Current Situation of Bacterial Infections and Antimicrobial Resistance Profiles in Pet Rabbits in Spain
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Database Collection and Management
2.2. Microbiological Diagnosis Techniques and Antimicrobial Susceptibility Testing
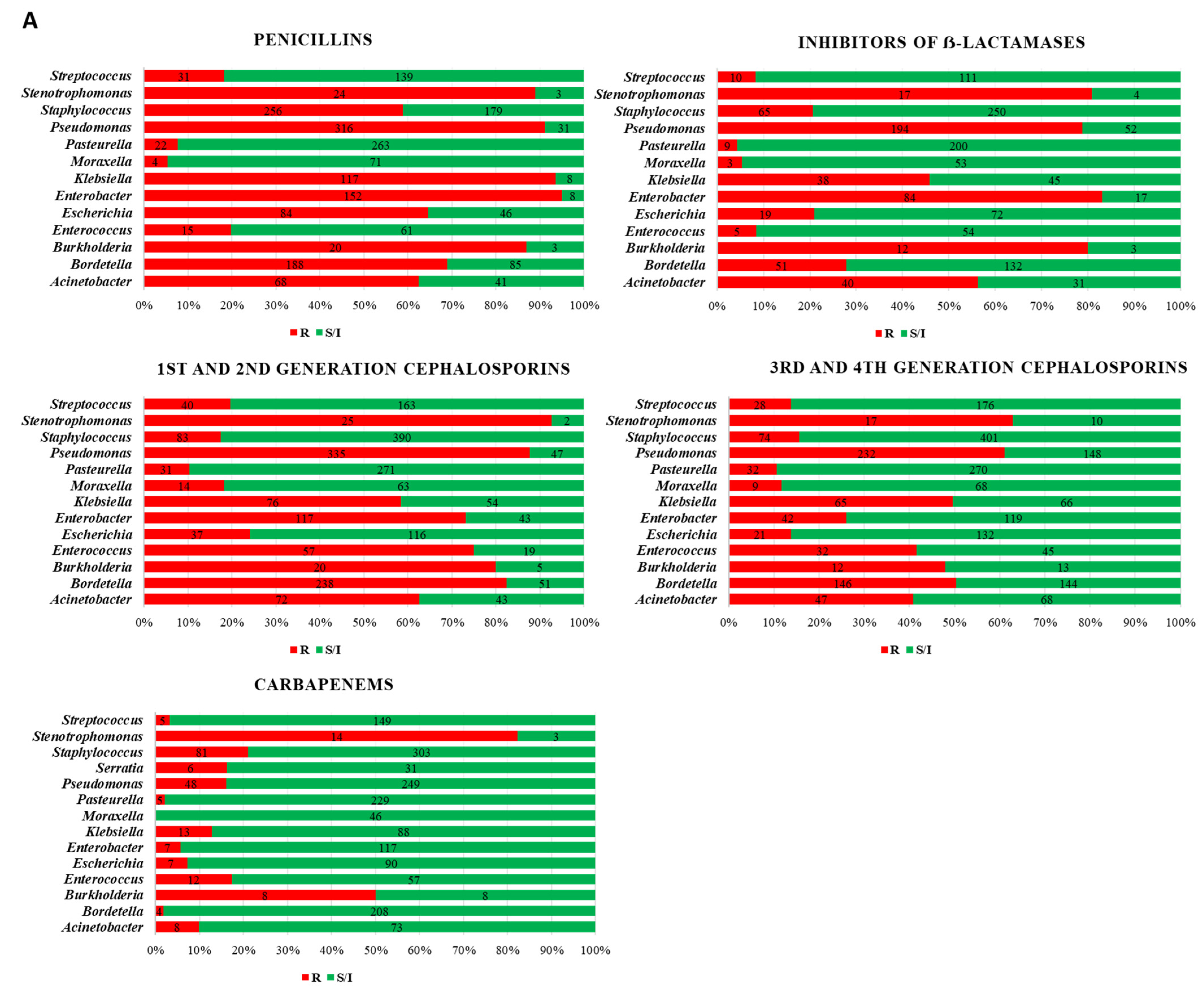
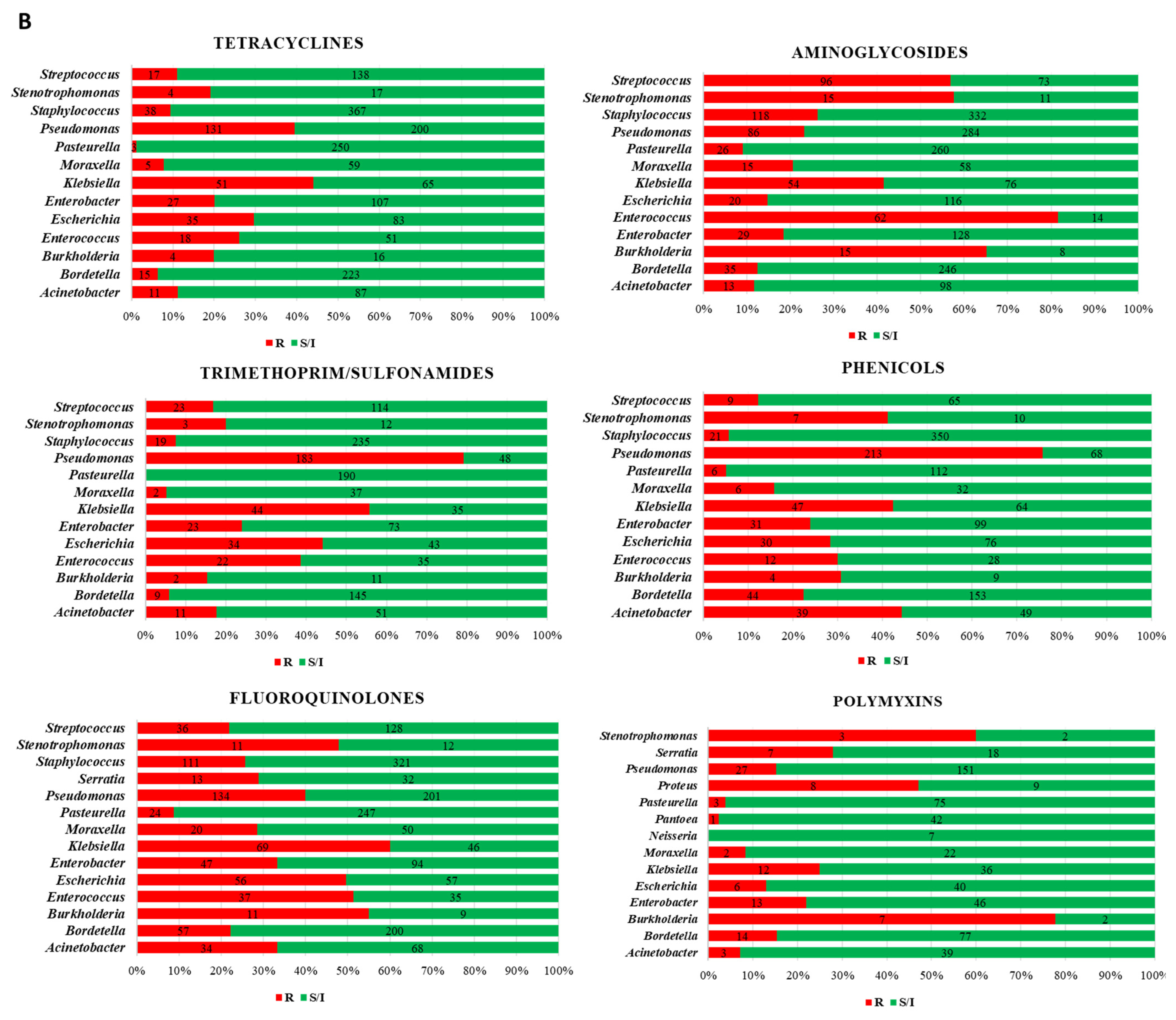
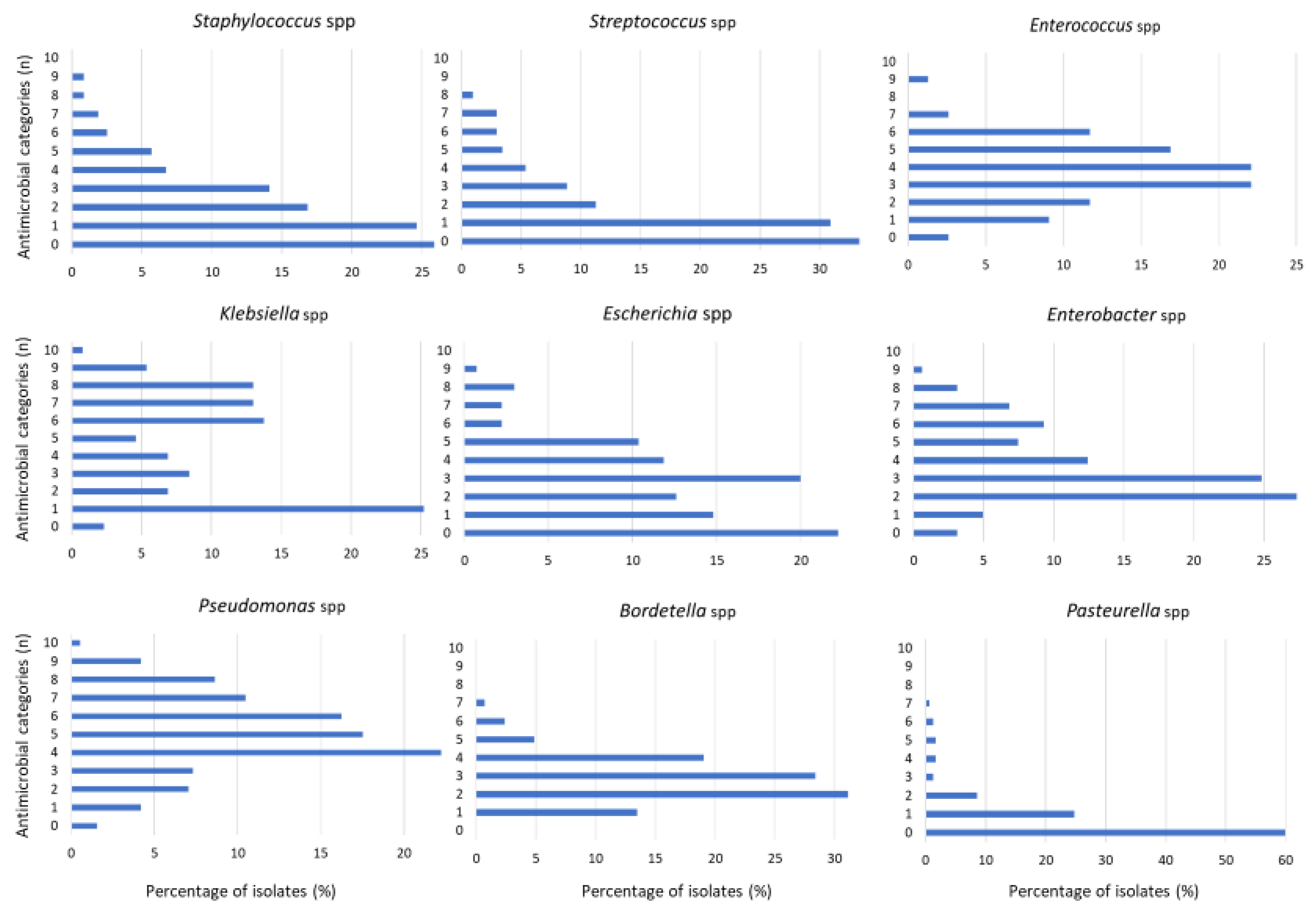
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giraldi, G.; Montesano, M.; Napoli, C.; Frati, P.; La Russa, R.; Santurro, A.; Scopetti, M.; Orsi, G.B. Healthcare-Associated Infections Due to Multidrug-Resistant Organisms: A Surveillance Study on Extra Hospital Stay and Direct Costs. Curr. Pharm. Biotechnol. 2019, 20, 643–652. [Google Scholar] [CrossRef]
- Available online: https://www.avma.org/resources-tools/reports-statistics/us-pet-ownership-statistics (accessed on 26 April 2023).
- DeMello, M. Rabbits Multiplying Like Rabbits: The Rise in the Worldwide Popularity of Rabbits as Pets. In Companion Animals in Everyday Life; Pręgowski, M., Ed.; Palgrave Macmillan: New York, NY, USA, 2016. [Google Scholar]
- D’Amico, F.; Casalino, G.; Bozzo, G.; Camarda, A.; Lombardi, R.; Dimuccio, M.M.; Circella, E. Spreading of Pasteurella multocida Infection in a Pet Rabbit Breeding and Possible Implications on Healed Bunnies. Vet. Sci. 2022, 9, 301. [Google Scholar] [CrossRef] [PubMed]
- Centers of Disease Control and Prevention. Available online: https://www.cdc.gov/onehealth/basics/zoonotic-diseases.html (accessed on 31 March 2023).
- Guardabassi, L.; Schwarz, S.; Lloyd, D.H. Pet animals as reservoirs of antimicrobial-resistant bacteria. J. Antimicrob. Chemother. 2004, 66, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, D.H. Reservoirs of antimicrobial resistance in pet animals. Clin. Infect. Dis. 2007, 45, S148–S152. [Google Scholar] [CrossRef] [PubMed]
- Wedley, A.L.; Dawson, S.; Maddox, T.W.; Coyne, K.P.; Pinchbeck, G.L.; Clegg, P.; Nuttall, T.; Kirchner, M.; Williams, N.J. Carriage of antimicrobial resistant Escherichia coli in dogs: Prevalence, associated risk factors and molecular characteristics. Vet. Microbiol. 2017, 199, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fernández, R.; Durán, I.; Molina-López, R.A.; Darwich, L. Antimicrobial Resistance in Bacteria Isolated from Cats and Dogs from the Iberian Peninsula. Front. Microbiol. 2021, 11, 621597. [Google Scholar] [CrossRef]
- Darwich, L.; Seminati, C.; Burballa, A.; Nieto, A.; Durán, I.; Tarradas, N.; Molina-López, R.A. Antimicrobial susceptibility of bacterial isolates from urinary tract infections in companion animals in Spain. Vet. Rec. 2021, 188, e60. [Google Scholar] [CrossRef]
- Schirò, G.; Gambino, D.; Mira, F.; Vitale, M.; Guercio, A.; Purpari, G.; Antoci, F.; Licitra, F.; Chiaramonte, G.; La Giglia, M.; et al. Antimicrobial Resistance (AMR) of Bacteria Isolated from Dogs with Canine Parvovirus (CPV) Infection: The Need for a Rational Use of Antibiotics in Companion Animal Health. Antibiotics 2022, 11, 142. [Google Scholar] [CrossRef]
- Muñoz-Ibarra, E.; Molina-López, R.A.; Durán, I.; Garcias, B.; Martín, M.; Darwich, L. Antimicrobial Resistance in Bacteria Isolated from Exotic Pets: The Situation in the Iberian Peninsula. Animals 2022, 12, 1912. [Google Scholar] [CrossRef]
- Carpenter, J.W. (Ed.) Exotic Animal Formulary, 3rd ed.; Elsevier Publishing: St. Louis, MO, USA, 2005; 564p. [Google Scholar]
- Ivey, E.S.; Morrisey, J.K. Therapeutics for rabbits. Vet. Clin. N. Am. 2000, 3, 183 220. [Google Scholar] [CrossRef]
- Quesenberry, K.E.; Carpenter, J.W. (Eds.) Ferrets Rabbits, and Rodents: Clinical Medicine and Surgery; WB Saunders Co.: Philadelphia, PA, USA, 2004; 461p. [Google Scholar]
- M31-A3; Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals. 3rd ed. CLSI: Wayne, PA, USA, 2008.
- M100-S26; Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Sixth Informational Supplement. CLSI: Wayne, PA, USA, 2016.
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 26 April 2023).
- Berends, M.S.; Luz, C.F.; Friedrich, A.W.; Sinha, B.N.M.; Albers, C.J.; Glasner, C. AMR: An R Package for Working with Antimicrobial Resistance Data. J. Stat. Softw. 2022, 104, 1–31. [Google Scholar] [CrossRef]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- EMA/CVMP/CHMP. Categorisation of Antibiotics in the European Union; European Medicines Agency: Amsterdam, The Netherlands, 2019.
- Deeb, B. Update for veterinary practitioners on pasteurellosis in rabbits. J. Small Exotic Anim. Med. 1993, 2, 112–113. [Google Scholar]
- Rzewuska, M.; Kwiecień, E.; Chrobak-Chmiel, D.; Kizerwetter-Świda, M.; Stefańska, I.; Gieryńska, M. Pathogenicity and Virulence of Trueperella pyogenes: A Review. Int. J. Mol. Sci. 2019, 20, 2737. [Google Scholar] [CrossRef] [PubMed]
- Kimura, R.; Hayashi, Y.; Takeuchi, T.; Shimizu, M.; Iwata, M.; Tanahashi, J.; Makoto, I. Pasteurella multocida septicemia caused by close contact with a domestic cat: Case report and literature review. J. Infect. Chemother. 2004, 10, 250–252. [Google Scholar] [CrossRef]
- Weber, D.J.; Wolfson, J.S.; Swartz, M.N.; Hooper, D.C. Pasteurella multocida infections. Report of 34 cases and review of the literature. Medicine 1984, 63, 133–154. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.A.; Ho, M. Pasteurella multocida: From zoonosis to cellular microbiology. Clin. Microbiol. Rev. 2013, 26, 631–655. [Google Scholar] [CrossRef]
- Abrahamian, F.M.; Goldstein, E.J. Microbiology of animal bite wound infections. Clin. Microbiol. Rev. 2011, 24, 231–246. [Google Scholar] [CrossRef]
- Wilkie, I.W.; Harper, M.; Boyce, J.D.; Adler, B. Pasteurella Multocida: Diseases and Pathogenesis; Springer: Heidelberg/Berlin, Germany, 2012; pp. 1–22. [Google Scholar]
- Körmöndi, S.; Terhes, G.; Pál, Z.; Varga, E.; Harmati, M.; Buzás, K.; Urbán, E. Human Pasteurellosis Health Risk for Elderly Persons Living with Companion Animals. Emerg. Infect. Dis. 2019, 25, 229–235. [Google Scholar] [CrossRef]
- Rougier, S.; Galland, D.; Boucher, S.; Boussarie, D.; Vallé, M. Epidemiology and susceptibility of pathogenic bacteria responsible for upper respiratory tract infections in pet rabbits. Vet. Microbiol. 2006, 115, 192–198. [Google Scholar] [CrossRef]
- Wisplinghoff, H. Pseudomonas spp., Acinetobacter spp. and Miscellaneous Gram-Negative Bacilli; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1579–1599.e2. [Google Scholar]
- Wareth, G.; Neubauer, H. The Animal-Foods-Environment Interface of Klebsiella Pneumoniae in Germany: An Observational Study on Pathogenicity, Resistance Development and the Current Situation. Vet. Res. 2021, 52, 16. [Google Scholar] [CrossRef]
- Schmiedel, J.; Falgenhauer, L.; Domann, E.; Bauerfeind, R.; Prenger-Berninghoff, E.; Imirzalioglu, C.; Chakraborty, T. Multiresistant Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae from Humans, Companion Animals and Horses in Central Hesse, Germany. BMC Microbiol. 2014, 14, 187. [Google Scholar] [CrossRef]
- Soteras, A.R.; Mackay, A.S.; Borràs-Maixenchs, N.; Martí, J.A. Catheter related necrotizing fascitiis in haematological patients: Case report and implications for nursing. Ann. Oncol. 2019, 30, v843. [Google Scholar] [CrossRef]
- Al-Hamad, A.; Burnie, J.; Upton, M. Enhancement of antibiotic susceptibility of Stenotrophomonas maltophilia using a polyclonal antibody developed against an ABC multidrug efflux pump. Can. J. Microbiol. 2011, 57, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Peykov, S.; Strateva, T. Whole-Genome Sequencing-Based Resistome Analysis of Nosocomial Multidrug-Resistant Non-Fermenting Gram-Negative Pathogens from the Balkans. Microorganisms 2023, 11, 651. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial resistance in ESKAPE pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef] [PubMed]
- WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. Available online: https://www.who.int/en/news-room/detail/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 31 March 2023).

| Bacteria Isolates | Number (% in spp.) | Overall % (N = 2998) |
|---|---|---|
| Staphylococcus spp. | n = 475 | 15.8 |
| S. aureus | 171 (36) | 5.70 |
| S. xylosus | 33 (7) | 1.10 |
| S. epidermidis | 26 (5.5) | 0.86 |
| S. lugdunensis | 14 (3) | 0.46 |
| S. pseudointermedius | 13 (2.8) | 0.43 |
| S. sciuri | 9 (1.9) | 0.30 |
| S. capitis | 9 (1.9) | 0.30 |
| S. chromogenes | 7 (1.5) | 0.23 |
| S. intermedius | 6 (1.3) | 0.20 |
| S. simulans | 6 (1.3) | 0.20 |
| S. chleiferi | 6 (1.3) | 0.20 |
| S. cohnii | 5 (1.1) | 0.16 |
| S. saprofhyticus | 5 (1.1) | 0.16 |
| S. succinus | 5 (1.1) | 0.16 |
| Others | 160 (33.7) | 5.33 |
| Pseudomonas spp. | n = 382 | 12.7 |
| P. aeruginosa | 264 (69) | 8.80 |
| P. putida | 19 (5) | 0.63 |
| P. fluorescens | 14 (3.7) | 0.46 |
| P. korensis | 7 (1.8) | 0.23 |
| P. fulva | 5 (1.3) | 0.16 |
| P. libaniensis | 5 (1.3) | 0.16 |
| P. monteilii | 5 (1.3) | 0.16 |
| Others | 63 (16.5) | 2.10 |
| Pasteurella spp. | n = 302 | 10.1 |
| P. multocida | 230 (76.2) | 7.7 |
| P. canis | 10 (3.3) | 0.3 |
| Others | 62 (20.5) | 2.1 |
| Bordetella spp. | n = 289 | 9.6 |
| B. bronchiseptica | 278 (96.2) | 9.3 |
| Others | 11 (3.8) | 0.4 |
| Streptococcus spp. | n = 204 | 6.8 |
| S. intermedius | 32 (15.7) | 1.1 |
| S. anginosus | 6 (3) | 0.2 |
| S. oralis | 6 (3) | 0.2 |
| Others | 160 (78.4) | 5.3 |
| Enterobacter spp. | n = 161 | 5.4 |
| E. cloacae | 123 (76.39) | 4.10 |
| E. kobei | 13 (8.07) | 0.43 |
| E. bugandensis | 6 (3.72) | 0.20 |
| Others | 19 (11.80) | 0.63 |
| Escherichia spp. | n = 153 | 5.1 |
| E. coli | 141(92.15) | 4.70 |
| E. vulneris | 7 (4.15) | 0.23 |
| Others | 5 (3.26) | 0.16 |
| Klebsiella spp. | n = 131 | 4.4 |
| K. pneumoniae | 75 (57.3) | 2.5 |
| K. oxytoca | 43 (32.8) | 1.4 |
| Others | 13 (9.9) | 0.4 |
| Acinetobacter spp. | n = 115 | 3.8 |
| A. iwoffii | 28 (24.4) | 0.9 |
| A. baumannii | 16 (13.9) | 0.5 |
| A. johnsonii | 10 (8.7) | 0.3 |
| A. pitti | 10 (8.7) | 0.3 |
| Others | 51(44.3) | 1.7 |
| Pantoea spp. | n = 90 | 3.0 |
| P. agglomerans | 69 (76.7) | 2.3 |
| Others | 21 (23.3) | 0.7 |
| Enterococcus spp. | n = 77 | 2.6 |
| E. faecalis | 40 (51.9) | 1.3 |
| Others | 37 (48.05) | 1.23 |
| Moraxella spp. | n = 77 | 2.6 |
| M. branhamella | 32 (41.6) | 1.1 |
| M. catarrhalis | 25 (32.5) | 0.8 |
| Others | 20 (26) | 0.7 |
| Serratia spp. | n = 54 | 1.8 |
| S. marcescens | 39 (72.2) | 1.3 |
| S. liquefaciens | 5 (9.3) | 0.2 |
| S. odorífera | 5 (9.3) | 0.2 |
| Others | 5 (9.3) | 0.2 |
| Neisseria spp. | n = 37 | 1.6 |
| N. animaloris/zoodegmatis | 1 (2.7) | 0.03 |
| N. gonorrheae | 1 (2.7) | 0.03 |
| N. species | 1 (2.7) | 0.03 |
| Others | 34 (92) | 1.1 |
| Proteus spp. | n = 34 | 1.1 |
| P. mirabilis | 28 (82.4) | 0.9 |
| P. vulgaris | 4 (11.7) | 0.1 |
| P. penneri | 2 (5.9) | 0.1 |
| Trueperella spp. | n = 32 | 1.1 |
| T. pyogenes | 32 (100) | 1.1 |
| Strenotrophomonas spp. | n = 27 | 0.9 |
| S. maltophilia | 26 (96.3) | 0.9 |
| Others | 1 (3.7) | 0.0 |
| Burkholderia spp. | n = 25 | 0.8 |
| B. cepacia | 19 (76) | 0.6 |
| Others | 6 (24) | 0.2 |
| Genus | Isolates | Number of AMR Categories/Families | MDR * Profile | |
|---|---|---|---|---|
| Gram-Negative | n | Mean | Median | % |
| Pseudomonas | 381 | 5.0 | 5 | 8.1 |
| Stenotrophomonas | 26 | 5.2 | 5 | 0 |
| Burkholderia | 25 | 4.6 | 5 | 0 |
| Acinetobacter | 115 | 3.0 | 3 | 11.3 |
| Bordetella | 289 | 2.8 | 3 | 0 |
| Pasteurella | 299 | 0.7 | 0 | 0 |
| Moraxella | 77 | 1.2 | 1 | 0 |
| Escherichia | 129 | 2.7 | 3 | 47.9 |
| Klebsiella | 134 | 4.4 | 4 | 57.5 |
| Enterobacter | 157 | 3.6 | 3 | 35.7 |
| Proteus | 34 | 3.1 | 3 | 47.1 |
| Serratia | 54 | 3.3 | 3 | 33.3 |
| Gram-Positive | n | Mean | Median | % |
| Staphylococcus | 466 | 2.0 | 1 | 5 |
| Streptococcus | 204 | 1.6 | 1 | 0 |
| Enterococcus | 77 | 3.7 | 4 | 6.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández, M.; Garcias, B.; Duran, I.; Molina-López, R.A.; Darwich, L. Current Situation of Bacterial Infections and Antimicrobial Resistance Profiles in Pet Rabbits in Spain. Vet. Sci. 2023, 10, 352. https://doi.org/10.3390/vetsci10050352
Fernández M, Garcias B, Duran I, Molina-López RA, Darwich L. Current Situation of Bacterial Infections and Antimicrobial Resistance Profiles in Pet Rabbits in Spain. Veterinary Sciences. 2023; 10(5):352. https://doi.org/10.3390/vetsci10050352
Chicago/Turabian StyleFernández, Mercedes, Biel Garcias, Inma Duran, Rafael A. Molina-López, and Laila Darwich. 2023. "Current Situation of Bacterial Infections and Antimicrobial Resistance Profiles in Pet Rabbits in Spain" Veterinary Sciences 10, no. 5: 352. https://doi.org/10.3390/vetsci10050352
APA StyleFernández, M., Garcias, B., Duran, I., Molina-López, R. A., & Darwich, L. (2023). Current Situation of Bacterial Infections and Antimicrobial Resistance Profiles in Pet Rabbits in Spain. Veterinary Sciences, 10(5), 352. https://doi.org/10.3390/vetsci10050352






