The Single Intra-Articular Injection of Platelet-Rich Plasma vs. Non-Steroidal Anti-Inflammatory Drugs as Treatment Options for Canine Cruciate Ligament Rupture and Patellar Luxation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Groups
2.2. Performed Examinations and Tests
3. Results
3.1. TNF-α Concentration
3.2. Degree of Lameness
3.3. Painfulness of Manipulations
3.4. Goniometry in Flexion
3.5. Goniometry in Extension
3.6. Muscle Strength
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spinella, G.; Arcamone, G.; Valentini, S. Cranial cruciate ligament rupture in dogs: Review on biomechanics, etiopathogenetic factors and rehabilitation. Vet. Sci. 2021, 8, 186. [Google Scholar] [CrossRef]
- Alvarez, L.X.; Repac, J.A.; Kirkby Shaw, K.; Compton, N. Systematic review of postoperative rehabilitation interventions after cranial cruciate ligament surgery in dogs. Vet. Surg. 2022, 51, 233–243. [Google Scholar] [CrossRef]
- Rudd Garces, G.; Arizmendi, A.; Barrientos, L.S.; Crespi, J.A.; Morales, H.; García, P.P.; Padula, G.; Giovambattista, G. Epidemiology of cranial cruciate ligament rupture and patellar luxation in dogs from the province of Buenos Aires, Argentina. Vet. Comp. Orthop. Traumatol. 2021, 34, 24. [Google Scholar] [CrossRef]
- Witsberger, T.H.; Villamil, J.A.; Schultz, L.G.; Hahn, A.W.; Cook, J.L. Prevalence of and risk factors for hip dysplasia and cranial cruciate ligament deficiency in dogs. J. Am. Vet. Med. Assoc. 2008, 232, 1818–1824. [Google Scholar] [CrossRef]
- Dymond, N.; Goldsmid, S.; Simpson, D. Tibial tuberosity advancement in 92 canine stifles: Initial results, clinical outcome and owner evaluation. Aust. Vet. J. 2010, 88, 381–385. [Google Scholar] [CrossRef]
- Hayashi, K.; Bhandal, J.; Kim, S.Y.; Rodriguez, C.O., Jr.; Entwistle, R.; Naydan, D.; Kapatkin, A.; Stover, S.M. Immunohistochemical and histomorphometric evaluation of vascular distribution in intact canine cranial cruciate ligament. Vet. Surg. 2011, 40, 192–197. [Google Scholar] [CrossRef]
- Chauvet, A.E.; Johnson, A.L.; Pijanowski, G.J.; Homco, L.; Smith, R. Evaluation of fibular head transposition, lateral fabellar suture, and conservative treatment of cranial cruciate ligament rupture in large dogs: A retrospective study. J. Am. Anim. Hosp. Assoc. 1996, 32, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Vasseur, P.B.; Berry, C.R. Progression of stifle osteoarthrosis following reconstruction of the cranial cruciate ligament in 21 dogs. J. Am. Anim. Hosp. Assoc. 1992, 28, 129–136. [Google Scholar]
- Nanda, A.; Hans, E.C. Tibial plateau leveling osteotomy for cranial cruciate ligament rupture in canines: Patient selection and reported outcomes. Vet. Med. Res. Rep. 2019, 10, 249–255. [Google Scholar] [CrossRef]
- Duerr, F.M.; Martin, K.W.; Rishniw, M.; Palmer, R.H.; Selmic, L.E. Treatment of canine cranial cruciate ligament disease. A survey of ACVS diplomates and primary care veterinarians. Vet. Comp. Orthop. Traumatol. 2014, 27, 478–483. [Google Scholar] [PubMed]
- Blackford-Winders, C.L.; Daubert, M.; Rendahl, A.K.; Conzemius, M.G. Comparison of semi-cylindrical recession trochleoplasty and trochlear block recession for the treatment of canine medial patellar luxation: A pilot study. Vet. Comp. Orthop. Traumatol. 2021, 34, 183–190. [Google Scholar] [CrossRef]
- Bula, E.; Perry, K.L. Tibial tuberosity transposition advancement for treatment of concomitant cranial cruciate ligament rupture and medial patellar luxation in four feline stifles. JFMS Open Rep. 2021, 7, 20551169211044695. [Google Scholar] [CrossRef]
- Hulse, D.A. Pathophysiology and management of medial patellar luxation in the dog. Vet. Med. Small Anim. Clin. 1981, 76, 43–51. [Google Scholar] [PubMed]
- Roy, R.G.; Wallace, L.J.; Johston, G.R.; Wickstrom, S.L. A retrospective evaluation of stifle osteoarthritis in dogs with bilateral medial patellar luxation and unilateral surgical repair. Vet. Surg. 1992, 21, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Roush, J.K. Canine patellar luxation. Vet. Clin. N. Am. Small Anim. Pract. 1993, 23, 855–868. [Google Scholar] [CrossRef]
- Di Dona, F.; Della Valle, G.; Balestriere, C.; Lamagna, B.; Meomartino, L.; Napoleone, G.; Lamagna, F.; Fatone, G. Lateral patellar luxation in nine small breed dogs. Open Vet. J. 2016, 6, 255–258. [Google Scholar] [CrossRef]
- Di Dona, F.; Della Valle, G.; Fatone, G. Patellar luxation in dogs. Vet. Med. Res. Rep. 2018, 9, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Bosio, F.; Bufalari, A.; Peirone, B.; Petazzoni, M.; Vezzoni, A. Prevalence, treatment and outcome of patellar luxation in dogs in Italy. A retrospective multicentric study (2009–2014). Vet. Comp. Orthop. Traumatol. 2017, 30, 364–370. [Google Scholar] [CrossRef]
- Hayes, A.G.; Boudrieau, R.J.; Hungerford, L.L. Frequency and distribution of medial and lateral patellar luxation in dogs: 124 cases (1982–1992). J. Am. Vet. Med. Assoc. 1994, 205, 716–720. [Google Scholar]
- Harasen, G. Patellar luxation: Pathogenesis and surgical correction. Can. Vet. J. 2006, 47, 1037–1039. [Google Scholar]
- Lopez de la Oliva Cases, P.; Grierson, J. Patellar luxation in dogs. Companion Anim. 2019, 24, 293–298. [Google Scholar] [CrossRef]
- Doom, M.; de Bruin, T.; de Rooster, H.; van Bree, H.; Cox, E. Immunopathological mechanisms in dogs with rupture of the cranial cruciate ligament. Vet. Immunol. Immunopathol. 2008, 125, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Markova, D.; Anderson, D.G.; Zheng, Z.; Shapiro, I.M.; Risbud, M.V. TNF-α and IL-1β promote a disintegrin-like and metalloprotease with thrombospondin type I motif-5-mediated aggrecan degradation through syndecan-4 in intervertebral disc. J. Biol. Chem. 2011, 286, 39738–39749. [Google Scholar] [CrossRef] [PubMed]
- Kammermann, J.R.; Kincaid, S.A.; Rumph, P.F.; Baird, D.K.; Visco, D.M. Tumor necrosis factor-α (TNF-α) in canine osteoarthritis: Immunolocalization of TNF-α, stromelysin and TNF receptors in canine osteoarthritic cartilage. Osteoarthr. Cartil. 1996, 4, 23–34. [Google Scholar] [CrossRef]
- Schneider, M.; Dron, F.; Cuinet, E.; Woehrlé, F. Comparative pharmacokinetic profile of cimicoxib in dogs and cats after IV administration. Vet. J. 2021, 270, 105625. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, T. Molecular mechanism for various pharmacological activities of NSAIDS. Pharmaceuticals 2010, 3, 1614–1636. [Google Scholar] [CrossRef]
- Giorgi, M.; Kim, T.; Saba, A.; Rouini, M.-R.; Yun, H.; Ryschanova, R.; Owen, H. Detection and quantification of cimicoxib, a novel COX-2 inhibitor, in canine plasma by HPLC with spectrofluorimetric detection: Development and validation of a new methodology. J. Pharm. Biomed. Anal. 2013, 83, 28–33. [Google Scholar] [CrossRef]
- Nakagawa, K.; Yamagami, T.; Takemura, N. Hepatocellular toxicosis associated with the alternate administration of carprofen and meloxicam in a siberian husky. J. Vet. Med. Sci. 2005, 67, 1051–1053. [Google Scholar] [CrossRef]
- Johnston, S.A.; McLaughlin, R.M.; Budsberg, S.C. Nonsurgical management of osteoarthritis in dogs. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 1449–1470. [Google Scholar] [CrossRef]
- Cheng, H.F.; Harris, R.C. Renal effects of non-steroidal anti-inflammatory drugs and selective cyclooxygenase-2 inhibitors: The administration of non-steroidal anti-inflammatory drugs and selective cyclooxygenase-2 inhibitors in dentistry. Curr. Pharm. Des. 2005, 11, 1795–1804. [Google Scholar] [CrossRef]
- KuKanich, B.; Bidgood, T.; Knesl, O. Clinical pharmacology of nonsteroidal anti-inflammatory drugs in dogs. Vet. Anaesth. Analg. 2012, 39, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Abrams, G.; Frank, R.; Fortier, L.; Cole, B. Platelet-rich plasma for articular cartilage repair. Sports Med. Arthrosc. Rev. 2013, 21, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, M.H.; Malhotra, A.; Brighton, T.; Walsh, W.R.; Lindeman, R. Platelet function and constituents of platelet rich plasma. Int. J. Sports Med. 2013, 34, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.K.; Mishra, A.; Rodeo, S.R.; Fu, F.; Terry, M.A.; Randelli, P.; Canale, S.T.; Kelly, F.B. Platelet-rich plasma in orthopaedic applications: Evidence-based recommendations for treatment. J. Am. Acad. Orthop. Surg. 2013, 21, 739–748. [Google Scholar] [CrossRef]
- Boswell, S.G.; Cole, B.J.; Sundman, E.A.; Karas, V.; Fortier, L.A. Platelet-rich plasma: A milieu of bioactive factors. Arthroscopy 2012, 28, 429–439. [Google Scholar] [CrossRef]
- Arnoczky, S.; Shebani-Rad, S. The basic science of platelet-rich plasma (PRP): What clinicians need to know. Sports Med. Arthrosc. Rev. 2013, 21, 180–185. [Google Scholar] [CrossRef]
- Stief, M.; Gottschalk, J.; Ionita, J.C.; Einspanier, A.; Oechtering, G.; Böttcher, P. Concentration of platelets and growth factors in canine autologous conditioned plasma. Vet. Comp. Orthop. Traumatol. 2011, 24, 122–125. [Google Scholar] [CrossRef]
- Jaegger, G.; Marcellin-Little, D.J.; Levine, D. Reliability of goniometry in Labrador retrievers. Am. J. Vet. Res. 2002, 63, 979–986. [Google Scholar] [CrossRef]
- Duerr, F. Canine Lameness, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2004; pp. 81–83. [Google Scholar]
- Perry, J.; Weiss, W.B.; Burnfield, J.M.; Gronley, J.K. The supine hip extensor manual muscle test: A reliability and validity study. Arch. Phys. Med. Rehabil. 2004, 85, 1345–1350. [Google Scholar] [CrossRef]
- McCarrel, T.M.; Mall, N.A.; Lee, A.S.; Cole, B.J.; Butty, D.C.; Fortier, L.A. Considerations for the use of platelet-rich plasma in orthopedics. Sports Med. 2014, 44, 1025–1036. [Google Scholar] [CrossRef]
- Ahmad, M.R.; Badar, W.; Ullah Khan, M.A.; Mahmood, A.; Latif, N.; Iqbal, T.; Assir, M.Z.K.; Sleem, M.A. Combination of preconditioned adipose-derived mesenchymal stem cells and platelet-rich plasma improves the repair of osteoarthritis in rat. Regen. Med. 2020, 15, 2285–2295. [Google Scholar] [CrossRef]
- Arican, M.; Şimşek, A.; Parlak, K.; Atli, K.; Sönmez, G. Matrix metalloproteinases 2 and 9 activity after intra-articular injection of autologous platelet-rich plasma for the treatment of osteoarthritis in dogs. Acta Vet. Brno. 2018, 87, 127–135. [Google Scholar] [CrossRef]
- Wang, Z.; Zhai, C.; Fei, H.; Hu, J.; Cui, W.; Li, Z.; Fan, W. Intraarticular injection autologous platelet-rich plasma and bone marrow concentrate in a goat osteoarthritis model. J. Orthop. Res. 2018, 36, 2140–2146. [Google Scholar] [CrossRef] [PubMed]
- Xin, F.; Wang, H.; Yuan, F.; Ding, Y. Platelet-rich plasma combined with alendronate reduces pain and inflammation in induced osteoarthritis in rats by inhibiting the nuclear factor-kappa B signaling pathway. BioMed Res. Int. 2020, 20, 10. [Google Scholar] [CrossRef] [PubMed]
- Arican, M.; Şimşek, A.; Parlak, K.; Atli, K.; Sönmez, G. Effect of inflammatory marker activity after intra-articular injection of autologous platelet-rich plasma in dogs with osteoarthritis. Med. Weter. 2019, 75, 744–748. [Google Scholar] [CrossRef]
- Franklin, S.P.; Stoker, A.M.; Bozynski, C.C.; Kuroki, K.; Clarke, K.M.; Johnson, J.K.; Cook, J.L. Comparison of platelet-rich plasma, stromal vascular fraction (SVF), or SVF with an injectable PLGA nanofiber scaffold for the treatment of osteochondral injury in dogs. J. Knee Surg. 2018, 31, 686–697, Erratum in J. Knee Surg. 2018, 31, e2. [Google Scholar] [PubMed]
- Wanstrath, A.W.; Hettlich, B.F.; Su, L.; Smith, A.; Zekas, L.J.; Allen, M.J.; Bertone, A.L. Evaluation of a single intra-articular injection of autologous protein solution for treatment of osteoarthritis in a canine population. Vet. Surg. 2016, 45, 764–774. [Google Scholar] [CrossRef] [PubMed]
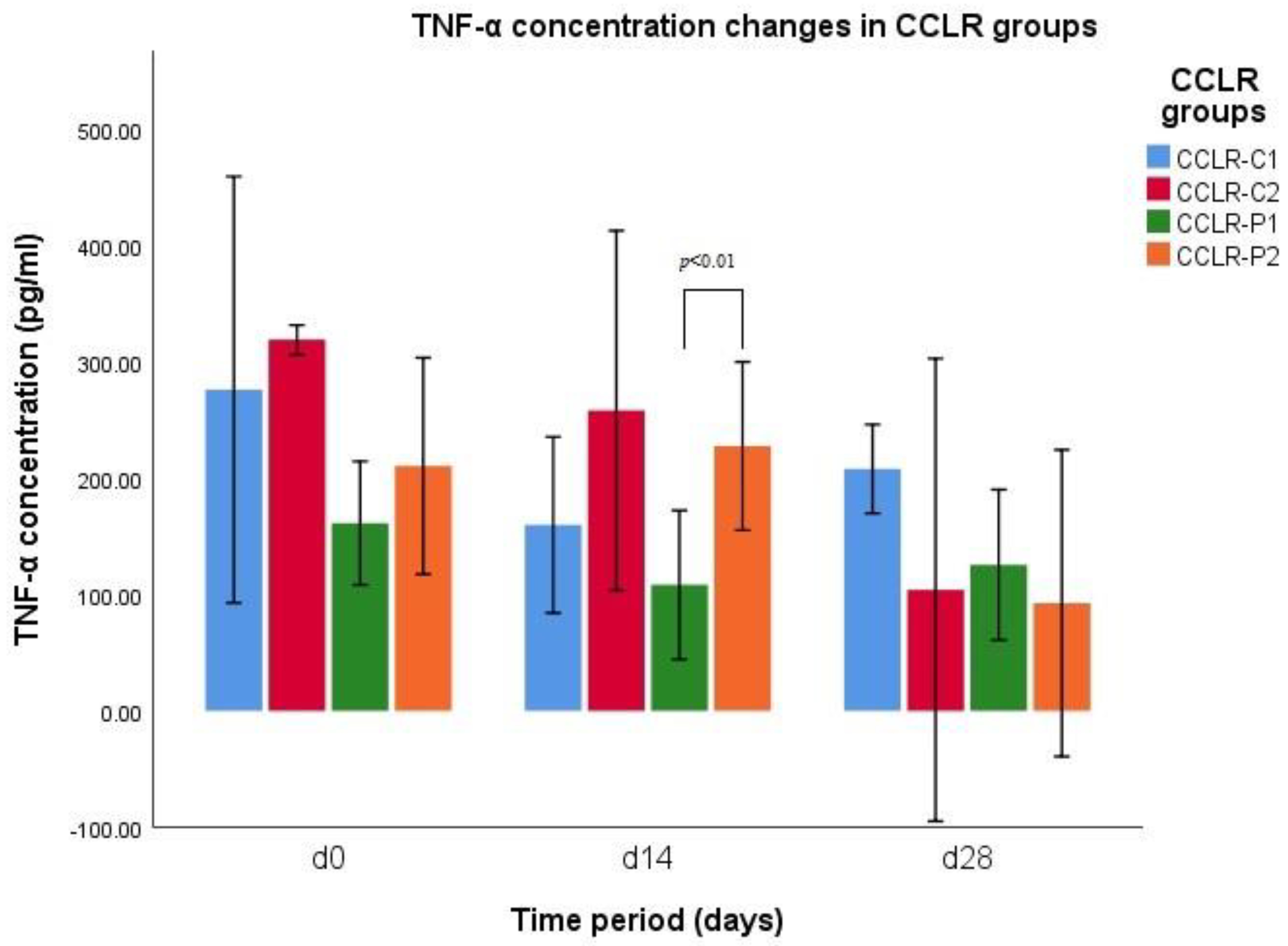
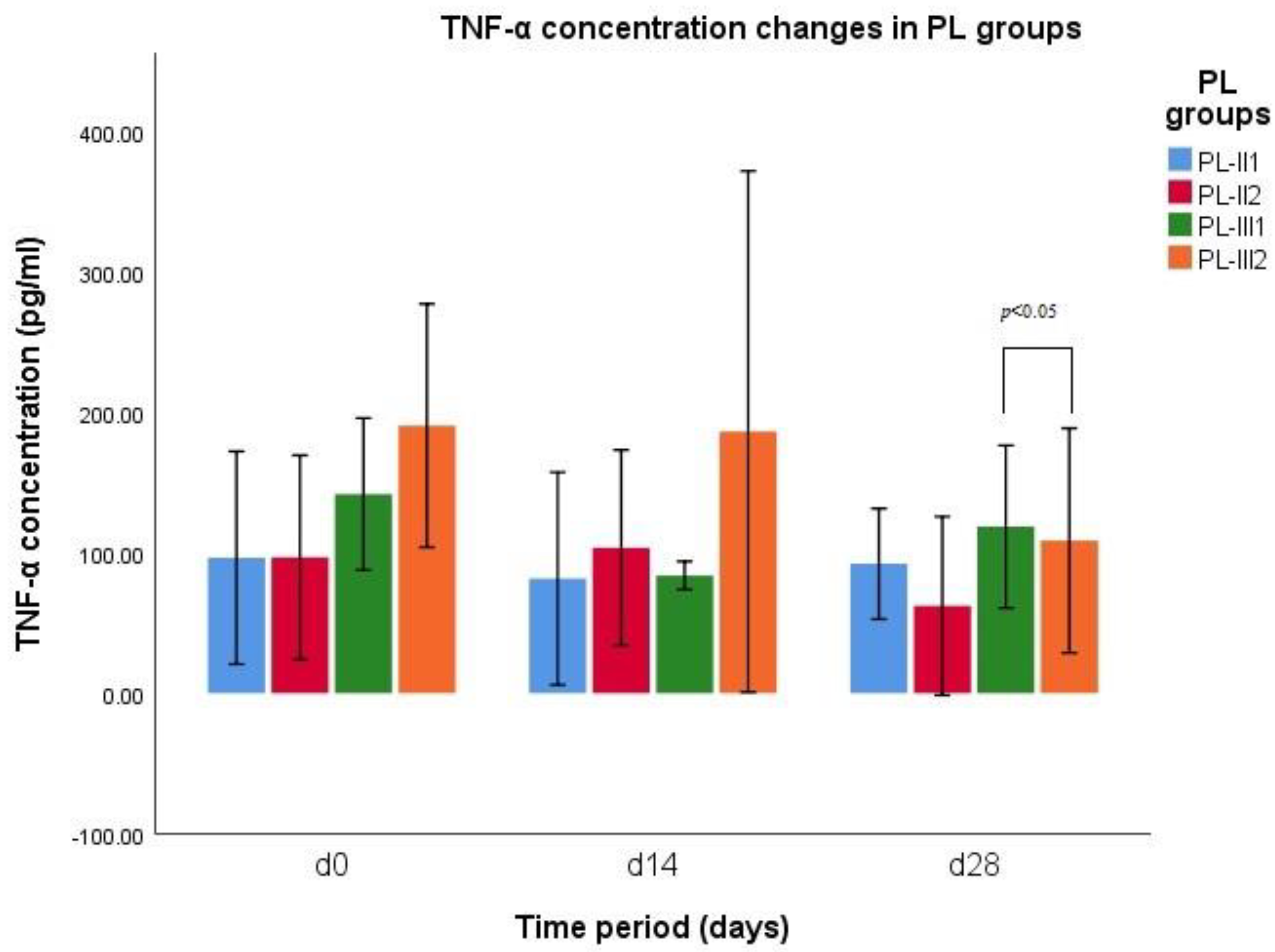
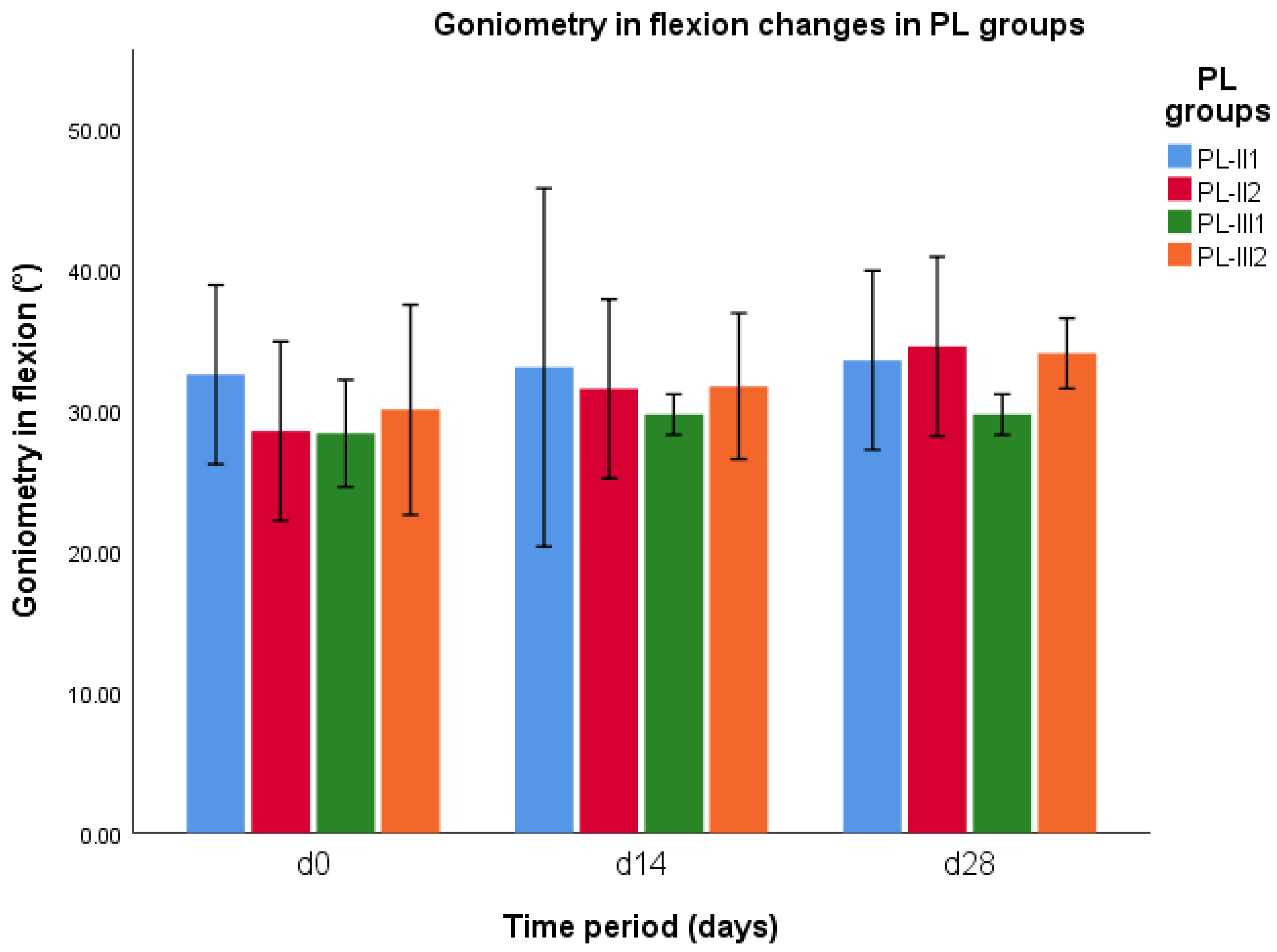
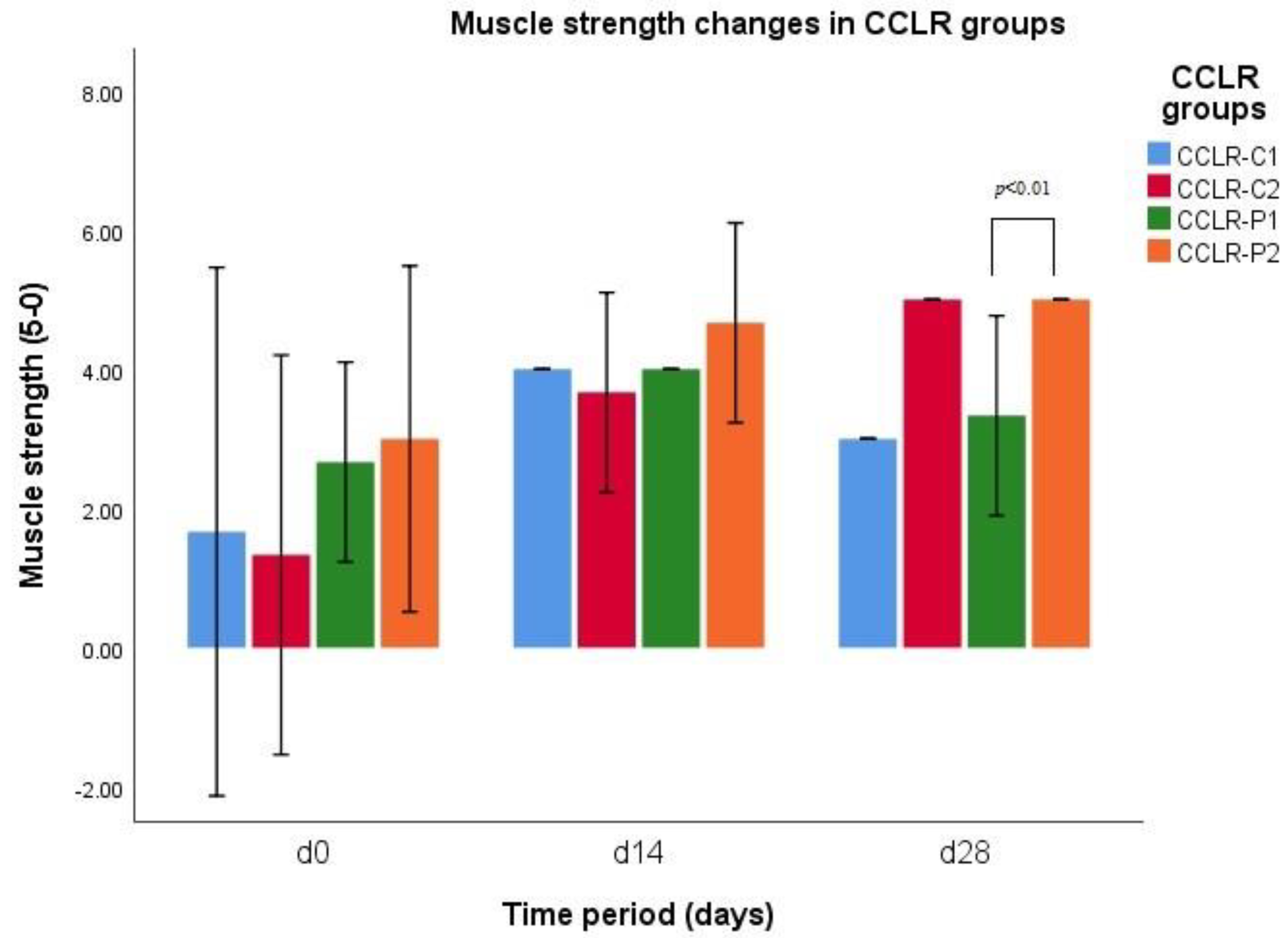
| Parameter | CCLR Groups | d0 | d14 | d28 |
|---|---|---|---|---|
| Degree of lameness (0–5) | CCLR-P1 | 3.33 ± 0.58 a | 0.67 ± 0.58 a | 1.33 ± 1.15 |
| CCLR-P2 | 3.33 ± 0.58 b | 0.67 ± 0.58 b | 0.00 | |
| CCLR-C1 | 3.00 ± 1.00 | 2.00 ± 0.00 | 2.00 ± 0.31 | |
| CCLR-C2 | 4.33 ± 0.58 c | 2.00 ± 0.00 c | 0.33 ± 0.58 c |
| Parameter | PL Groups | d0 | d14 | d28 |
|---|---|---|---|---|
| Degree of lameness (0–5) | PL-II1 | 2.50 ± 0.7 | 0.50 ± 0.71 | 0.50 ± 0.71 |
| PL-II2 | 2.50 ± 0.7 a | 0.50 ± 0.71 | 0.00 a | |
| PL-III1 | 3.67 ± 0.58 b,c | 1.33 ± 0.58 b | 1.33 ± 1.15 b,c | |
| PL-III2 | 3.67 ± 0.58 d,e | 1.67 ± 0.58 d | 0.33 ± 0.58 d,e |
| Parameter | CCLR Groups | d0 | d14 | d28 |
|---|---|---|---|---|
| Painfulness of manipulations (1–3) | CCLR-P1 | 2.00 ± 0.00 a,b | 0.00 a | 0.67 ± 0.58 b |
| CCLR-P2 | 2.33 ± 0.58 c,d | 0.33 ± 0.58 c | 0.00 d | |
| CCLR-C1 | 2.67 ± 0.58 e | 0.33 ±0.58 e | 1.00 ± 1.00 | |
| CCLR-C2 | 2.67 ± 0.58 f,g | 0.67 ± 0.58 f | 0.00 g |
| Parameter | PL Groups | d0 | d14 | d28 |
|---|---|---|---|---|
| Painfulness of manipulations (1–3) | PL-II1 | 1.00 ± 0.00 | 0.50 ± 0.78 | 0.00 |
| PL-II2 | 1.00 ± 0.00 | 0.00 | 0.00 | |
| PL-III1 | 2.33 ± 0.58 a,b | 0.33 ±0.58 a | 0.67 ± 0.58 b | |
| PL-III2 | 2.00 ± 1.00 c | 0.67 ± 0.58 | 0.00 c |
| Parameter | CCLR Groups | d0 | d14 | d28 |
|---|---|---|---|---|
| Goniometry in extension (°) | CCLR-P1 | 149.00 ± 1.00 | 150.67 ± 1.15 | 151.33 ± 1.53 |
| CCLR-P2 | 146.00 ± 1.73 | 148.67 ± 1.53 a | 153.00 ± 2.00 a | |
| CCLR-C1 | 146.67 ± 1.53 b | 149.00 ± 1.00 | 150.00 ± 1.00 b | |
| CCLR-C2 | 144.67 ± 1.15 c,d | 148.33 ± 1.53 d | 151.33 ± 1.53 d |
| Parameter | PL Groups | d0 | d14 | d28 |
|---|---|---|---|---|
| Goniometry in extension (°) | PL-II1 | 147.50 ± 2.12 | 148.50 ± 2.12 | 149.00 ± 2.83 |
| PL-II2 | 149.50 ± 0.71 a | 151.50 ± 0.71 | 154.50 ± 0.71 a | |
| PL-III1 | 145.67 ± 3.79 | 146.67 ± 3.06 | 147.33 ± 3.21 | |
| PL-III2 | 147.33 ± 2.52 | 148.33 ± 1.53 | 150.67 ± 1.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raulinaitė, K.; Želvytė, R.; Škėmienė, K.; Burbaitė, E.; Karvelienė, B.; Monkevičienė, I. The Single Intra-Articular Injection of Platelet-Rich Plasma vs. Non-Steroidal Anti-Inflammatory Drugs as Treatment Options for Canine Cruciate Ligament Rupture and Patellar Luxation. Vet. Sci. 2023, 10, 555. https://doi.org/10.3390/vetsci10090555
Raulinaitė K, Želvytė R, Škėmienė K, Burbaitė E, Karvelienė B, Monkevičienė I. The Single Intra-Articular Injection of Platelet-Rich Plasma vs. Non-Steroidal Anti-Inflammatory Drugs as Treatment Options for Canine Cruciate Ligament Rupture and Patellar Luxation. Veterinary Sciences. 2023; 10(9):555. https://doi.org/10.3390/vetsci10090555
Chicago/Turabian StyleRaulinaitė, Kristina, Rasa Želvytė, Kristina Škėmienė, Evelina Burbaitė, Birutė Karvelienė, and Ingrida Monkevičienė. 2023. "The Single Intra-Articular Injection of Platelet-Rich Plasma vs. Non-Steroidal Anti-Inflammatory Drugs as Treatment Options for Canine Cruciate Ligament Rupture and Patellar Luxation" Veterinary Sciences 10, no. 9: 555. https://doi.org/10.3390/vetsci10090555
APA StyleRaulinaitė, K., Želvytė, R., Škėmienė, K., Burbaitė, E., Karvelienė, B., & Monkevičienė, I. (2023). The Single Intra-Articular Injection of Platelet-Rich Plasma vs. Non-Steroidal Anti-Inflammatory Drugs as Treatment Options for Canine Cruciate Ligament Rupture and Patellar Luxation. Veterinary Sciences, 10(9), 555. https://doi.org/10.3390/vetsci10090555







