Marine Microalgae as a Nutritive Tool to Mitigate Ruminal Greenhouse Gas Production: In Vitro Fermentation Characteristics of Fresh and Ensiled Maize (Zea mays L.) Forage
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Treatments
2.2. Forage Production and Elaboration of Microsilages
2.3. Chemical Composition
2.4. In Vitro Incubation
2.4.1. Measurement of Biogas, Methane, Carbon Monoxide, and Hydrogen Sulfide Production
2.4.2. Ruminal Hydrogen Potential and Dry Matter Degradability
2.4.3. Calculations
2.5. Statistical Analysis
3. Results
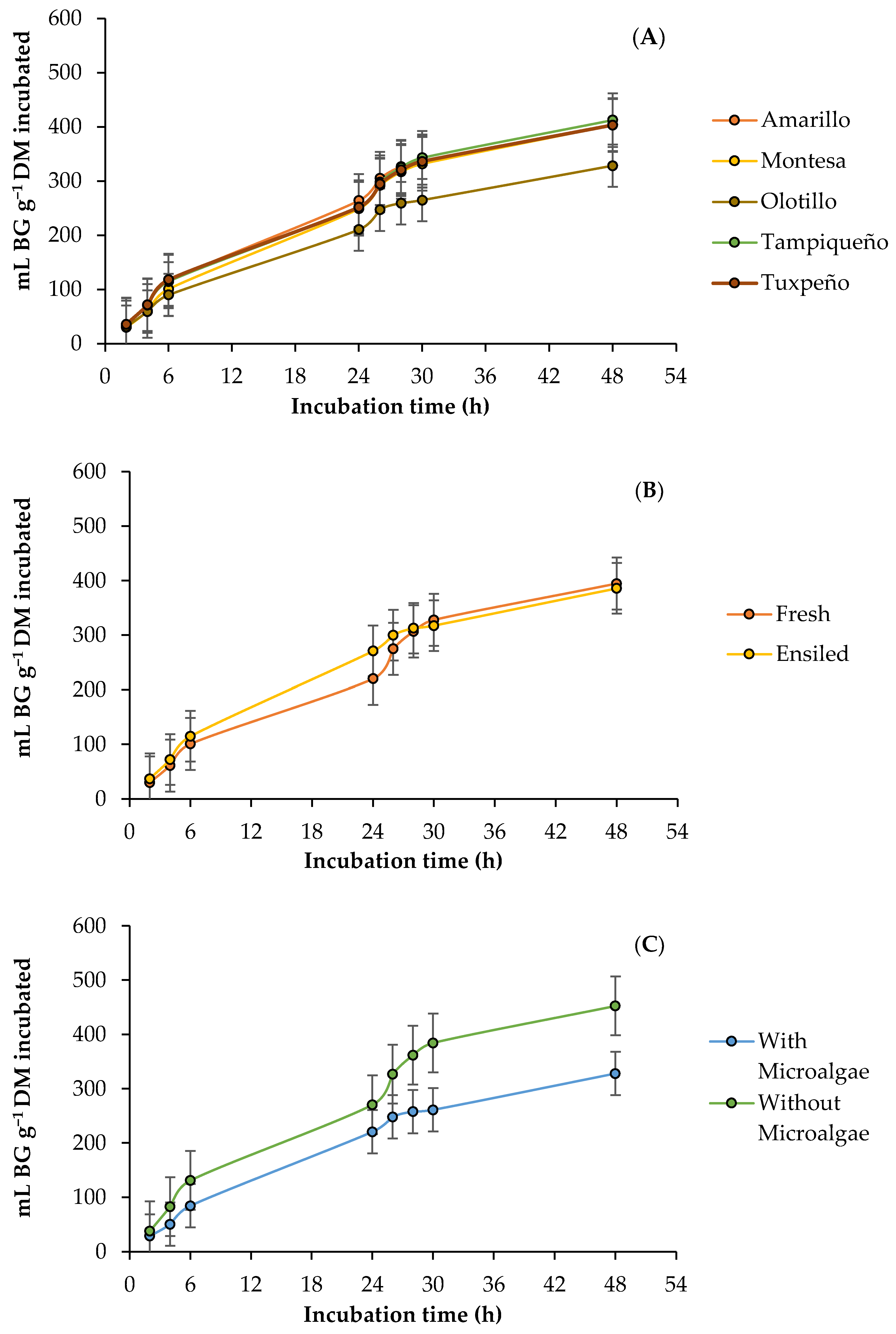
3.1. Ruminal Biogas Production
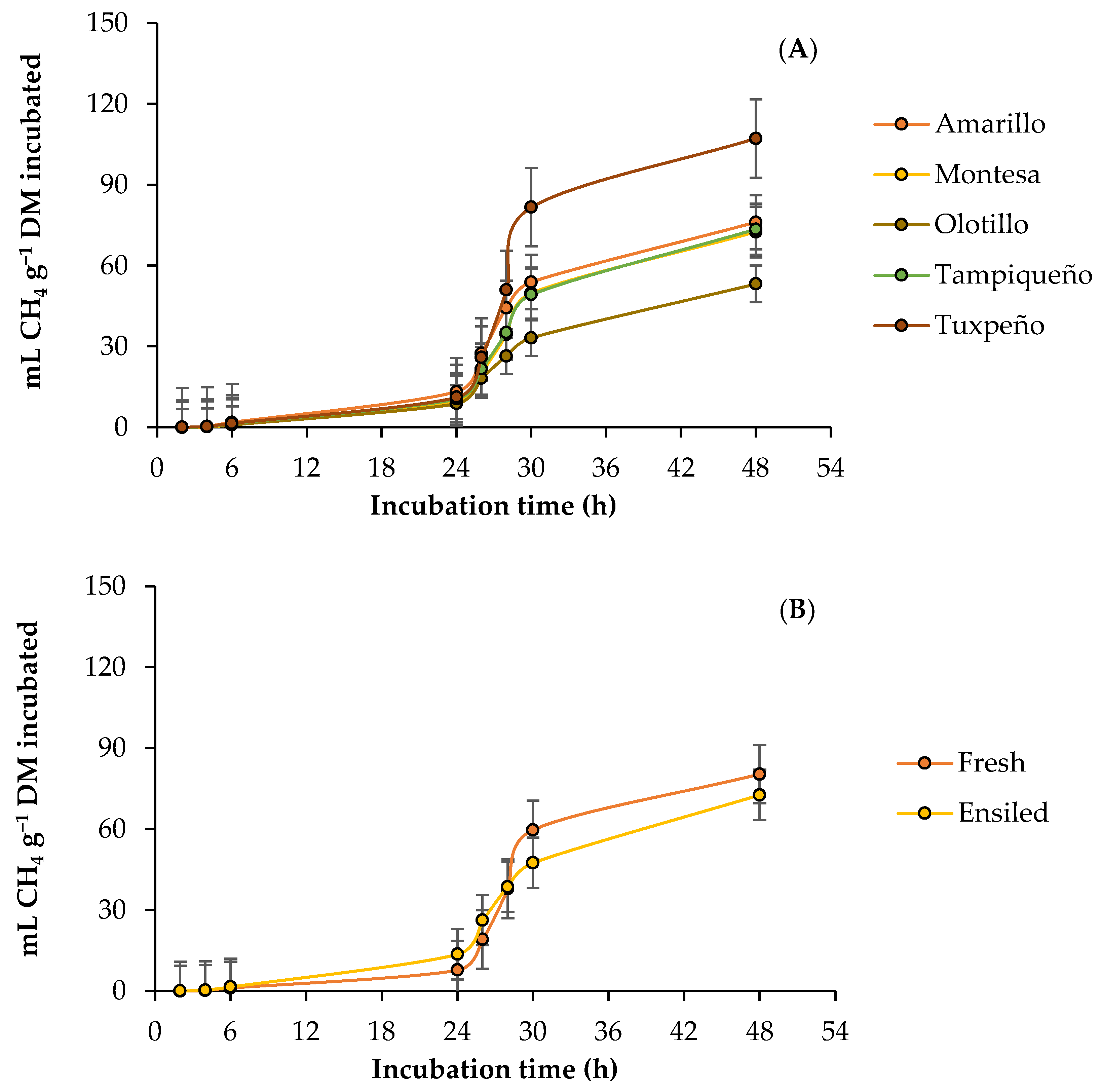
3.2. Ruminal Methane Production
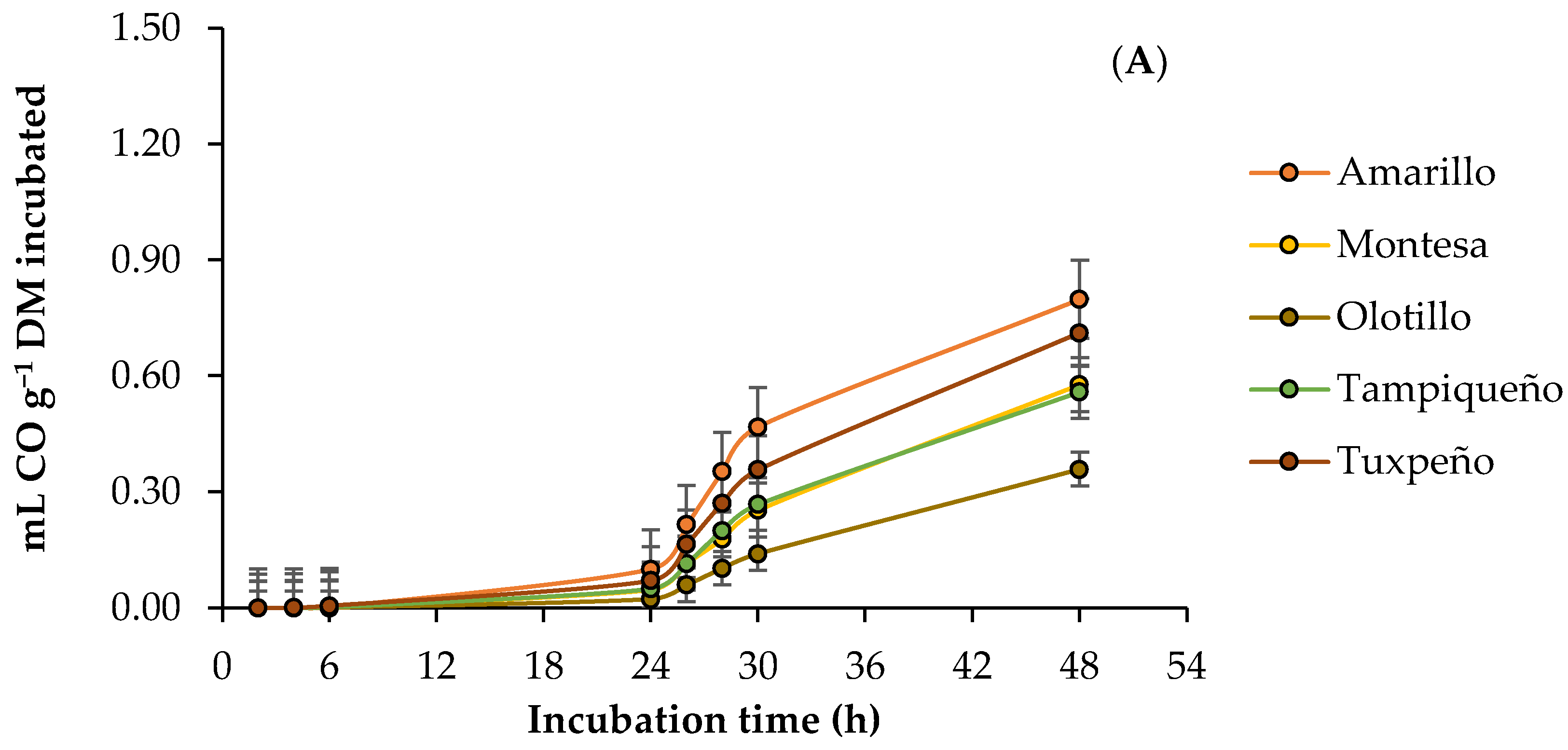
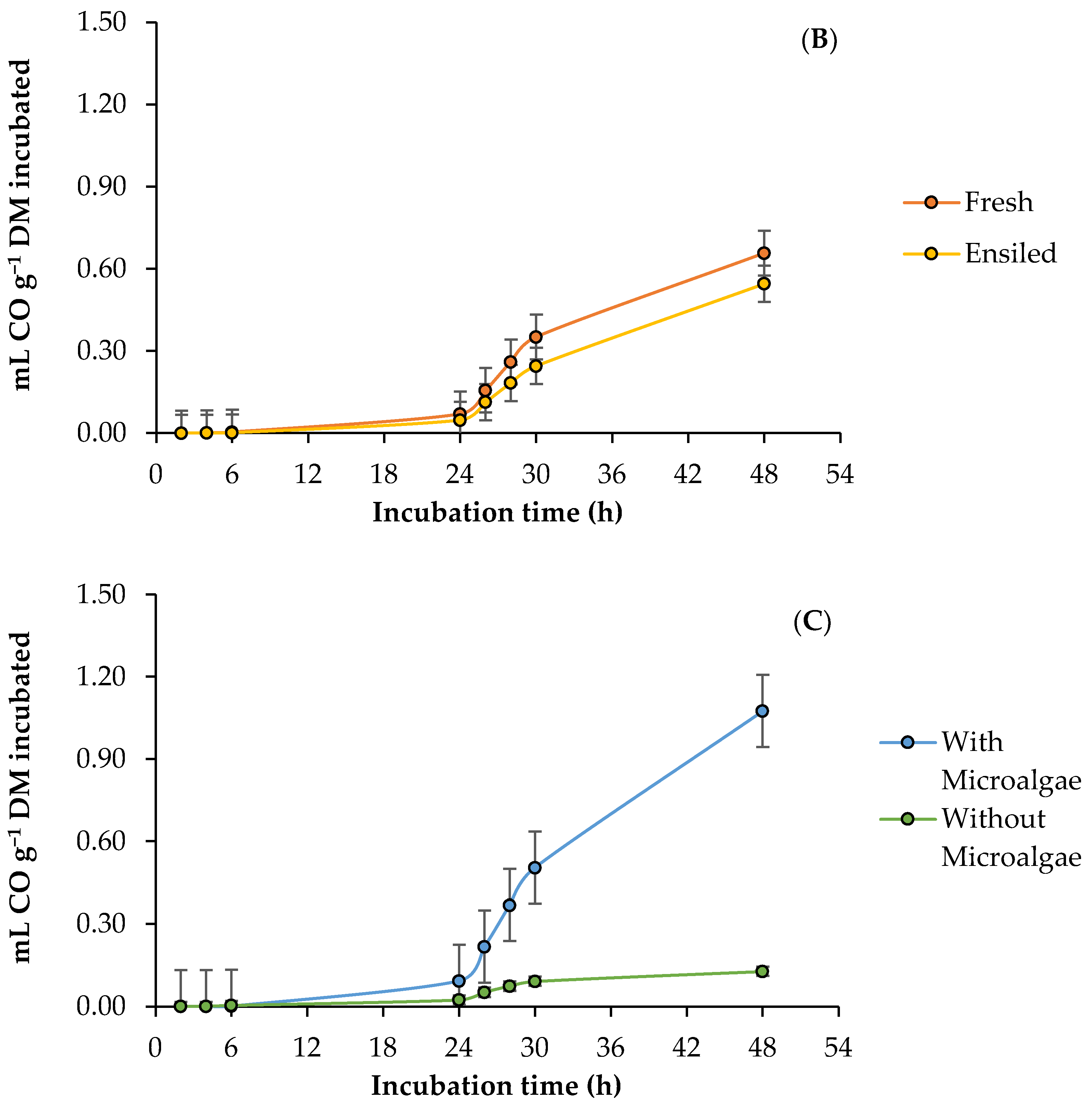
3.3. Ruminal Carbon Monoxide Production
3.4. Ruminal Hydrogen Sulfide Production
3.5. Ruminal Fermentation Characteristics and CH4 Conversion Efficiency
4. Discussion
4.1. Ruminal Biogas Production
4.2. Ruminal Methane Production
4.3. Ruminal Carbon Monoxide Production
4.4. Ruminal Hydrogen Sulfide Production
4.5. Ruminal Fermentation Characteristics and CH4 Conversion Efficiency
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Molotoks, A.; Smith, P.; Dawson, T.P. Impacts of land use, population, and climate change on global food security. Food Energy Secur. 2021, 10, e261. [Google Scholar] [CrossRef]
- Grossi, G.; Goglio, P.; Vitali, A.; Williams, A.G. Livestock and climate change: Impact of livestock on climate and mitigation strategies. Anim. Front. 2019, 9, 69–76. [Google Scholar] [CrossRef]
- Godde, C.M.; Mason-D’Croz, D.; Mayberry, D.E.; Thornton, P.K.; Herrero, M. Impacts of climate change on the livestock food supply chain; a review of the evidence. Glob. Food Sec. 2021, 28, 100488. [Google Scholar] [CrossRef] [PubMed]
- Maggiolino, A.; Dahl, G.E.; Bartolomeo, N.; Bernabucci, U.; Vitali, A.; Serio, G.; Cassandro, M.; Centoducati, G.; Santus, E.; De Palo, P. Estimation of maximum thermo-hygrometric index thresholds affecting milk production in Italian Brown Swiss cattle. J. Dairy Sci. 2020, 103, 8541–8553. [Google Scholar] [CrossRef]
- Michalk, D.L.; Kemp, D.R.; Badgery, W.B.; Wu, J.; Zhang, Y.; Thomassin, P.J. Sustainability and future food security-A global perspective for livestock production. Land Degrad. Dev. 2019, 30, 561–573. [Google Scholar] [CrossRef]
- Caro, D.; Davis, S.J.; Bastianoni, S.; Caldeira, K. Global and regional trends in greenhouse gas emissions from livestock. Clim. Change 2014, 126, 203–216. [Google Scholar] [CrossRef]
- Maggiolino, A.; Lorenzo, J.M.; Quiñones, J.; Latorre, M.A.; Blando, F.; Centoducati, G.; Dahl, G.E.; De Palo, P. Effects of dietary supplementation with Pinus taeda hydrolyzed lignin on in vivo performances, in vitro nutrient apparent digestibility, and gas emission in beef steers. Anim. Feed Sci. Technol. 2019, 255, 114217. [Google Scholar] [CrossRef]
- Immig, I. The rumen and hindgut as source of ruminant methanogenesis. Environ. Monit. Assess. 1996, 42, 57–72. [Google Scholar] [CrossRef]
- Vasta, V.; Daghio, M.; Cappucci, A.; Buccioni, A.; Serra, A.; Viti, C.; Mele, M. Invited review: Plant polyphenols and rumen microbiota responsible for fatty acid biohydrogenation, fiber digestion, and methane emission: Experimental evidence and methodological approaches. J. Dairy Sci. 2019, 102, 3781–3804. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; Ungerfeld, E.M.; Eckard, R.J.; Wang, M. Review: Fifty years of research on rumen methanogenesis: Lessons learned and future challenges for mitigation. Animals 2020, 14, s2–s16. [Google Scholar] [CrossRef]
- Sun, X.; Cheng, L.; Jonker, A.; Munidasa, S.; Pacheco, D. A review: Plant carbohydrate types-The potential impact on ruminant methane emissions. Front. Vet. Sci. 2022, 9, 880115. [Google Scholar] [CrossRef]
- Ungerfeld, E.M. Metabolic hydrogen flows in rumen fermentation: Principles and possibilities of interventions. Front. Microbiol. 2020, 11, 589. [Google Scholar] [CrossRef]
- Santillán, M.K.G.; Khusro, A.; Salem, A.Z.M.; Pliego, A.B.; Elghandour, M.M.M.Y. Potential and valorization of Salix babylonica waste leaf extract to mitigate equine fecal production of methane, carbon monoxide, and hydrogen sulphide. Waste Biomass Valorization 2022, 14, 1079–1095. [Google Scholar] [CrossRef]
- Sobieraj, K.; Stegenta-Dąbrowska, S.; Luo, G.; Koziel, J.A.; Białowiec, A. Carbon monoxide fate in the environment as an inspiration for biorefinery industry: A review. Front. Environ. Sci. 2022, 10, 822463. [Google Scholar] [CrossRef]
- Pereira, A.M.; de Lurdes Nunes Enes Dapkevicius, M.; Borba, A.E. Alternative pathways for hydrogen sink originated from the ruminal fermentation of carbohydrates: Which microorganisms are involved in lowering methane emission? Anim. Microbiome 2022, 4, 5. [Google Scholar] [CrossRef]
- Saksrithai, K.; King, A.J. Controlling hydrogen sulphide emissions during poultry productions. J. Anim. Res. Nutr. 2018, 3, 2. [Google Scholar] [CrossRef]
- McCauley, J.I.; Labeeuw, L.; Jaramillo-Madrid, A.C.; Nguyen, L.N.; Nghiem, L.D.; Chaves, A.V.; Ralph, P.J. Management of enteric methanogenesis in ruminants by algal-derived feed additives. Curr. Pollut. Rep. 2020, 6, 188–205. [Google Scholar] [CrossRef]
- Morais, T.; Inácio, A.; Coutinho, T.; Ministro, M.; Cotas, J.; Pereira, L.; Bahcevandziev, K. Seaweed potential in the animal feed: A Review. J. Mar. Sci. Eng. 2020, 8, 559. [Google Scholar] [CrossRef]
- Souza, C.M.; Bastos, T.S.; dos Santos, M.C. Microalgae use in animal nutrition. Res. Soc. Dev. 2021, 6, e53101622986. [Google Scholar] [CrossRef]
- de Oliveira, A.P.F.; Bragotto, A.P.A. Microalgae-based products: Food and public health. Futur. Foods. 2022, 6, 100157. [Google Scholar] [CrossRef]
- Souza, C.M.M.; de Lima, D.C.; Bastos, T.S.; de Oliveira, S.G.; Beirão, B.C.B.; Félix, A.P. Microalgae Schizochytrium sp. as a source of docosahexaenoic acid (DHA): Effects on diet digestibility, oxidation and palatability and on immunity and inflammatory indices in dogs. Anim. Sci. J. 2019, 90, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Anele, U.Y.; Yang, W.Z.; McGinn, P.J.; Tibbetts, S.M.; McAllister, T.A. Ruminal in vitro gas production, dry matter digestibility, methane abatement potential, and fatty acid biohydrogenation of six species of microalgae. Can. J. Anim. Sci. 2016, 96, 35–363. [Google Scholar] [CrossRef]
- Elghandour, M.M.Y.; Vallejo, L.H.; Salem, A.Z.M.; Salem, M.Z.M.; Camacho, L.M.; Buendía, R.; Odongo, N.E. Effects of Schizochytrium microalgae and sunflower oil as sources of unsaturated fatty acids for the sustainable mitigation of ruminal biogases methane and carbon dioxide. J. Clean. Prod. 2017, 168, 1389–1397. [Google Scholar] [CrossRef]
- Mahata, C.; Das, P.; Khan, S.; Thaher, M.I.A.; Abdul, Q.M.; Annamalai, S.N.; Al Jabri, H. The potential of marine microalgae for the production of food, feed, and fuel (3F). Fermentation 2022, 8, 316. [Google Scholar] [CrossRef]
- Masoero, F.; Gallo, A.; Zanfi, C.; Giuberti, G.; Spanghero, M. Effect of nitrogen fertilization on chemical composition and rumen fermentation of different parts of plants of three corn hybrids. Anim. Feed Sci. Technol. 2011, 164, 207–216. [Google Scholar] [CrossRef]
- Vargas, V.; Hernández, M.E.; Gutiérrez, L.J.; Plácido, J.M.; Jiménez, C.A. Clasificación climática del Estado de Tamaulipas, México. Ciencia UAT 2007, 2, 15–19. [Google Scholar]
- Thiex, N.; Novotny, L.; Crawford, A. Determination of ash in animal feed: AOAC official method 942.05 revisited. J. AOAC Int. 2012, 95, 1392–1397. [Google Scholar] [CrossRef] [PubMed]
- De Bellis, P.; Maggiolino, A.; Albano, C.; De Palo, P.; Blando, F. Ensiling grape pomace with and without addition of a Lactiplantibacillus plantarum Strain: Effect on polyphenols and microbiological characteristics, in vitro nutrient apparent digestibility, and gas emission. Front. Vet. Sci. 2022, 9, 808293. [Google Scholar] [CrossRef]
- Van Soest, P.V.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Faichney, G.; White, G. Methods for the Analysis of Feeds Eaten by Ruminants; Division of Animal Production, Ian Clunies Ross Animal Research Laboratory and Commonwealth Scientific and Industrial Research Organization: Melbourne, VIC, Australia, 1983. [Google Scholar]
- Padmore, J.M. Animal Feed. In Official Methods of Analysis of the Association of Official Analytical Chemists; Helrick, K., Ed.; AOAC: Arlington, VA, USA, 1990; pp. 69–90. [Google Scholar]
- Mertens, D.R. Creating a system for meeting the fiber requirements of dairy cows. J. Dairy Sci. 1997, 80, 1463–1481. [Google Scholar] [CrossRef] [PubMed]
- Sniffen, C.J.; O’connor, J.D.; Van Soest, P.J.; Fox, D.G.; Russell, J.B. A net carbohydrate and protein system for evaluating cattle diets: II. Carbohydrate and protein availability. J. Anim. Sci. 1992, 70, 3562–3577. [Google Scholar] [CrossRef] [PubMed]
- Goering, M.K.; Van Soest, P.J. Forage Fibre Analysis (Apparatus, Reagents, Procedures and Some Applications); Agricultural Research Service USDA: Washington, DC, USA, 1970; pp. 1–24. [CrossRef]
- Theodorou, M.K.; Williams, B.A.; Dhanoa, M.S.; McAllan, A.B.; France, J. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim. Feed Sci. Technol. 1994, 48, 185–197. [Google Scholar] [CrossRef]
- Acosta, J.A.D.; Elghandour, M.M.; Mariezcurrena-Berasain, M.D.; Adegbeye, M.J.; Fajemisin, A.N.; Pliego, A.B.; Salem, A.Z. Effect of mid-term dietary administration of the Caesalpinia coriaria extract on the sustainable mitigation of equine fecal methane, carbon monoxide and hydrogen sulfide production. J. Equine Vet. Sci. 2022, 115, 104021. [Google Scholar] [CrossRef] [PubMed]
- Tagliapietra, F.; Cattani, M.; Bailoni, L.; Schiavon, S. In vitro rumen fermentation: Effect of headspace pressure on the gas production kinetics of corn meal and meadow hay. Anim. Feed Sci. Technol. 2010, 158, 197–201. [Google Scholar] [CrossRef]
- Alvarado-Ramírez, E.R.; Maggiolino, A.; Elghandour, M.M.M.Y.; Rivas-Jacobo, M.A.; Ballesteros-Rodea, G.; Palo, P.D.; Salem, A.Z.M. Impact of co-ensiling of maize with Moringa oleifera on the production of greenhouse gases and the characteristics of fermentation in ruminants. Animals 2023, 13, 764. [Google Scholar] [CrossRef]
- Elghandour, M.M.; Chagoyán, J.C.V.; Salem, A.Z.; Kholif, A.E.; Castañeda, J.S.M.; Camacho, L.M.; Cerrillo-Soto, M.A. Effects of Saccharomyces cerevisiae at direct addition or pre-incubation on in vitro gas production kinetics and degradability of four fibrous feeds. Ital. J. Anim. Sci. 2014, 13, 3075. [Google Scholar] [CrossRef]
- SAS Institute, Cary SAS. User’s Guide: Statistics, Version 9.0; SAS Institute: Cary, NC, USA, 2002. [Google Scholar]
- France, J.; Dijkstra, J.; Dhanoa, M.S.; Lopez, S.; Bannink, A. Estimating the extent of degradation of ruminant feeds from a description of their gas production profiles observed in vitro: Derivation of models and other mathematical considerations. Br. J. Nutr. 2000, 83, 143–150. [Google Scholar] [CrossRef]
- Menke, K.H.; Raab, L.; Salewski, A.; Steingass, H.; Fritz, D.; Schneider, W. The estimation of the digestibility and metabolizable energy content of ruminant feeding stuffs from the gas production when they are incubated with rumen liquor in vitro. J. Agric. Sci. 1979, 93, 217–222. [Google Scholar] [CrossRef]
- Getachew, G.; Makkar, H.P.S.; Becker, K. Tropical browses: Contents of phenolic compounds, in vitro gas production and stoichiometric relationship between short chain fatty acid and in vitro gas production. J. Agric. Sci. 2002, 139, 341–352. [Google Scholar] [CrossRef]
- Getachew, G.; Blümmel, M.; Makkar, H.P.S.; Becker, K. In vitro gas measuring techniques for assessment of nutritional quality of feeds: A review. Anim. Feed Sci. Technol. 1998, 72, 261–281. [Google Scholar] [CrossRef]
- Sucu, E. In Vitro studies on rumen fermentation and methanogenesis of different microalgae and their effects on acidosis in dairy cows. Fermentation 2023, 9, 229. [Google Scholar] [CrossRef]
- Kholif, A.E.; Gouda, G.A.; Morsy, T.A.; Matloup, O.H.; Sallam, S.M.; Patra, A.K. Associative effects between Chlorella vulgaris microalgae and Moringa oleifera leaf silage used at different levels decreased in vitro ruminal greenhouse gas production and altered ruminal fermentation. Environ. Sci. Pollut. Res. 2023, 30, 6001–6020. [Google Scholar] [CrossRef]
- Scholz, B.; Liebezeit, G. Screening for biological activities and toxicological effects of 63 phytoplankton species isolated from freshwater, marine and brackish water habitats. Harmful Algae 2012, 20, 58–70. [Google Scholar] [CrossRef]
- Burnett, V.F.; Jacobs, J.L.; Norng, S.; Ponnampalam, E.N. Feed intake, liveweight gain and carcass traits of lambs offered pelleted annual pasture hay supplemented with flaxseed (Linum usitatissimum) flakes or algae (Schizochytrium sp.). Anim. Prod. Sci. 2016, 57, 877–883. [Google Scholar] [CrossRef]
- Meehan, D.J.; Cabrita, A.R.; Silva, J.L.; Fonseca, A.J.; Maia, M.R. Effects of Chlorella vulgaris, Nannochloropsis oceanica and Tetraselmis sp. supplementation levels on in vitro rumen fermentation. Algal Res. 2021, 56, 102284. [Google Scholar] [CrossRef]
- Palmonari, A.; Federiconi, A.; Cavallini, D.; Sniffen, C.J.; Mammi, L.; Turroni, S.; D’Amico, F.; Holder, P.; Formigoni, A. Impact of molasses on ruminal volatile fatty acid production and microbiota composition in vitro. Animals 2023, 13, 728. [Google Scholar] [CrossRef] [PubMed]
- Horst, E.H.; López, S.; Neumann, M.; Giráldez, F.J.; Bumbieris, J.V.H. Effects of hybrid and grain maturity stage on the ruminal degradation and the nutritive value of maize forage for silage. Agriculture 2020, 10, 251. [Google Scholar] [CrossRef]
- Hall, M.B. Challenges with nonfiber carbohydrate methods. J. Anim. Sci. 2003, 81, 3226–3232. [Google Scholar] [CrossRef]
- Zhao, X.H.; Gong, J.M.; Zhou, S.; Fu, C.B.; Liu, C.J.; Xu, L.J.; Pan, K.; Qu, M.R. Effects of degradable protein and non-fibre carbohydrates on microbial growth and fermentation in the rumen simulating fermenter (Rusitec). Ital. J. Anim. Sci. 2015, 14, 3771. [Google Scholar] [CrossRef][Green Version]
- Xu, N.; Wang, D.; Liu, J. Variance of zein protein and starch granule morphology between corn and steam flaked products determined starch ruminal degradability through altering starch hydrolyzing bacteria attachment. Animals 2019, 9, 626. [Google Scholar] [CrossRef]
- Hatew, B.; Bannink, A.; Van Laar, H.; De Jonge, L.H.; Dijkstra, J. Increasing harvest maturity of whole-plant corn silage reduces methane emission of lactating dairy cows. J. Dairy Sci. 2016, 99, 354–368. [Google Scholar] [CrossRef]
- Drewery, M.L.; Sawyer, J.E.; Pinchak, W.E.; Wickersham, T.A. Effect of increasing amounts of postextraction algal residue on straw utilization in steers. J. Anim. Sci. 2014, 92, 4642–4649. [Google Scholar] [CrossRef]
- Kholif, A.E.; Elghandour, M.M.Y.; Salem, A.Z.M.; Barbabosa, A.; Márquez, O.; Odongo, N.E. The effects of three total mixed rations with different concentrate to maize silage ratios and different levels of microalgae Chlorella vulgaris on in vitro total gas, methane and carbon dioxide production. J. Agric. Sci. 2017, 155, 494–507. [Google Scholar] [CrossRef]
- Mavrommatis, A.; Skliros, D.; Simoni, M.; Righi, F.; Flemetakis, E.; Tsiplakou, E. Alterations in the rumen particle-associated microbiota of goats in response to dietarys supplementation levels of Schizochytrium spp. Sustainability 2021, 13, 607. [Google Scholar] [CrossRef]
- Min, B.R.; Parkerb, D.; Brauerb, D.; Waldrip, H.; Lockardb, C.; Halesc, K.; Akbay, A.; Augyte, S. The role of seaweed as a potential dietary supplementation for enteric methane mitigation in ruminants: Challenges and opportunities. Anim. Nutr. 2021, 7, 1371–1387. [Google Scholar] [CrossRef]
- Sheng, P.; Ribeiro, G.O.; Wang, Y.; McAllister, T.A. Humic substances reduce ruminal methane production and increase the efficiency of microbial protein synthesis in vitro. J. Sci. Food Agric. 2019, 99, 2152–2157. [Google Scholar] [CrossRef]
- Hassan, A.A.; Salem, A.Z.M.; Elghandour, M.M.Y.; Hafsa, S.A.; Reddy, P.R.K.; Atia, S.E.S.; Vidu, L. Humic substances isolated from clay soil may improve the ruminal fermentation, milk yield, and fatty acid profile: A novel approach in dairy cows. Anim. Feed Sci. Technol. 2020, 268, 114601. [Google Scholar] [CrossRef]
- Ranilla, M.J.; Jouany, J.P.; Morgavi, D.P. Methane production and substrate degradation by rumen microbial communities containing single protozoal species in vitro. Lett. Appl. Microbiol. 2007, 45, 675–680. [Google Scholar] [CrossRef]
- Ruiz, P.E.H.; Mellado, M.; Adegbeye, M.J.; Salem, A.Z.M.; Covarrubias, J.L.P.; Elghandour, M.M.M.Y.; Omotoso, O.B. Effects of long-term supplementation of Caesalpinia coriaria fruit extract on ruminal methane, carbon monoxide, and hydrogen sulfide production in sheep. Biomass Convers. Biorefin. 2022, 1–14. [Google Scholar] [CrossRef]
- Haarstad, K.; Bergersen, O.; Sørheim, R. Occurrence of carbon monoxide during organic waste degradation. J. Air Waste Manag. Assoc. 2006, 56, 575–580. [Google Scholar] [CrossRef]
- Mand, T.D.; Metcalf, W.W. Energy conservation and hydrogenase function in methanogenic archaea, in particular the genus Methanosarcina. Microbiol. Mol. Biol. 2019, 83, e00020-19. [Google Scholar] [CrossRef] [PubMed]
- Biester, A.; Marcano-Delgado, A.N.; Drennan, C.L. Structural insights into microbial one-carbon ketabolic enzymes ni–fe–s-dependent carbon monoxide dehydrogenases and Acetyl-CoA synthases. Biochemistry 2022, 61, 2797–2805. [Google Scholar] [CrossRef]
- Wu, H.; Meng, Q.; Yu, Z. Effect of pH buffering capacity and sources of dietary sulfur on rumen fermentation, sulfide production, methane production, sulfate reducing bacteria, and total Archaea in in vitro rumen cultures. Bioresour. Technol. 2015, 186, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, G. Decreasing ruminal methane production through enhancing the sulfate reduction pathway. Anim. Nutr. 2022, 9, 320–326. [Google Scholar] [CrossRef]
- Schlegel, P.; Wyss, U.; Arrigo, Y.; Hess, H.D. Changes in macro-and micromineral concentrations in herbage during the harvesting and conservation processes. Grass Forage Sci. 2018, 73, 918–925. [Google Scholar] [CrossRef]
- Palangi, V.; Taghizadeh, A.; Abachi, S.; Lackner, M. Strategies to mitigate enteric methane emissions in ruminants: A review. Sustainability 2022, 14, 13229. [Google Scholar] [CrossRef]
- Dar, S.A.; Kleerebezem, R.; Stams, A.J.; Kuenen, J.G.; Muyzer, G. Competition and coexistence of sulfate-reducing bacteria, acetogens and methanogens in a lab-scale anaerobic bioreactor as affected by changing substrate to sulfate ratio. Appl. Microbiol. Biotechnol. 2008, 78, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.; Yang, C. Ruminal methane production: Associated microorganisms and the potential of applying hydrogen-utilizing bacteria for mitigation. Sci. Total Environ. 2019, 654, 1270–1283. [Google Scholar] [CrossRef]
- Van Zijderveld, S.M.; Gerrits, W.J.J.; Apajalahti, J.A.; Newbold, J.R.; Dijkstra, J.; Leng, R.A.; Perdok, H.B. Nitrate and sulfate: Effective alternative hydrogen sinks for mitigation of ruminal methane production in sheep. J. Dairy Sci. 2010, 93, 5856–5866. [Google Scholar] [CrossRef]
- Kovács, L.; Rózsa, L.; Pálffy, M.; Hejel, P.; Baumgartner, W.; Szenci, O. Subacute ruminal acidosis in dairy cows-physiological background, risk factors and diagnostic methods. Vet. Stanica. 2020, 51, 5–17. [Google Scholar] [CrossRef]
- Miranda-Romero, L.A.; Tirado-González, D.N.; Tirado-Estrada, G.; Améndola-Massiotti, R.; Sandoval-González, L.; Ramírez-Valverde, R.; Salem, A.Z. Quantifying non-fibrous carbohydrates, acid detergent fiber and cellulose of forage through an in vitro gas production technique. J. Sci. Food Agric. 2020, 100, 3099–3110. [Google Scholar] [CrossRef] [PubMed]
- Ghasimi, D.S.; Aboudi, K.; de Kreuk, M.; Zandvoort, M.H.; van Lier, J.B. Impact of lignocellulosic-waste intermediates on hydrolysis and methanogenesis under thermophilic and mesophilic conditions. Chem. Eng. J. 2016, 295, 181–191. [Google Scholar] [CrossRef]
- Astudillo-Neira, R.; Muñoz-Nuñez, E.; Quiroz-Carreno, S.; Avila-Stagno, J.; Alarcon-Enos, J. Bioconversion in ryegrass-fescue hay by Pleurotus ostreatus to increase their nutritional value for ruminant. Agriculture 2022, 12, 534. [Google Scholar] [CrossRef]


| Num. | Genotypes | States | Marine Microalgae 1 |
|---|---|---|---|
| 1 | Amarillo | Fresh | Without |
| 2 | With | ||
| 3 | Ensiled | Without | |
| 4 | With | ||
| 5 | Montesa | Fresh | Without |
| 6 | With | ||
| 7 | Ensiled | Without | |
| 8 | With | ||
| 9 | Olotillo | Fresh | Without |
| 10 | With | ||
| 11 | Ensiled | Without | |
| 12 | With | ||
| 13 | Tampiqueño | Fresh | Without |
| 14 | With | ||
| 15 | Ensiled | Without | |
| 16 | With | ||
| 17 | Tuxpeño | Fresh | Without |
| 18 | With | ||
| 19 | Ensiled | Without | |
| 20 | With |
| Item 1 | Genotypes of Maize 2,3 | Marine Microalgae (D. salina) 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Amarillo | Montesa | Olotillo | Tampiqueño | Tuxpeño | |||||||
| FRE | ENS | FRE | ENS | FRE | ENS | FRE | ENS | FRE | ENS | ||
| OM (%) | 92.1 | 92.8 | 92.7 | 93.3 | 92.8 | 93.0 | 92.1 | 93.6 | 91.6 | 92.1 | 30.0–33.0 |
| CP (%) | 10.8 | 8.3 | 10.5 | 8.3 | 10.3 | 8.4 | 10.5 | 8.6 | 10.3 | 8.6 | 12.0–13.0 |
| EE (%) | 2.4 | 3.6 | 2.6 | 3.9 | 2.6 | 3.8 | 2.2 | 3.4 | 2.5 | 3.6 | 3.8 |
| NDF (%) | 59.7 | 47.6 | 52.6 | 50.4 | 66.2 | 59.8 | 61.7 | 59.5 | 58.9 | 52.9 | - |
| ADF (%) | 31.7 | 26.9 | 30.3 | 26.2 | 36.4 | 36.1 | 35.1 | 32.3 | 30.5 | 28.9 | - |
| ADL (%) | 3.8 | 4.1 | 3.7 | 3.9 | 4.4 | 4.9 | 4.3 | 4.9 | 3.7 | 4.2 | - |
| NFC (%) | 19.2 | 33.3 | 26.9 | 34.6 | 13.6 | 21.0 | 17.8 | 22.2 | 20.5 | 27.0 | - |
| TC (%) | 13.2 | 17.2 | 21.5 | 27.2 | 3.7 | 9.0 | 6.1 | 12.5 | 3.7 | 5.3 | 12.0–13.0 |
| FV (%) | 5.0–6.0 | ||||||||||
| TN (%) | 0.3–0.4 | ||||||||||
| NH3 (%) | 0.05–0.07 | ||||||||||
| Nitrates (ppm) | 50.0–60.0 | ||||||||||
| Nitrites (ppm) | 100.0–120.0 | ||||||||||
| Phosphorus (%) | 0.8–1.0 | ||||||||||
| Potassium (%) | 0.3–0.5 | ||||||||||
| Calcium (%) | 14.0 | ||||||||||
| Magnesium (%) | 8.0–9.0 | ||||||||||
| Iron (ppm) | 450.0–950.0 | ||||||||||
| Boron (ppm) | 200.0 | ||||||||||
| Silica (ppm) | 20.0 | ||||||||||
| Copper (ppm) | 10.0–15.0 | ||||||||||
| Manganese (ppm) | 15.0–20.0 | ||||||||||
| Zinc (ppm) | 10.0–15.0 | ||||||||||
| Vanadium (ppm) | 1.0–2.0 | ||||||||||
| Genotypes | States | Marine Microalgae | BG Production | |||||
|---|---|---|---|---|---|---|---|---|
| Parameters 1 | mL BG g−1 DM Incubated | |||||||
| b | c | Lag | 6 h | 24 h | 48 h | |||
| Amarillo | Fresh | Without | 564.37 | 0.0283 | 3.09 | 136.81 | 265.16 | 526.81 |
| With | 305.03 | 0.0268 | 1.67 | 83.12 | 203.23 | 296.41 | ||
| Ensiled | Without | 398.20 | 0.0302 | 2.18 | 129.52 | 298.08 | 396.64 | |
| With | 398.90 | 0.0309 | 2.18 | 113.81 | 290.68 | 396.05 | ||
| Montesa | Fresh | Without | 582.63 | 0.0285 | 3.19 | 132.11 | 255.39 | 540.31 |
| With | 303.23 | 0.0262 | 1.66 | 75.83 | 193.08 | 292.32 | ||
| Ensiled | Without | 394.83 | 0.0301 | 2.16 | 107.10 | 273.02 | 388.15 | |
| With | 396.57 | 0.0322 | 2.17 | 89.09 | 275.05 | 392.98 | ||
| Olotillo | Fresh | Without | 304.13 | 0.0213 | 1.66 | 95.04 | 183.04 | 282.48 |
| With | 288.90 | 0.0263 | 1.58 | 67.96 | 159.76 | 273.65 | ||
| Ensiled | Without | 396.07 | 0.0283 | 2.17 | 125.55 | 283.41 | 390.61 | |
| With | 380.80 | 0.0336 | 2.08 | 71.73 | 216.47 | 367.02 | ||
| Tampiqueño | Fresh | Without | 616.13 | 0.0316 | 3.37 | 115.00 | 248.92 | 574.06 |
| With | 322.67 | 0.0301 | 1.77 | 83.10 | 225.63 | 318.92 | ||
| Ensiled | Without | 449.90 | 0.0250 | 2.46 | 168.40 | 312.37 | 436.93 | |
| With | 327.40 | 0.0286 | 1.79 | 92.73 | 222.43 | 320.68 | ||
| Tuxpeño | Fresh | Without | 579.07 | 0.0285 | 3.17 | 141.82 | 265.05 | 538.93 |
| With | 312.60 | 0.0277 | 1.71 | 78.77 | 203.17 | 303.12 | ||
| Ensiled | Without | 463.70 | 0.0258 | 2.54 | 161.37 | 321.54 | 452.19 | |
| With | 326.63 | 0.0286 | 1.79 | 90.33 | 217.55 | 319.59 | ||
| Pooled SEM 2 | 42.523 | 0.00093 | 0.23264 | 6.139 | 16.267 | 39.330 | ||
| p-value | ||||||||
| Genotype | 0.0385 | 0.0166 | 0.0385 | <0.0001 | 0.0005 | 0.0245 | ||
| State | 0.2036 | <0.0001 | 0.2037 | <0.0001 | <0.0001 | 0.6269 | ||
| Microalgae | <0.0001 | 0.0024 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| Genotype × State | 0.0579 | <0.0001 | 0.0579 | 0.0030 | 0.2391 | 0.0462 | ||
| Genotype × Microalgae | 0.0190 | 0.0010 | 0.0190 | 0.0026 | 0.1727 | 0.0262 | ||
| State × Microalgae | <0.0001 | 0.0005 | <0.0001 | 0.9350 | 0.6471 | 0.0002 | ||
| Genotype × State × Microalgae | 0.1554 | 0.3778 | 0.1554 | <0.0001 | 0.0144 | 0.1361 | ||
| Genotypes | States | Marine Microalgae | CH4 Production | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameters 1 | mL CH4 g−1 DM Incubated | mL CH4 100 mL−1 BG | |||||||||
| b | c | Lag | 6 h | 24 h | 48 h | 6 h | 24 h | 48 h | |||
| Amarillo | Fresh | Without | 103.30 | 0.1504 | 17.89 | 3.10 | 13.46 | 105.10 | 2.27 | 5.07 | 19.92 |
| With | 37.33 | 0.0791 | 6.47 | 0.83 | 6.62 | 37.29 | 1.00 | 3.25 | 12.57 | ||
| Ensiled | Without | 85.01 | 0.0886 | 14.72 | 1.40 | 16.20 | 85.28 | 1.08 | 5.43 | 21.50 | |
| With | 76.65 | 0.0865 | 13.28 | 2.10 | 16.60 | 76.75 | 1.83 | 5.70 | 19.38 | ||
| Montesa | Fresh | Without | 109.29 | 0.1740 | 18.93 | 0.77 | 5.41 | 108.80 | 0.58 | 2.11 | 20.23 |
| With | 37.63 | 0.0738 | 6.52 | 0.67 | 7.48 | 37.56 | 0.88 | 3.87 | 12.78 | ||
| Ensiled | Without | 80.01 | 0.0884 | 13.86 | 1.14 | 13.87 | 80.18 | 1.07 | 5.08 | 20.67 | |
| With | 63.42 | 0.0787 | 10.98 | 1.23 | 12.68 | 63.42 | 1.38 | 4.60 | 16.10 | ||
| Olotillo | Fresh | Without | 50.16 | 0.0753 | 8.69 | 0.84 | 7.81 | 50.10 | 0.88 | 4.28 | 17.72 |
| With | 34.17 | 0.0771 | 5.92 | 0.86 | 5.86 | 34.10 | 1.27 | 3.67 | 12.47 | ||
| Ensiled | Without | 77.96 | 0.0867 | 13.50 | 1.28 | 12.82 | 78.20 | 1.02 | 4.50 | 19.95 | |
| With | 50.47 | 0.0930 | 8.74 | 0.88 | 8.89 | 50.59 | 1.22 | 4.10 | 13.77 | ||
| Tampiqueño | Fresh | Without | 106.85 | 0.1539 | 18.51 | 0.73 | 5.78 | 106.74 | 0.62 | 2.27 | 19.44 |
| With | 45.09 | 0.0776 | 7.81 | 0.90 | 8.47 | 45.06 | 1.08 | 3.75 | 14.15 | ||
| Ensiled | Without | 93.37 | 0.08540 | 16.17 | 2.47 | 17.86 | 93.63 | 1.47 | 5.72 | 21.43 | |
| With | 48.53 | 0.0781 | 8.41 | 1.55 | 10.21 | 48.52 | 1.67 | 4.60 | 15.13 | ||
| Tuxpeño | Fresh | Without | 138.46 | 0.2183 | 11.30 | 1.78 | 9.13 | 138.88 | 1.30 | 3.72 | 25.76 |
| With | 43.45 | 0.0699 | 7.53 | 0.95 | 8.24 | 43.28 | 1.20 | 4.05 | 14.28 | ||
| Ensiled | Without | 96.20 | 0.0832 | 16.66 | 2.10 | 17.70 | 96.54 | 1.30 | 5.50 | 21.32 | |
| With | 53.37 | 0.0813 | 9.24 | 1.37 | 9.72 | 53.43 | 1.52 | 4.47 | 16.70 | ||
| Pooled SEM 2 | 18.618 | 0.00996 | 3.225 | 0.141 | 0.926 | 19.093 | 0.102 | 0.308 | 1.948 | ||
| p-value | |||||||||||
| Genotype | 0.0044 | 0.0028 | 0.0044 | <0.0001 | <0.0001 | 0.1224 | <0.0001 | 0.0008 | <0.0001 | ||
| State | 0.3380 | <0.0001 | 0.3380 | <0.0001 | <0.0001 | 0.9040 | <0.0001 | <0.0001 | 0.9761 | ||
| Microalgae | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0026 | 0.2425 | <.0001 | ||
| Genotype × State | 0.0180 | 0.0001 | 0.0180 | <0.0001 | 0.1372 | 0.3775 | <0.0001 | 0.0016 | 0.0001 | ||
| Genotype × Microalgae | 0.0074 | <0.0001 | 0.0074 | 0.0005 | 0.0100 | 0.2282 | 0.0006 | 0.0173 | 0.0005 | ||
| State × Microalgae | 0.0024 | <0.0001 | 0.0024 | 0.0085 | 0.0006 | 0.0234 | 0.0002 | 0.0072 | 0.0014 | ||
| Genotype × State × Microalgae | 0.0406 | 0.0004 | 0.0406 | <0.0001 | <0.0001 | 0.4401 | <0.0001 | <0.0001 | 0.0002 | ||
| Genotypes | States | Microalgae | CO Production | |||||
|---|---|---|---|---|---|---|---|---|
| Parameters 1 | mL CO g−1 DM Incubated | |||||||
| b | c | Lag | 6 h | 24 h | 48 h | |||
| Amarillo | Fresh | Without | 0.6273 | 0.0001 | 0.0008 | 0.0029 | 0.0209 | 0.1411 |
| With | 4.7611 | 0.0070 | 0.0064 | 0.0007 | 0.1999 | 1.6132 | ||
| Ensiled | Without | 0.0763 | 0.0001 | 0.0001 | 0.0007 | 0.0066 | 0.0414 | |
| With | 1.9180 | 0.0064 | 0.0518 | 0.0024 | 0.1755 | 1.3986 | ||
| Montesa | Fresh | Without | 0.7742 | 0.0011 | 0.0076 | 0.0054 | 0.0354 | 0.2078 |
| With | 5.7051 | 0.0056 | 0.0010 | 0.0007 | 0.0529 | 0.7300 | ||
| Ensiled | Without | 0.0224 | 0.0004 | 0.0000 | 0.0006 | 0.0069 | 0.0454 | |
| With | 1.7999 | 0.0007 | 0.9307 | 0.0013 | 0.0954 | 1.3290 | ||
| Olotillo | Fresh | Without | 0.0535 | 0.0000 | 0.0001 | 0.0005 | 0.0074 | 0.0356 |
| With | 0.2461 | 0.0006 | 0.0048 | 0.0007 | 0.0406 | 0.7284 | ||
| Ensiled | Without | 0.0215 | 0.0001 | 0.0000 | 0.0005 | 0.0055 | 0.0404 | |
| With | 1.0734 | 0.0089 | 0.0014 | 0.0013 | 0.0388 | 0.6317 | ||
| Tampiqueño | Fresh | Without | 0.1236 | 0.0009 | 0.0002 | 0.0034 | 0.0210 | 0.1465 |
| With | 1.8035 | 0.0011 | 0.0024 | 0.0009 | 0.1275 | 1.3743 | ||
| Ensiled | Without | 0.0277 | 0.0002 | 0.0000 | 0.0010 | 0.0059 | 0.0408 | |
| With | 0.3877 | 0.0002 | 0.2600 | 0.0024 | 0.0448 | 0.6751 | ||
| Tuxpeño | Fresh | Without | 1.2500 | 0.0001 | 1.3104 | 0.0185 | 0.1245 | 0.5351 |
| With | 1.3275 | 0.0016 | 0.0053 | 0.0010 | 0.0656 | 1.0586 | ||
| Ensiled | Without | 0.0259 | 0.0001 | 0.0000 | 0.0008 | 0.0048 | 0.0371 | |
| With | 4.7608 | 0.0013 | 0.0064 | 0.0026 | 0.0906 | 1.2169 | ||
| Pooled SEM 2 | 1.04294 | 0.00246 | 0.35748 | 0.00112 | 0.02611 | 0.18557 | ||
| p-value | ||||||||
| Genotype | 0.0669 | 0.4891 | 0.6193 | <0.0001 | 0.0024 | 0.0210 | ||
| State | 0.1674 | 0.9815 | 0.9561 | 0.0001 | 0.0660 | 0.1870 | ||
| Microalgae | 0.0243 | 0.0088 | 0.9757 | 0.0002 | <0.0001 | <0.0001 | ||
| Genotype × State | 0.1361 | 0.3791 | 0.2914 | <0.0001 | 0.4407 | 0.2278 | ||
| Genotype × Microalgae | 0.0332 | 0.3630 | 0.2949 | <0.0001 | 0.0010 | 0.0711 | ||
| State × Microalgae | 0.0722 | 0.7781 | 0.1187 | <0.0001 | 0.2443 | 0.4680 | ||
| Genotype × State × Microalgae | 0.0169 | 0.4836 | 0.6176 | <0.0001 | 0.0608 | 0.0676 | ||
| Genotypes | States | Marine Microalgae | H2S Production | |||||
|---|---|---|---|---|---|---|---|---|
| Parameters 1 | mL H2S g−1 DM Incubated | |||||||
| b | c | Lag | 6 h | 24 h | 48 h | |||
| Amarillo | Fresh | Without | 0.0907 | 0.00019 | 0.0007 | 0.0040 | 0.0237 | 0.1678 |
| With | 0.0395 | 0.00014 | 0.0003 | 0.0027 | 0.0195 | 0.1082 | ||
| Ensiled | Without | 0.1230 | 0.00023 | 0.0009 | 0.0112 | 0.0530 | 0.1776 | |
| With | 0.0590 | 0.00017 | 0.0004 | 0.0071 | 0.0312 | 0.1348 | ||
| Montesa | Fresh | Without | 0.1138 | 0.00011 | 0.0008 | 0.0049 | 0.0331 | 0.2128 |
| With | 0.0347 | 0.00014 | 0.0003 | 0.0023 | 0.0170 | 0.0942 | ||
| Ensiled | Without | 0.0951 | 0.00021 | 0.0007 | 0.0079 | 0.0443 | 0.1522 | |
| With | 0.0530 | 0.00017 | 0.0004 | 0.0045 | 0.0268 | 0.1364 | ||
| Olotillo | Fresh | Without | 0.0618 | 0.00026 | 0.0005 | 0.0066 | 0.0261 | 0.0910 |
| With | 0.0614 | 0.00013 | 0.0005 | 0.0047 | 0.0249 | 0.1072 | ||
| Ensiled | Without | 0.1080 | 0.00023 | 0.0008 | 0.0091 | 0.0453 | 0.1669 | |
| With | 0.0423 | 0.00015 | 0.0003 | 0.0040 | 0.0217 | 0.1317 | ||
| Tampiqueño | Fresh | Without | 0.0741 | 0.00025 | 0.0006 | 0.0036 | 0.0253 | 0.2122 |
| With | 0.0530 | 0.00012 | 0.0004 | 0.0039 | 0.0256 | 0.1232 | ||
| Ensiled | Without | 0.1311 | 0.00019 | 0.0010 | 0.0145 | 0.0513 | 0.1638 | |
| With | 0.0620 | 0.00014 | 0.0005 | 0.0046 | 0.0269 | 0.1179 | ||
| Tuxpeño | Fresh | Without | 0.1480 | 0.00020 | 0.0011 | 0.0070 | 0.0379 | 0.2263 |
| With | 0.0470 | 0.00018 | 0.0004 | 0.0024 | 0.0212 | 0.1037 | ||
| Ensiled | Without | 0.1303 | 0.00011 | 0.0010 | 0.0155 | 0.0492 | 0.1871 | |
| With | 0.0591 | 0.00014 | 0.0004 | 0.0036 | 0.0272 | 0.1156 | ||
| Pooled SEM 2 | 0.01702 | 0.000020 | 0.00013 | 0.00100 | 0.00412 | 0.01778 | ||
| p-value | ||||||||
| Genotype | 0.2252 | 0.1262 | 0.2242 | 0.0412 | 0.6025 | 0.0847 | ||
| State | 0.0758 | 0.1452 | 0.0776 | <0.0001 | <0.0001 | 0.6414 | ||
| Microalgae | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| Genotype × State | 0.5024 | 0.0496 | 0.5099 | 0.0036 | 0.2079 | 0.0304 | ||
| Genotype × Microalgae | 0.2656 | 0.0520 | 0.2610 | 0.0021 | 0.6565 | 0.0215 | ||
| State × Microalgae | 0.4399 | 0.2361 | 0.4510 | <0.0001 | 0.0004 | 0.0477 | ||
| Genotype × State × Microalgae | 0.1506 | 0.0729 | 0.1542 | 0.0125 | 0.1943 | 0.0577 | ||
| Genotypes | States | Marine Microalgae | Ruminal Fermentation Characteristics 1 | CH4 Conversion Efficiency 2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| pH | DMD | SCFA | ME | CH4:SCFA | CH4:ME | CH4:OM | |||
| Amarillo | Fresh | Without | 7.09 | 39.42 | 3.73 | 5.59 | 33.63 | 3.61 | 4.74 |
| With | 7.04 | 39.88 | 3.54 | 5.50 | 72.20 | 7.54 | 9.75 | ||
| Ensiled | Without | 6.93 | 51.62 | 5.34 | 6.08 | 69.61 | 9.60 | 13.41 | |
| With | 6.88 | 69.59 | 5.05 | 5.93 | 111.39 | 15.10 | 20.68 | ||
| Montesa | Fresh | Without | 7.15 | 35.39 | 3.56 | 5.47 | 79.59 | 8.23 | 10.40 |
| With | 7.14 | 35.60 | 4.11 | 5.75 | 86.90 | 10.01 | 13.44 | ||
| Ensiled | Without | 6.97 | 47.12 | 4.67 | 5.74 | 40.15 | 5.25 | 6.98 | |
| With | 6.95 | 66.42 | 5.51 | 6.17 | 37.66 | 5.40 | 7.69 | ||
| Olotillo | Fresh | Without | 7.25 | 45.14 | 2.68 | 4.98 | 60.01 | 5.18 | 6.00 |
| With | 7.38 | 44.78 | 3.76 | 5.54 | 34.45 | 3.75 | 4.82 | ||
| Ensiled | Without | 7.09 | 44.30 | 4.21 | 5.51 | 148.36 | 18.26 | 23.31 | |
| With | 7.10 | 60.58 | 3.63 | 5.21 | 64.80 | 7.39 | 9.03 | ||
| Tampiqueño | Fresh | Without | 7.20 | 40.55 | 3.70 | 5.54 | 72.18 | 7.82 | 10.17 |
| With | 7.28 | 41.53 | 4.08 | 5.73 | 121.30 | 14.47 | 19.95 | ||
| Ensiled | Without | 7.08 | 39.23 | 3.62 | 5.23 | 35.28 | 3.92 | 4.71 | |
| With | 7.06 | 61.68 | 4.43 | 5.65 | 45.07 | 5.68 | 7.39 | ||
| Tuxpeño | Fresh | Without | 7.10 | 40.28 | 4.23 | 5.78 | 37.71 | 4.39 | 5.91 |
| With | 7.09 | 40.41 | 5.08 | 6.21 | 91.74 | 11.84 | 17.00 | ||
| Ensiled | Without | 7.10 | 47.88 | 3.69 | 5.41 | 101.72 | 11.10 | 14.07 | |
| With | 7.02 | 67.37 | 4.34 | 5.74 | 40.16 | 4.84 | 6.51 | ||
| Pooled SEM 3 | 0.130 | 2.806 | 0.361 | 0.185 | 4.041 | 0.525 | 1.029 | ||
| p-value | |||||||||
| Genotype | 0.0015 | 0.1390 | 0.0005 | 0.0005 | 0.0009 | <0.0001 | <0.0001 | ||
| State | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| Microalgae | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.2525 | <0.0001 | <0.0001 | ||
| Genotype × State | 0.0016 | 0.0016 | 0.2391 | 0.2391 | 0.0016 | 0.0266 | 0.1372 | ||
| Genotype × Microalgae | 0.0345 | 0.9176 | 0.1728 | 0.1728 | 0.0175 | 0.0094 | 0.0100 | ||
| State × Microalgae | <0.0001 | <0.0001 | 0.6472 | 0.6472 | 0.0074 | 0.0008 | 0.0006 | ||
| Genotype × State × Microalgae | 0.7751 | 0.9784 | 0.0144 | 0.0144 | <0.0001 | <0.0001 | <0.0001 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elghandour, M.M.M.Y.; Maggiolino, A.; Alvarado-Ramírez, E.R.; Hernández-Meléndez, J.; Rivas-Cacerese, R.R.; Hernández-Ruiz, P.E.; Khusro, A.; De Palo, P.; Salem, A.Z.M. Marine Microalgae as a Nutritive Tool to Mitigate Ruminal Greenhouse Gas Production: In Vitro Fermentation Characteristics of Fresh and Ensiled Maize (Zea mays L.) Forage. Vet. Sci. 2023, 10, 556. https://doi.org/10.3390/vetsci10090556
Elghandour MMMY, Maggiolino A, Alvarado-Ramírez ER, Hernández-Meléndez J, Rivas-Cacerese RR, Hernández-Ruiz PE, Khusro A, De Palo P, Salem AZM. Marine Microalgae as a Nutritive Tool to Mitigate Ruminal Greenhouse Gas Production: In Vitro Fermentation Characteristics of Fresh and Ensiled Maize (Zea mays L.) Forage. Veterinary Sciences. 2023; 10(9):556. https://doi.org/10.3390/vetsci10090556
Chicago/Turabian StyleElghandour, Mona Mohamed Mohamed Yasseen, Aristide Maggiolino, Edwin Rafael Alvarado-Ramírez, Javier Hernández-Meléndez, Raymundo Rene Rivas-Cacerese, Pedro Enrique Hernández-Ruiz, Ameer Khusro, Pasquale De Palo, and Abdelfattah Zeidan Mohamed Salem. 2023. "Marine Microalgae as a Nutritive Tool to Mitigate Ruminal Greenhouse Gas Production: In Vitro Fermentation Characteristics of Fresh and Ensiled Maize (Zea mays L.) Forage" Veterinary Sciences 10, no. 9: 556. https://doi.org/10.3390/vetsci10090556
APA StyleElghandour, M. M. M. Y., Maggiolino, A., Alvarado-Ramírez, E. R., Hernández-Meléndez, J., Rivas-Cacerese, R. R., Hernández-Ruiz, P. E., Khusro, A., De Palo, P., & Salem, A. Z. M. (2023). Marine Microalgae as a Nutritive Tool to Mitigate Ruminal Greenhouse Gas Production: In Vitro Fermentation Characteristics of Fresh and Ensiled Maize (Zea mays L.) Forage. Veterinary Sciences, 10(9), 556. https://doi.org/10.3390/vetsci10090556










