairy Sci. 2021, 104, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Afonso, J.S.; Oikonomou, G.; Carter, S.; Clough, H.E.; Griffiths, B.E.; Rushton, J. Diagnosis of Bovine Digital Dermatitis: Exploring the Usefulness of Indirect ELISA. Front. Vet. Sci. 2021, 8, 728691. [Google Scholar] [CrossRef]
- Evans, N.J.; Blowey, R.W.; Timofte, D.; Isherwood, D.R.; Brown, J.M.; Murray, R.; Paton, R.J.; Carter, S.D. Association between bovine digital dermatitis treponemes and a range of ‘non-healing’ bovine hoof disorders. Vet. Rec. 2011, 168, 214. [Google Scholar] [CrossRef]
- Persson Waller, K.; Bengtsson, M.; Nyman, A.-K. Prevalence and risk factors for udder cleft dermatitis in dairy cattle. J. Dairy Sci. 2014, 97, 310–318. [Google Scholar] [CrossRef]
- Clegg, S.R.; Bell, J.; Ainsworth, S.; Blowey, R.W.; Bell, N.J.; Carter, S.D.; Evans, N.J. Isolation of digital dermatitis treponemes from cattle hock skin lesions. Vet. Dermatol. 2016, 27, 106-e30. [Google Scholar] [CrossRef]
- Oliveira, V.H.S.; Sørensen, J.T.; Thomsen, P.T. Assessing farmer awareness of digital dermatitis prevalence in Danish dairy herds. Vet. Rec. 2017, 182, 325. [Google Scholar] [CrossRef]
- Solano, L.; Barkema, H.W.; Jacobs, C.; Orsel, K. Validation of the M-stage scoring system for digital dermatitis on dairy cows in the milking parlor. J. Dairy. Sci. 2017, 100, 1592–1603. [Google Scholar] [CrossRef] [PubMed]
- Stamm, L.V.; Bergen, H.L.; Walker, R.L. Molecular typing of papillomatous dermatitis-associated Treponema analysis of 16S–23S ribosomal DNA intergenic spacer regions. J. Clin. Microbiol. 2002, 40, 3463–3469. [Google Scholar] [CrossRef] [PubMed]
- Pringle, M.; Bergsten, C.; Fernström, L.L.; Höök, H.; Johansson, K.E. Isolation and characterization of Treponema phagedenis-like spirochetes from digital dermatitis lesions in Swedish dairy cattle. Acta Vet. Scand. 2008, 50, 40. [Google Scholar] [CrossRef] [PubMed]
- Caddey, B.; De Buck, J. Meta-analysis of bovine digital dermatitis microbiota reveals distinct microbial community structures associated with lesions. Front. Cell. Infect. Microbiol. 2021, 11, 685861. [Google Scholar] [CrossRef]
- Bruckbauer, H.R.; Preac-Mursic, V.; Fuchs, R.; Wilske, B. Cross-reactive proteins of Borrelia burgdorferi. Eur. J. Clin. Microbiol. Infect. Dis. 1992, 11, 224–232. [Google Scholar] [CrossRef]
- Magnarelli, L.A.; Anderson, J.F.; Johnson, R.C. Cross-reactivity in serological tests for Lyme disease and other spirochetal infections. J. Infect. Dis. 1987, 156, 183–188. [Google Scholar] [CrossRef]
- Clegg, S.R.; Carter, S.D.; Stewart, J.P.; Amin, D.M.; Blowey, R.W.; Evans, N.J. Bovine ischaemic teat necrosis: A further potential role for digital dermatitis treponemes. Vet. Rec. 2016, 178, 71–73. [Google Scholar] [CrossRef]
- Adjadj, N.R.; Cargnel, M.; Ribbens, S.; Quinet, C.; Malandrin, L.; Mignon, B.; Mori, M. Prevalence of Anaplasma phagocytophilum, Borrelia burgdorferi sensu lato, Rickettsia spp. and Babesia spp. in cattle serum and questing ticks from Belgium. Ticks Tick Borne Dis. 2023, 14, 102146. [Google Scholar] [CrossRef]
- Holmøy, I.H.; Ahlén, L.; Frössling, J.; Sølverød, L.; Holzhauer, M.; Nødtvedt, A.; Fjeldaas, T. Evaluation of test characteristics of 2 ELISA tests applied to bulk tank milk and claw-trimming records for herd-level diagnosis of bovine digital dermatitis using latent class analysis. J. Dairy Sci. 2021, 104, 10111–10120. [Google Scholar] [CrossRef]
- Biemans, F.; Bijma, P.; Boots, N.M.; de Jong, M.C.M. Digital Dermatitis in dairy cattle: The contribution of different disease classes to transmission. Epidemics 2018, 23, 76–84. [Google Scholar] [CrossRef]




| M-Stage | Prevalence | Frequency |
|---|---|---|
| M0 | 61.4% | 13,246 |
| M1 | 1.4% | 295 |
| M2 | 7.1% | 1534 |
| M3 | 0.3% | 75 |
| M4 | 20.9% | 4512 |
| M4.1 | 8.8% | 1891 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holzhauer, M.; Mars, J.; Holstege, M.; van der Heijden, H. An In-House ELISA for Treponema Antibodies in Bulk Milk as Part of a Monitoring Tool for Claw Health in Dairy Herds. Vet. Sci. 2023, 10, 571. https://doi.org/10.3390/vetsci10090571
Holzhauer M, Mars J, Holstege M, van der Heijden H. An In-House ELISA for Treponema Antibodies in Bulk Milk as Part of a Monitoring Tool for Claw Health in Dairy Herds. Veterinary Sciences. 2023; 10(9):571. https://doi.org/10.3390/vetsci10090571
Chicago/Turabian StyleHolzhauer, Menno, Jet Mars, Manon Holstege, and Harold van der Heijden. 2023. "An In-House ELISA for Treponema Antibodies in Bulk Milk as Part of a Monitoring Tool for Claw Health in Dairy Herds" Veterinary Sciences 10, no. 9: 571. https://doi.org/10.3390/vetsci10090571
APA StyleHolzhauer, M., Mars, J., Holstege, M., & van der Heijden, H. (2023). An In-House ELISA for Treponema Antibodies in Bulk Milk as Part of a Monitoring Tool for Claw Health in Dairy Herds. Veterinary Sciences, 10(9), 571. https://doi.org/10.3390/vetsci10090571







