Evaluation of the Microbiological Status of Cattle Carcasses in Mongolia: Considering the Hygienic Practices of Slaughter Establishments
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Slaughter Establishments
2.2. Carcass Sampling
2.3. Bacteriological Analyses
2.4. DNA Extraction and PCR Analysis
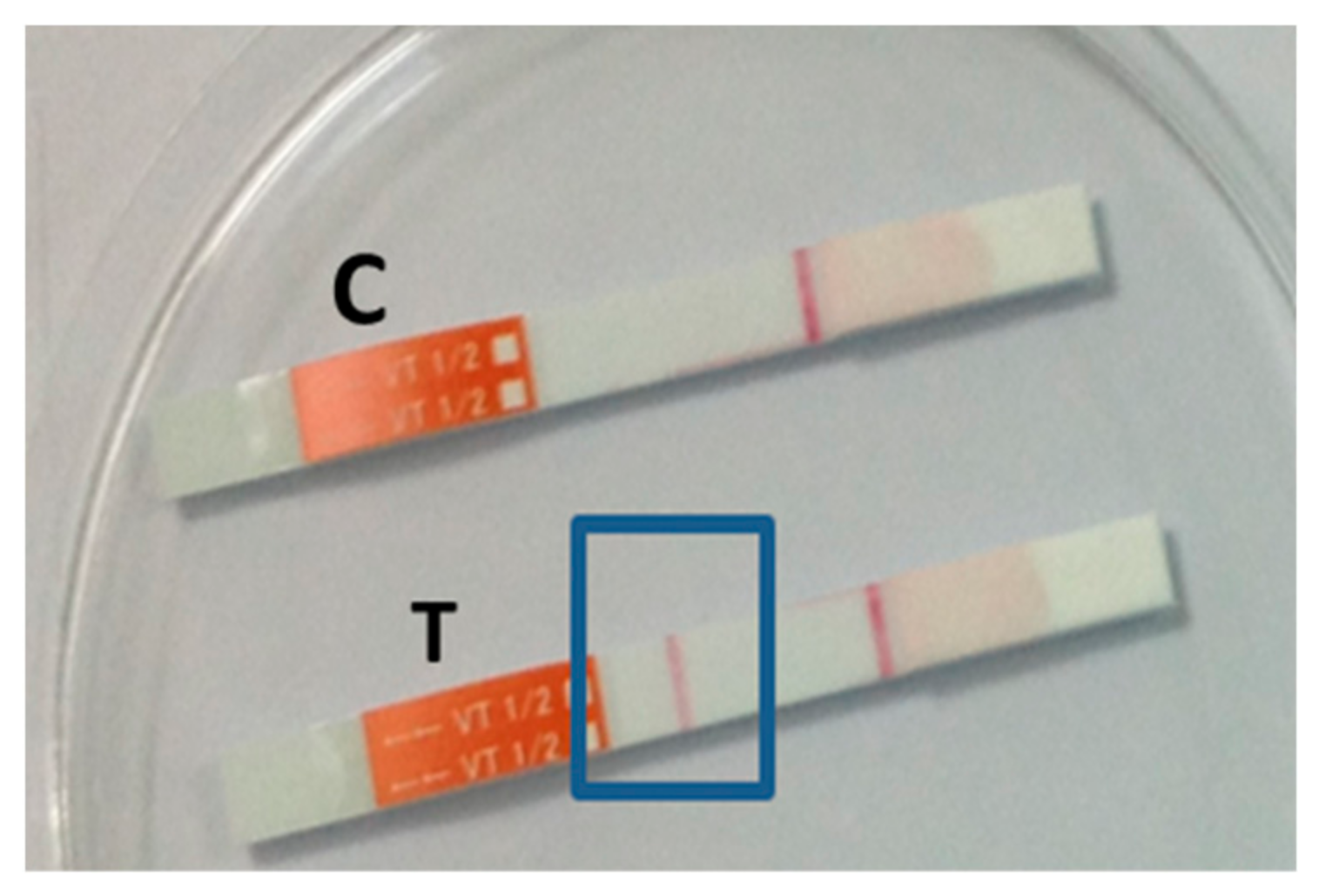
2.5. Immunochromatographic Test (ICT)
2.6. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anuujin, G. Survey on the Domestic and Export Meat Value Chain in Mongolia; United Nations Conference on Trade and Development: Geneva, Switzerland, 2021. [Google Scholar]
- Van Gelder, R.; Brown, N.; Shombodon, D. Mongolian Pastoralism–Nomadism and Marketing. In Proceedings of the 22nd International Grassland Congress, Sydney, Australia, 15–19 September 2013. [Google Scholar]
- United Nations for Industrial Development Organization. Strategic Directions on Industrial Policy in Mongolia; UNIDO: Vienna, Austria, 2011. [Google Scholar]
- McDonald, K.; Sun, D.W. Predictive food microbiology for the meat industry: A review. Int. J. Food Microbiol. 1999, 52, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Steele, T.M.; McMullin, D.Q. The examination of surface contamination on beef carcasses during slaughter and aging in a small-scale meat packaging operation equipped with an organic acid carcass washer. J. Anim. Vet. Adv. 2007, 6, 927–931. [Google Scholar]
- Bohaychuk, V.M.; Checkley, S.L.; Gensler, G.E.; Barrios, P.R. Microbiological baseline study of poultry slaughtered in provincially inspected abattoirs in Alberta, Canada. Can. Vet. J. 2009, 50, 173. [Google Scholar] [PubMed]
- Kim, H.J.; Kim, D.; Kim, H.J.; Song, S.O.; Song, Y.H.; Jang, A. Evaluation of the microbiological status of raw beef in Korea: Considering the suitability of aerobic plate count guidelines. Korean J. Food Sci. Anim. Resour. 2018, 38, 43. [Google Scholar] [PubMed]
- Sadiq, A.; Samad, M.; Basharat, N.; Ali, S.; Saad, Z.; Khan, A.N.; Ahmad, Y.; Khan, A.; Khan, J. Methicillin-Resistant Staphylococcus aureus (MRSA) in Slaughter Houses and Meat Shops in Capital Territory of Pakistan During 2018–2019. Front. Microbiol. 2020, 11, 577707. [Google Scholar] [CrossRef] [PubMed]
- Teshome, G.; Assefa, Z.; Keba, A. Assessment of microbial quality status of raw beef around Addis Ababa city, Ethiopia. Afr. J. Food Sci. 2020, 14, 209–214. [Google Scholar]
- Bosilevac, J.M.; Wang, R.; Luedtke, B.E.; Hinkley, S.; Wheeler, T.L.; Koohmaraie, M. Characterization of Enterohemorrhagic Escherichia coli on veal hides and carcasses. J. Food Prot. 2017, 80, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Terajima, J.; Iyoda, S.; Ohnishi, M.; Watanabe, H. Shiga toxin (verotoxin)-producing Escherichia coli in Japan. In Enterohemorrhagic Escherichia coli and Other Shiga Toxin-Producing E. coli; American Society for Microbiology: Washington, DC, USA, 2015; pp. 197–209. [Google Scholar]
- Armstrong, G.L.; Hollingsworth, J.; Morris Jr, J.G. Emerging foodborne pathogens: Escherichia coli O157: H7 as a model of entry of a new pathogen into the food supply of the developed world. Epidemiol. Rev. 1996, 18, 29–51. [Google Scholar] [CrossRef] [PubMed]
- Idland, L.; Granquist, E.G.; Aspholm, M.; Lindbäck, T. The prevalence of Campylobacter spp., Listeria monocytogenes and Shiga toxin-producing Escherichia coli in Norwegian dairy cattle farms: A comparison between free stall and tie stall housing systems. J. Appl. Microbiol. 2022, 132, 3959–3972. [Google Scholar] [CrossRef] [PubMed]
- Bettelheim, K.A. 2000. Role of non-O157 VTEC. J. Appl. Microbiol. 2000, 88, 38S–50S. [Google Scholar] [CrossRef] [PubMed]
- Blanco, M.; Blanco, J.E.; Blanco, J.; Mora, A.; Prado, C.; Alonso, M.P.; Mouriño, M.; Madrid, C.; Balsalobre, C.; Juárez, A. Distribution and characterization of fecal verotoxin-producing Escherichia coli (VTEC) isolated from healthy cattle. Vet. Microbiol. 1997, 54, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Laury, A.; Echeverry, A.; Brashears, M. Fate of Escherichia coli O157: H7 in Meat. Safety of Meat and Processed Meat; Springer: New York, NY, USA, 2009; pp. 31–53. [Google Scholar]
- Stromberg, Z.R.; Lewis, G.L.; Aly, S.S.; Lehenbauer, T.W.; Bosilevac, J.M.; Cernicchiaro, N.; Moxley, R.A. Prevalence and level of enterohemorrhagic Escherichia coli in culled dairy cows at harvest. J. Food Prot. 2016, 79, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Stromberg, Z.R.; Baumann, N.W.; Lewis, G.L.; Sevart, N.J.; Cernicchiaro, N.; Renter, D.G.; Moxley, R.A. Prevalence of enterohemorrhagic Escherichia coli O26, O45, O103, O111, O121, O145, and O157 on hides and preintervention carcass surfaces of feedlot cattle at harvest. Foodborne Pathog. Dis. 2015, 12, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Gill, C.O. Microbiological contamination of meat during slaughter and butchering of cattle, sheep, and pigs. Micobiol. Meat Poult. 1998, 31, 118–157. [Google Scholar]
- National Statistics Office of Mongolia. Statistical Bulletin on Food Safety; National Statistics Office of Mongolia: Ulaanbaatar, Mongolia, 2021.

| A | B | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range, CFU/cm2 | Plant A, n = 33 | Plant B, n = 62 | Variables | Plant A, n = 33 | Plant B, n = 62 | Independent t-Test | ||||||
| Rump | Brisket | Flank | Rump | Brisket | Flank | Mean | SD | Mean | SD | p Value | ||
| ≤103 | 0.0 | 9.1 | 0.0 | 0.0 | 3.3 | 0.0 | Rump | 3.92 | 0.50 | 4.79 | 0.64 | <0.001 |
| 103–104 | 54.5 | 0.0 | 0.0 | 10.0 | 0.0 | 0.0 | Brisket | 4.66 | 1.14 | 5.26 | 0.90 | ns |
| 104–105 | 45.5 | 9.1 | 0.0 | 43.3 | 3.3 | 3.3 | Flank | 4.48 | 0.33 | 4.95 | 0.74 | <0.05 |
| 105–106 | 0.0 | 45.5 | 90.9 | 46.7 | 23.3 | 53.3 | ANOVA | ns | ns | |||
| 106–107 | 0.0 | 36.4 | 9.1 | 0.0 | 53.3 | 30.0 | Two-way ANOVA | |||||
| 107–108 | 0.0 | 0.0 | 0.0 | 0.0 | 13.3 | 13.3 | Plant types | <0.001 | ||||
| Above detection | 0.0 | 0.0 | 0.0 | 0.0 | 3.3 | 0.0 | Sites | <0.05 | ||||
| 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | Plant type x Sites | ns | |||||
| A | B | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range, CFU/cm2 | Plant A, n = 33 | Plant B, n = 62 | Variables | Plant A, n = 33 | Plant B, n = 62 | Independent t-Test | ||||||
| Rump | Brisket | Flank | Rump | Brisket | Flank | Mean | SD | Mean | SD | p Value | ||
| Undetected | 9.1 | 27.3 | 18.2 | 3.3 | 13.3 | 0.0 | Rump | 1.35 | 1.06 | 1.91 | 0.76 | ns |
| ≤101 | 45.5 | 45.5 | 36.4 | 13.3 | 3.3 | 6.7 | Brisket | 1.11 | 0.67 | 2.31 | 0.92 | <0.001 |
| 101–102 | 27.3 | 18.2 | 27.3 | 46.7 | 33.3 | 60.0 | Flank | 1.42 | 0.95 | 1.80 | 0.59 | ns |
| 102–103 | 9.1 | 9.1 | 9.1 | 30.0 | 33.3 | 30.0 | ANOVA | ns | <0.05 | |||
| 103–104 | 9.1 | 0.0 | 9.1 | 6.7 | 6.7 | 3.3 | Two-way ANOVA | |||||
| 104–105 | 0.0 | 0.0 | 0.0 | 0.0 | 6.7 | 0.0 | Plant types | <0.001 | ||||
| Above detection | 0.0 | 0.0 | 0.0 | 0.0 | 3.3 | 0.0 | Sites | ns | ||||
| 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | Plant type x Sites | ns | |||||
| A | B | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range, CFU/cm2 | Plant A, n = 33 | Plant B, n = 62 | Variables | Plant A, n = 33 | Plant B, n = 62 | Independent t-Test | ||||||
| Rump | Brisket | Flank | Rump | Brisket | Flank | Mean | SD | Mean | SD | p Value | ||
| ≤101 | 18.2 | 18.2 | 0.0 | 6.7 | 0.0 | 0.0 | Rump | 1.97 | 0.89 | 3.73 | 0.81 | <0.001 |
| 101–102 | 27.3 | 45.5 | 45.5 | 13.3 | 0.0 | 23.3 | Brisket | 1.73 | 0.64 | 3.62 | 0.75 | <0.001 |
| 102–103 | 45.5 | 36.4 | 54.5 | 40.0 | 16.7 | 43.3 | Flank | 2.04 | 0.45 | 3.54 | 0.78 | <0.001 |
| 103–104 | 9.1 | 0.0 | 0.0 | 26.7 | 30.0 | 23.3 | ANOVA | ns | ns | |||
| 104–105 | 0.0 | 0.0 | 0.0 | 3.3 | 26.7 | 3.3 | Two-way ANOVA | |||||
| 105–106 | 0.0 | 0.0 | 0.0 | 10.0 | 3.3 | 6.7 | Plant types | <0.001 | ||||
| Above detection | 0.0 | 0.0 | 0.0 | 0.0 | 23.3 | 0.0 | Sites | ns | ||||
| 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | Plant type x Sites | ns | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bayarsaikhan, M.; Purevdorj, N.-O.; Kim, B.H.; Jung, J.H.; Cho, G.J. Evaluation of the Microbiological Status of Cattle Carcasses in Mongolia: Considering the Hygienic Practices of Slaughter Establishments. Vet. Sci. 2023, 10, 563. https://doi.org/10.3390/vetsci10090563
Bayarsaikhan M, Purevdorj N-O, Kim BH, Jung JH, Cho GJ. Evaluation of the Microbiological Status of Cattle Carcasses in Mongolia: Considering the Hygienic Practices of Slaughter Establishments. Veterinary Sciences. 2023; 10(9):563. https://doi.org/10.3390/vetsci10090563
Chicago/Turabian StyleBayarsaikhan, Munkhgerel, Nyam-Osor Purevdorj, Byoung Hoon Kim, Jae Hun Jung, and Gil Jae Cho. 2023. "Evaluation of the Microbiological Status of Cattle Carcasses in Mongolia: Considering the Hygienic Practices of Slaughter Establishments" Veterinary Sciences 10, no. 9: 563. https://doi.org/10.3390/vetsci10090563
APA StyleBayarsaikhan, M., Purevdorj, N.-O., Kim, B. H., Jung, J. H., & Cho, G. J. (2023). Evaluation of the Microbiological Status of Cattle Carcasses in Mongolia: Considering the Hygienic Practices of Slaughter Establishments. Veterinary Sciences, 10(9), 563. https://doi.org/10.3390/vetsci10090563





