Evaluation of the Immune Response to Transport Stress in the Aosta Valley Breed
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Complete Blood Count (CBC) Analysis
2.3. Clinical Chemistry Analysis
2.4. Serum Protein Electrophoresis
2.5. Immunoenzymatic Analysis
- -
- “Bovine Interleukin 6 ELISA Kit” (Standard curve range: 20–6000 ng/L; Sensitivity: 10.5 ng/L),
- -
- “Bovine Tumor Necrosis Factor Alpha ELISA Kit” (Standard curve range: 10–3000 ng/L; Sensitivity: 5.56 ng/L),
- -
- “Bovine Serum Amyloid A ELISA Kit” (Standard curve range: 0.4–40 µg/mL; Sensitivity: 0.054 µg/mL).
- -
- “Bovine Haptoglobin ELISA Kit” (Standard curve range: 3–900 µg/mL; Sensitivity: 1.69 µg/mL).
2.6. Statistical Analysis
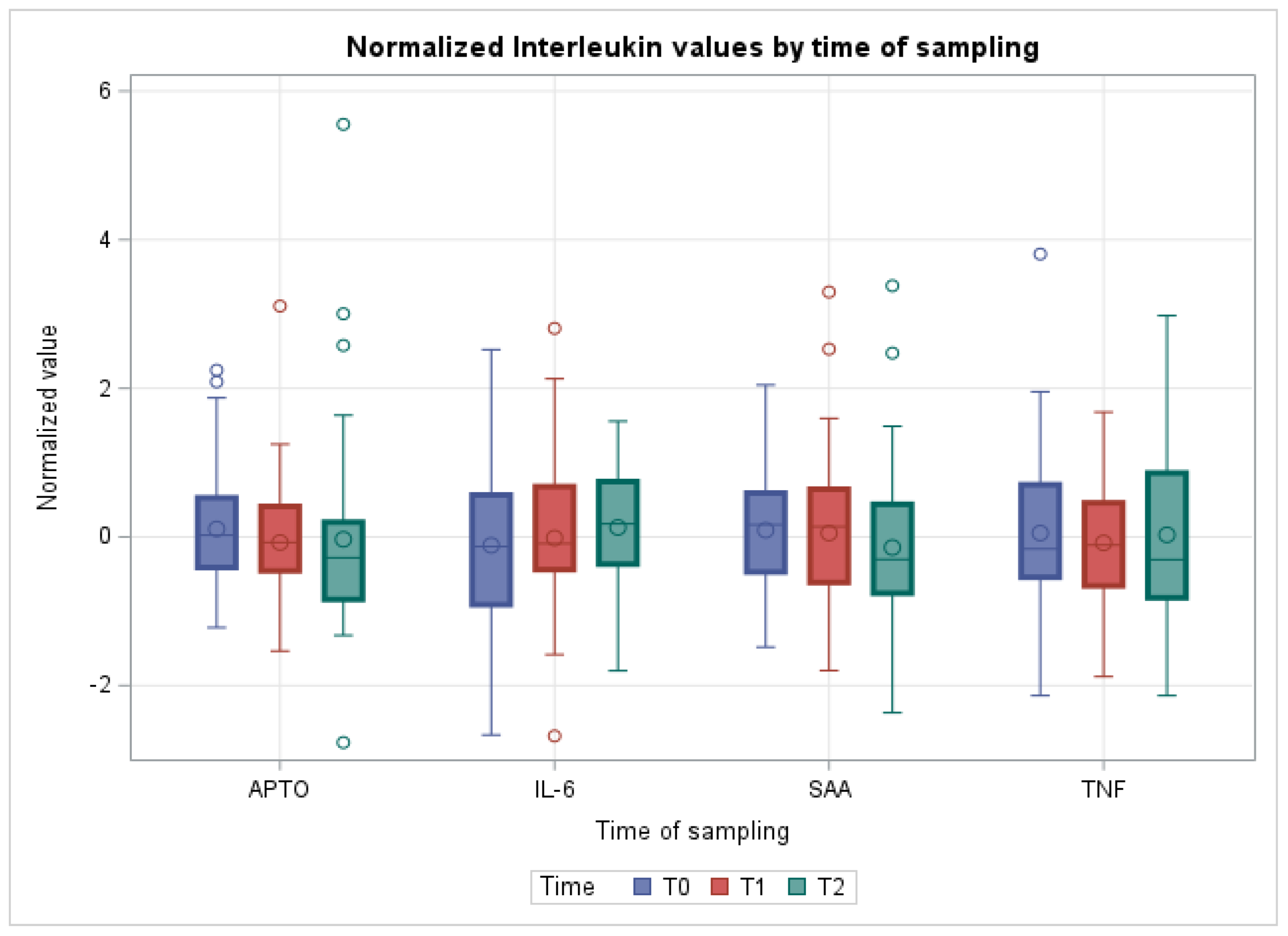
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cafazzo, S.; Magnani, D.; Calà, P.; Razzuoli, E.; Gerardi, G.; Bernardini, D.; Amadori, M.; Nanni Costa, L. Effect of short road journeys on behaviour and some blood variables related to welfare in young bulls. Appl. Anim. Behav. Sci. 2012, 139, 26–34. [Google Scholar] [CrossRef]
- Swanson, J.C.; Morrow-Tesch, J. Cattle transport: Historical, research, and future perspectives. J. Anim. Sci. 2001, 79, E102–E109. [Google Scholar] [CrossRef]
- European Parliament, Council of the European Union. Regulation (EU) 2005/1 of the European parliament and of council of 22 december 2004 on the protection of animals during transport and related operations. Off. J. Eur. Union 2005, 3, 1–44. Available online: http://data.europa.eu/eli/reg/2005/1/oj (accessed on 7 March 2023).
- Fazio, E.; Ferlazzo, A. Evaluation of stress during transport. Vet. Res. Commun. 2003, 27 (Suppl. S1), 519–524. [Google Scholar] [CrossRef] [PubMed]
- Earley, B.; Murray, M.; Prendiville, D.J.; Pintado, B.; Borque, C.; Canali, E. The effect of transport by road and sea on physiology, immunity and behaviour of beef cattle. Res. Vet. Sci. 2012, 92, 531–541. [Google Scholar] [CrossRef]
- Meléndez, D.M.; Marti, S.; Haley, D.B.; Schwinghamer, T.D.; Schwartzkopf-Genswein, K.S. Effect of transport and rest stop duration on welfare of conditioned cattle transported by road. PLoS ONE 2020, 15, e0228492. [Google Scholar] [CrossRef]
- Grandin, T. Assessment of stress during handling and transport. J. Anim. Sci. 1997, 75, 249–257. [Google Scholar] [CrossRef]
- Fraser, D.; Ritchie, J.S.; Fraser, A.F. The term “stress” in a veterinary context. Br. Vet. J. 1975, 131, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Earley, B.; Buckham Sporer, K.; Gupta, S. Invited review: Relationship between cattle transport, immunity and respiratory disease. Animal 2017, 11, 486–492. [Google Scholar] [CrossRef]
- Odore, R.; Badino, P.; Re, G.; Barbero, R.; Cuniberti, B.; D’Angelo, A.; Girardi, C.; Fraccaro, E.; Tarantola, M. Effects of housing and short-term transportqtion on hormone and lymphocite receptor concentrations in beef cattle. Res. Vet. Sci. 2011, 90, 341–345. [Google Scholar] [CrossRef]
- Murata, H.; Hirose, H. Effect of transportation stress o bovine lymphocyte and neutrophil functions. JARQ 1991, 25, 61–64. [Google Scholar]
- Cirone, F.; Padalino, B.; Tullio, D.; Capozza, P.; Losurdo, M.; Lanave, G.; Pratelli, A. Prevalence of pathogens related to bovine respiratory disease before and after transportatino in beef steers: Preliminary results. Animals 2019, 9, 1093. [Google Scholar] [CrossRef] [PubMed]
- Pratelli, A.; Cirone, F.; Capozza, P.; Trotta, A.; Corrente, M.; Balestrieri, A.; Buonavoglia, C. Bovine respiratory disease in beef calves supported long transport stress: An epidemiological study and strategies for control and prevention. Res. Vet. Sci. 2021, 135, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Kelley, K.W. Stress and immune function: A bibliographic review. Ann. Rech. Vet. 1980, 11, 445–478. [Google Scholar]
- Strillacci, M.G.; Vevey, M.; Blanchet, V.; Mantovani, R.; Sartori, C.; Bagnato, A. The genomic varation in the Aosta cattle breeds raised in an extensive alpine farming system. Animals 2020, 10, 2385. [Google Scholar] [CrossRef]
- Associazione Nazionale Allevatori Bovini di Razza Valdostana—A.N.A.Bo.Ra.Va. Available online: www.anaborava.it (accessed on 7 March 2023).
- Kaneko, J.J.; Harvey, J.W.; Bruss, M.L. Clinical Biochemistry of Domestic Animals, 6th ed.; Elvisier Inc.: New York, NY, USA, 2008. [Google Scholar]
- D. lgs. 7 Luglio 2011, n. 146 “Attuazione della Direttiva 2008/119/CE che Stabilisce le Norme Minime per la Protezione dei Vitelli”, Gazzetta Ufficiale della Repubblica Italiana, 2001, n.95. Available online: http://gazzettaufficiale.it/eli/id/2001/04/001G0202/sg (accessed on 7 March 2023).
- Razzuoli, E.; Olzi, E.; Calà, P.; Cafazzo, S.; Magnani, D.; Vitali, A.; Lacetera, N.; Archetti, L.; Lazzara, F.; Ferrari, A.; et al. Innate immune responses of young bulls to a novel environment. Vet. Immunol. Immunopathol. 2016, 172, 9–13. [Google Scholar] [CrossRef]
- Hughes, H.; Carroll, J.A.; Sanchez Burdick, N.C.; Richeson, J.T. Natural variations in the stress and acute phase response of cattle. Innate Immun. 2014, 20, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Lomborg, S.R.; Nielsen, L.R.; Heegaard, P.M.H.; Jacobsen, S. Acute phase proteins in cattle after exposure to complex stress. Vet. Res. Commun. 2008, 32, 575–582. [Google Scholar] [CrossRef]
- Van Engen, N.K.; Coetzee, J.F. Effects of transportation on cattle health and production: A review. Anim. Health Res. Rev. 2018, 19, 142–154. [Google Scholar] [CrossRef]
- Li, F.; Mujtabe Shah, A.; Wang, Z.; Peng, Q.; Hu, R.; Zou, H.; Tan, C.; Zhang, X.; Liao, Y.; Wang, Y.; et al. Effects of land transport stress on variations in ruminal microbe diversity and immune functions in different breeds of cattle. Animals 2019, 9, 599. [Google Scholar] [CrossRef]
- Calamari, L.; Bionaz, M.; Trevisi, E.; Bertoni, G. Preliminary study to validate a model animal welfare assessment in dairy farm. In Proceedings of the Fifth Congress of the European Society for Agricultural and Food Ethics (EURSAFE), Leuven, Belgium, 2–4 September 2004; pp. 38–42. [Google Scholar]
- Gruys, E.; Toussaint, M.J.M.; Niewold, T.A.; Koopmans, S.J. Acute phase reaction and acute phase proteins. J. Zhejiang Univ. Sci. 2005, 6B, 1045–1056. [Google Scholar] [CrossRef]
- Miller, W.J.; Pitts, W.J.; Clifton, C.M.; Morton, J.D. Effect of zinc deficienty per se on feed efficiency, serum alkaline phosphatase, zinc in skin, behavior, greying, and other measurements in the holstein calf. J. Dairy Sci. 1965, 48, 1329–1334. [Google Scholar] [CrossRef]
- Halse, K. Investigations on serum phosphatase in dairy cows during hypomagnesaemia. Scand. Vet. 1948, 6, 567–579. [Google Scholar]
- Beatty, D.T.; Barnes, A.; Taylor, E.; Pethick, D.; McCarthy, M.; Maloney, S.K. Physiological responses of Bos taurus and Bos indicus cattle to prolonged, continuous heat and humidity. J. Anim. Sci. 2006, 84, 972–985. [Google Scholar] [CrossRef] [PubMed]
- Mormede, P.; Soissons, J.; Bluthe, R.M.; Raoult, J.; Legarff, G.; Levieux, D.; Dantzer, R.; Chaillou, J.F.; Geffard, M.C.; Arnoux, D. Effect of transportation on blood serum composition, disease incidence, and production traits in young calves. Influence of the journey duration. Ann. Rech. Vét. 1982, 13, 369–384. [Google Scholar] [PubMed]
- Van Engen, N.K.; Stock, M.L.; Engelken, T.; Vann, R.C.; Wulf, L.W.; Karriker, L.A.; Busby, W.D.; Lakritz, J.; Carpentr, A.J.; Bradford, B.J.; et al. Impact of oral meloxicam on circulating biomarkers of stress and inflammation in beef steers after long-distance transportation. J. Anim. Sci. 2014, 92, 498–510. [Google Scholar] [CrossRef]
- Grandin, T.; Shivley, C. How farm animals react and perceive stressful situations such as handling, restraint, and transport. Animals 2015, 5, 1233–1251. [Google Scholar] [CrossRef]
- Gupta, S.; Earley, B.; Crowe, M.A. Effect of 12-hour road transportation on physiological, immunological and haematological parameters in bulls housed at different space allowances. Vet. J. 2007, 173, 605–616. [Google Scholar] [CrossRef]
- Early, B.; Drennan, M.; O’Riordan, E.G. The effect of road transport in comparison to a novel environment on the physiological, metabolic and behavioural responses of bulls. Res. Vet. Sci. 2013, 95, 811–818. [Google Scholar] [CrossRef]
- Fischer, A.D.; Colditz, I.G.; Lee, C.; Ferguson, D.M. The influence of land transport on animal welfare in extensive farming systems. J. Vet. Behav. 2009, 4, 157–162. [Google Scholar] [CrossRef]
| T0 | T1 | T2 | |
|---|---|---|---|
| BA % | 0.44 ± 0.26 | 0.44 ± 0.26 | 0.39 ± 0.26 |
| EO % | 1.32 ± 3.82 | 0.51 ± 1.12 | 0.33 ± 0.78 |
| WBC (m/mm3) | 10 ± 2.83 | ** 11.7 ± 2.5 | 9.77 ± 2.69 |
| RBC (m/mm3) | 10.1 ± 1.28 | 10.2 ± 1.18 | 9.73 ± 1.24 |
| HB (g/dL) | 12.8 ± 2.21 | 12.8 ± 2.01 | 12 ± 2 |
| HCT % | 34.3 ± 6.42 | 34.2 ± 6.05 | 32.8 ± 6.12 |
| LINF % | 51.7 ± 11.7 | 45.3 ± 13.2 | 51.3 ± 12.4 |
| MCH (pg) | 12.6 ± 1.33 | 12.5 ± 1.07 | 12.3 ± 0.93 |
| MCHC (g/dL) | 37.3 ± 3.17 | 37.6 ± 3.01 | 36.8 ± 2.77 |
| MCV (fL) | 33.9 ± 3.53 | 33.6 ± 3.39 | 33.6 ± 3.45 |
| MON % | 6.68 ± 2 | 6.4 ± 3.36 | 6.85 ± 2.51 |
| MPV (fL) | 10 ± 0.75 | 9.96 ± 0.77 | 9.77 ± 0.85 |
| NEU % | 39.9 ± 13.2 | * 47.3 ± 15.2 | 41.1 ± 13.9 |
| PCT % | 0.54 ± 0.35 | 0.59 ± 0.39 | 0.72 ± 1.16 |
| PDW | 8.99 ± 2 | 8.79 ± 1.96 | 8.62 ± 1.89 |
| PLT (m/mm3) | 518 ± 310 | 578 ± 347 | 549 ± 301 |
| RDW | 17.3 ± 2.3 | 17.2 ± 2.11 | 16.9 ± 2.29 |
| RRg | 3.45 ± 8.64 | 4.29 ± 9.52 | 1.65 ± 4.05 |
| T0 | T1 | T2 | |
|---|---|---|---|
| ALP (UI/L) | 568 ± 279 | 575 ± 271 | *** 246 ± 124 |
| ALT (UI/L) | 17.3 ± 4.83 | 17.2 ± 5.45 | 15.7 ± 5.41 |
| AST (UI/L) | 76.3 ± 19.1 | * 87 ± 24.5 | 73 ± 18.3 |
| BILT (mg/dL) | 0.2 ± 0.1 | 0.25 ± 0.13 | 0.23 ± 0.12 |
| CA (mg/dL) | 9.43 ± 3.45 | 9.45 ± 3.56 | 8.06 ± 2.82 |
| CHOL (mg/dL) | 125 ± 47 | 127 ± 49.4 | ** 91.1 ± 40.2 |
| CL (mEq/L) | 99.8 ± 7.73 | * 104 ± 9.59 | 99.3 ± 4.26 |
| CREA (mg/dL) | 0.92 ± 0.34 | 0.95 ± 0.34 | ** 1.12 ± 0.24 |
| FE (µg/dL) | 125 ± 95.7 | 120 ± 86.9 | 86.2 ± 57.4 |
| GGT (UI/L) | 23.1 ± 6.13 | 22.7 ± 7.16 | 19.9 ± 7.32 |
| K (mg/dL) | 9.31 ± 5.74 | 8.88 ± 1.91 | 12.3 ± 17.6 |
| MG (mg/dL) | 1.77 ± 0.65 | 1.66 ± 0.67 | 1.87 ± 1.03 |
| NA (mg/dL) | 143 ± 8.68 | 147 ± 10.7 | 140 ± 4.84 |
| PHOS (mg/dL) | 8.25 ± 3.32 | 7.89 ± 3.15 | 7.61 ± 3.14 |
| TP (g/dL) | 5.14 ± 0.78 | 5.32 ± 0.81 | 5.04 ± 0.64 |
| TRIG (mg/dL) | 29 ± 17.5 | ** 20.1 ± 13.3 | *** 13.4 ± 6.75 |
| UREA (mg/dL) | 13.6 ± 5.86 | 13.2 ± 5.75 | ** 19.3 ± 10 |
| T0 | T1 | T2 | |
|---|---|---|---|
| ALB (g/dL) | 2.57 ± 0.47 | 2.67 ± 0.51 | 2.49 ± 0.4 |
| α-G (g/dL) | 1.02 ± 0.16 | 1.03 ± 0.16 | 1.03 ± 0.14 |
| β-G (g/dL) | 0.73 ± 0.15 | 0.76 ± 0.2 | 0.71 ± 0.14 |
| γ-G (g/dL) | 0.84 ± 0.25 | 0.85 ± 0.26 | 0.82 ± 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagliasso, G.; Moriconi, M.; Fusi, F.; Vitale, N.; Vevey, M.; Dondo, A.; Razzuoli, E.; Bergagna, S. Evaluation of the Immune Response to Transport Stress in the Aosta Valley Breed. Vet. Sci. 2023, 10, 351. https://doi.org/10.3390/vetsci10050351
Pagliasso G, Moriconi M, Fusi F, Vitale N, Vevey M, Dondo A, Razzuoli E, Bergagna S. Evaluation of the Immune Response to Transport Stress in the Aosta Valley Breed. Veterinary Sciences. 2023; 10(5):351. https://doi.org/10.3390/vetsci10050351
Chicago/Turabian StylePagliasso, Giulia, Martina Moriconi, Francesca Fusi, Nicoletta Vitale, Mario Vevey, Alessandro Dondo, Elisabetta Razzuoli, and Stefania Bergagna. 2023. "Evaluation of the Immune Response to Transport Stress in the Aosta Valley Breed" Veterinary Sciences 10, no. 5: 351. https://doi.org/10.3390/vetsci10050351
APA StylePagliasso, G., Moriconi, M., Fusi, F., Vitale, N., Vevey, M., Dondo, A., Razzuoli, E., & Bergagna, S. (2023). Evaluation of the Immune Response to Transport Stress in the Aosta Valley Breed. Veterinary Sciences, 10(5), 351. https://doi.org/10.3390/vetsci10050351






