Simple Summary
This study aimed to evaluate the antimicrobial resistance patterns and biofilm production of clinical isolates of Pseudomonas aeruginosa which is a pathogenic bacterium that can cause infections in dogs. The results showed widespread resistance to various β-lactam antimicrobials, with amikacin and tobramycin being the only effective aminoglycosides. All isolates carried the oprD gene, which is essential in governing the entry of antibiotics into bacterial cells, and all isolates also carried virulence genes. The study emphasizes the importance of continued monitoring of antimicrobial resistance in veterinary medicine and responsible antibiotic use to prevent multi-drug resistance from emerging. The findings of this study have significant implications for the treatment and prevention of P. aeruginosa infections in dogs and highlight the need for further research to better understand the mechanisms underlying the emergence of multi-drug resistance.
Abstract
Pseudomonas aeruginosa is a pathogenic bacterium that can cause serious infections in both humans and animals, including dogs. Treatment of this bacterium is challenging because some strains have developed multi-drug resistance. This study aimed to evaluate the antimicrobial resistance patterns and biofilm production of clinical isolates of P. aeruginosa obtained from dogs. The study found that resistance to various β-lactam antimicrobials was widespread, with cefovecin and ceftiofur showing resistance in 74% and 59% of the isolates tested, respectively. Among the aminoglycosides, all strains showed susceptibility to amikacin and tobramycin, while gentamicin resistance was observed in 7% of the tested isolates. Furthermore, all isolates carried the oprD gene, which is essential in governing the entry of antibiotics into bacterial cells. The study also investigated the presence of virulence genes and found that all isolates carried exoS, exoA, exoT, exoY, aprA, algD, and plcH genes. This study compared P. aeruginosa resistance patterns worldwide, emphasizing regional understanding and responsible antibiotic use to prevent multi-drug resistance from emerging. In general, the results of this study emphasize the importance of the continued monitoring of antimicrobial resistance in veterinary medicine.
1. Introduction
Pseudomonas aeruginosa is a Gram-negative bacterium that is known to cause infections in humans, particularly in individuals with weakened immune systems or those with underlying medical conditions such as cystic fibrosis. This bacterium is highly adaptable and is capable of surviving in a wide range of environments, including in water, soil, and other moist environments [1]. It is also resistant to many antibiotics, making it difficult to treat. P. aeruginosa is known to excrete several virulence factors, including toxins and enzymes that contribute to tissue damage and disease [2].
One of the most relevant virulence factors of this pathogen is its ability to form biofilms, complex communities of bacteria encased in a protective matrix of extracellular polymeric substances (EPS). Biofilms can adhere to several surfaces like medical devices or lung tissue, for example, making it difficult for the host immune system or antibiotics to eliminate the infection [3,4]. In addition, the EPS matrix of the biofilm can facilitate the exchange of genetic material between bacteria, allowing for the spread of antibiotic resistance genes. The formation of a biofilm by P. aeruginosa involves several stages, including initial attachment, microcolony formation, EPS production, and maturation [5]. The EPS matrix is composed of a variety of polysaccharides, proteins, and extracellular DNA, helping to provide structural support and protection for the bacteria within the biofilm [6]. Therefore, strategies to prevent or disrupt biofilm formation in P. aeruginosa are important for public health, particularly in healthcare settings [7].
Pseudomonas also excrete a variety of toxins that contribute to the virulence of this bacterium [8]. These include exotoxin A, which inhibits protein synthesis, and pyocyanin, which generates reactive oxygen species that can damage host cells. The bacterium can also excrete elastase, which degrades host tissues and interferes with the immune response [9,10,11]. Finally, P. aeruginosa has several mechanisms for evading the host immune system, such as producing pigments that make it difficult for the immune cells to and modifying the bacterium’s lipopolysaccharide structure in order to evade recognition by the immune system [12].
Pseudomonas can cause infections in dogs, particularly those with weakened immune systems or underlying medical conditions. Dogs can acquire P. aeruginosa infections through a variety of means, such as contact with contaminated water, soil, or surfaces [13]. The process of an infection in dogs typically involves four stages: exposure, colonization, invasion, and dissemination. Exposure occurs when a dog comes into contact with a pathogen, such as through contact with contaminated surfaces, inhalation of infected droplets, or bites from infected animals. Colonization follows, during which the pathogen multiplies and takes up residence in a specific area of the dog’s body, such as the skin, respiratory tract, or gastrointestinal tract. During this stage, the dog may not show any signs of infection. The third stage, invasion, occurs when the pathogen invades the dog’s tissues and causes inflammation, swelling, and the production of pus or other fluids. Finally, in the dissemination stage, the pathogen may spread from the initial site of infection to other parts of the body, including the bloodstream or organs. This can lead to a severe, life-threatening infection if left untreated [14]. The bacteria can cause a range of symptoms in dogs, including skin and ear infections, urinary tract infections, and respiratory infections. The treatment of P. aeruginosa is challenging because some strains have developed multidrug resistance [15]. The bacterium’s high intrinsic antibiotic resistance is caused by several factors, including low outer membrane permeability, the production and derepression of the chromosomal AmpC β-lactamase, and the presence of numerous genes coding for multidrug resistance efflux pumps [16,17,18]. P. aeruginosa is typically resistant to many antibiotics, including penicillins, first- and second-generation cephalosporins, macrolides, chloramphenicol, and some aminoglycosides (such as streptomycin, neomycin, kanamycin, and spectinomycin), tetracyclines, and sulfonamides [16,19,20]. However, some antibiotics can be effective in treating P. aeruginosa infections, such as ureidopenicillins, carboxypenicillins, third- and fourth-generation cephalosporins, carbapenems, aminoglycosides, fluoroquinolones, and polymyxins [21,22,23].
Studying the relationship between P. aeruginosa and dogs is important for several reasons. Understanding the mechanisms of resistance can help veterinarians to choose the most effective treatment options for dogs with P. aeruginosa infections [24,25]. Another point is that dogs can act as reservoirs of P. aeruginosa, potentially spreading the bacteria to other animals or humans. Thus, understanding the transmission and prevention of P. aeruginosa infections in dogs can have implications for public health, as has been suggested by others [26]. Finally, P. aeruginosa is an important model organism for studying bacterial pathogenesis and antibiotic resistance mechanisms [27]. By studying P. aeruginosa infections in dogs, researchers can gain insights into the broader mechanisms of bacterial infections and resistance, which can lead to the development of new treatments and preventative measures [1]. The objective of this research was to concentrate on the P. aeruginosa that was obtained from samples of dogs at the INNO Veterinary Laboratory. The aim was to analyze the phenotype and genotype of antimicrobial resistance.
2. Materials and Methods
2.1. Samples and Bacterial Isolates
During the period from November 2021 to December 2021, a total of 27 P. aeruginosa isolates were obtained from various pathologies at the INNO Veterinary Laboratory (Table 1). INNO is the leading reference laboratory in providing specialized services for veterinary medicine in Braga, Portugal, and all isolates in this work originate from different areas of the country. The identification of each strain was confirmed using VITEK 2® COMPACT (bioMérieux, Marcy-l′Étoile, France); additionally, they were seeded on a Pseudomonas agar base supplemented with a CN (Liofilchem, Roseto Degli, Abruzzi, Italy) medium at 37 °C for 24–48 h in the medical microbiology laboratory. The isolates were subsequently cryopreserved at −20 °C in skim milk.

Table 1.
Characteristics of the study population and collection sites of P. aeruginosa strains isolated from dogs.
2.2. Antimicrobial Susceptibility Testing
The antimicrobial susceptibilities for all P. aeruginosa isolates were determined using th4e Kirby–Bauer disk diffusion method in accordance with EUCAST standards (2022). A total of 11 antibiotics were utilized in this study, including ceftazidime (CAZ, 30 μg/disk), cefepime (FEP, 30 μg/disk), doripenem (DOR, 10 μg/disk), imipenem (IMI, 10 μg/disk), meropenem (MEM, 10 μg/disk), aztreonam (ATM, 30 μg/disk), tobramycin (TOB, 10 μg/disk), ciprofloxacin (CIP, 5 μg/disk), gentamicin (CN, 10 μg/disk), amikacin (AK, 30 μg/disk) and ticarcillin-clavulanic acid (TTC, 85 μg/disk). The selection of these antibiotics and their corresponding cut-off values were based on EUCAST 2022 (EUCAST, 2022), with the exception of ceftazidime, which was based on CLSI 2021 (CLSI, 2021) due to the differing concentration of the disk from EUCAST standards. The antibiotics enrofloxacin (ENR), marbofloxacin (MAR), ceftiofur (CEFT), and cefovecin (CEF) were assessed by VITEK 2® COMPACT (bioMérieux).
2.3. Biofilm Formation and Biomass Quantification
The bacterial adhesion of all isolates was evaluated using a microtitre plate-based assay with modifications, as previously described [28]. To perform the assay, one colony from each bacterial culture that had grown overnight on brain–heart infusion (BHI) agar was suspended in Luria–Bertani (LB) broth and incubated for 24 h at 37 °C. Then, the bacterial suspension was diluted 0.5 on the McFarland scale using tryptic soy broth (TSB). Next, 100 µL of each bacterial suspension was added to eight wells of a flat-bottomed polystyrene microtitre plate, and the plate was incubated at 37 °C for 24 h. The negative control was sterile TSB, while the positive control was Pseudomonas aeruginosa ATCC 27853® with the ability to form biofilm. After incubation, the plate was washed two times with distilled water and allowed to dry at room temperature. Then, 100 µL of crystal violet (CV) at 0.1% (v/v) was added to each well for 10–15 min. The excess stain was removed by washing the plate two times with distilled water, and then the plate was left to dry for several hours or overnight. For qualitative assays, wells were photographed when dry. To quantify the biofilm biomass, 100 µL of 30% (v/v) acetic acid was added to solubilize the CV, and the optical density was measured at 570 nm using a blank of uninoculated 30% acetic acid and a microplate reader (BioTek ELx808U, BioTek, Winooski, VT, USA).
2.4. DNA Extraction
The method used was the boil method [29]. Briefly, in order to extract DNA, two to three colonies from each P. aeruginosa isolate were collected and suspended in 500 µL of sterile distilled water. The suspension was vortexed vigorously and then subjected to a heat bath at 100 °C for 8 min. The samples were centrifuged for 2 min at 12,000 rpm and the pellets were then discarded. The total DNA concentration was determined using a NanoDrop system. The DNA concentration was calculated for each sample and subsequently adjusted to 200 μg/mL. The DFS-Taq DNA polymerase from Bioron was used. This has a range of 10–500 μg/mL.
2.5. Antimicrobial Resistance and Virulence Genes
Antimicrobial resistance genes were screened in all isolates based on their phenotypic resistance results. The genomic DNA of all bacterial samples were utilized as templates for the PCR amplification of the 16S rDNA gene, which was subsequently used to confirm the presence of P. aeruginosa. The two primers used were 27F (5′ AGAGTTTGATCCTGGCTCAG-3′) and 1495R (5′ CTACGGCTACCTTGTTACGA-3′). These functions as forward primer and reverse primer, respectively [30]. Based on their phenotypic resistance profile, each isolate underwent PCR screening for the presence of the following antimicrobial resistance genes: blaTEM, blaSHV, blaCTX, blaPER, blaSME, blaKPC, blaIMP blaSmp, blaVim, blaVim-2, blaNDM, blaOXA, aac(6′)-Ie-aph(2″)-Ia, aph(3′)-IIIa, aac(3)-I, aac(3)-II, aac(3)-III, aac(3)-IV, ant(4′)-Ia and ant(2′)-Ia. All isolates were screened for genes encoding virulence factors by PCR: pilB, pilA, aprA, toxA, tssC, plcH, lasA, lasB, lasR, lasI, exoU, exoS, exoA, exoY, exoT, rhlR, rhlI, rhlA/B and algD. The primer sequences for all genes are presented in Table 2. Positive controls were established for each gene using multiple strains from the molecular genetics’ laboratory, while Mili Q water was employed as a negative control. We utilized conventional PCR (single PCR) to detect resistance genes in accordance with the PCR cycles provided in the respective references in Table 2. On the other hand, for the detection of virulence genes, we primarily employed conventional PCR (single PCR) with the exception of the exoA, exoY, exoT gene trio, and the rhlR and rhlI genes; these were identified using multiplex PCR, with the PCR cycles specified in the corresponding references.

Table 2.
Primer sequences for PCR used to amplify the different genes.
3. Results and Discussion
Several antimicrobial agents used in both human and veterinary medicine were tested for their effectiveness against 27 clinical isolates of P. aeruginosa. While our investigation demonstrated that none of the isolates were multidrug-resistant to the tested antibiotics (i.e., resistance to three or more antibiotic classes), it is important to note that many other antibiotic classes remain to be examined. It is possible that some of these untested classes may have multidrug resistance in these isolates. This is an important finding as it suggests that the isolates that were analyzed may still be susceptible to certain antibiotics and can be treated effectively with the appropriate use of therapy. Resistance to various β-lactam antimicrobials was observed among the isolates tested. In particular, cefovecin showed resistance in 74% of the isolates and 59% of the isolates were resistant to ceftiofur (Table 3). Ceftiofur and cefovecin are both antibiotics that belong to the third-generation cephalosporin class commonly used in veterinary medicine for the treatment of bacterial infections, including in otitis in dogs in Portugal. Ceftiofur is usually administered parenterally, while cefovecin is available as an injectable long-acting formulation, with a single dose providing protection for up to 14 days. As with any antibiotic, the inappropriate use or overuse of ceftiofur and cefovecin in veterinary medicine can lead to the emergence and spread of antibiotic-resistant bacteria, including those causing otitis in dogs. Moreover, resistance to these antibiotics may already exist in bacterial populations due to their use in livestock farming. Therefore, it is essential to use these antibiotics judiciously and only when necessary on the basis of the results of diagnostic tests and under the guidance of a qualified veterinarian [51]. Imipenem demonstrated resistance in 30% of the isolates, while 26% of the isolates were resistant to meropenem. In contrast, only 4% of the isolates demonstrated resistance to ticarcillin-clavulanic acid. All isolates showed intermediate susceptibility to doripenem. Among the aminoglycosides, all strains showed susceptibility to amikacin and tobramycin. However, gentamicin showed 7% resistance in the tested isolates. The susceptibility patterns exhibited significant variation overall. Out of the three fluoroquinolone antibiotics examined, only 7% of the isolates demonstrated resistance to ciprofloxacin and enrofloxacin, whereas marbofloxacin showed resistance in only 4% of the isolates. A study conducted by Harada et al. investigated antimicrobial susceptibility and resistance mechanisms to anti-pseudomonal agents in P. aeruginosa isolates collected from dogs and cats in Japan [52]. A total of 73 P. aeruginosa isolates were collected and tested for resistance against six different antimicrobial agents: orbifloxacin, enrofloxacin, ciprofloxacin, cefotaxime, aztreonam, and gentamicin. The study found that the resistance rates against orbifloxacin, enrofloxacin, ciprofloxacin, cefotaxime, aztreonam, and gentamicin were 34.2%, 31.5%, 20.5%, 17.8%, 12.3%, and 4.1%, respectively. This study is the first report on cephalosporin- and fluoroquinolone-resistant isolates of P. aeruginosa from Japanese companion animals. The findings highlight the importance of surveillance of antimicrobial resistance in veterinary medicine and the need for appropriate antimicrobial use. Another study by Shahini et al. investigated the resistance patterns of P. aeruginosa strains isolated from different regions of Iran. For example, in Tehran, the highest levels of resistance were observed for trimethoprim (100%) and ceftazidime (80%), while imipenem (60%) and cefepime (52%) had the lowest resistance. Indifferent states of America, Pseudomonas showed the least resistance to imipenem (15%) and ciprofloxacin (20%), whereas gentamicin (50%) showed the highest resistance [53]. The findings mentioned in the statement suggest that different populations in different countries may have different resistance patterns. This can be influenced by various factors such as the usage of different antibiotics and hygiene standards. For instance, the high resistance levels observed in Tehran may be attributed to the frequent use of certain antibiotics in that region or to the poor hygiene standards found in healthcare facilities. On the other hand, the low resistance levels observed in America may be due to better adherence to infection control measures and the judicious use of antibiotics [5,53]. Overall, our study and others highlight the importance of understanding P. aeruginosa resistance patterns in different populations and regions. It also emphasizes the need for responsible use of antibiotics and strict adherence to infection control measures to prevent the emergence and spread of multidrug-resistant strains of P. aeruginosa.

Table 3.
Antimicrobial resistance phenotypes of Pseudomonas aeruginosa isolated from dogs (n = 27).
The genotypic results for the rDNA 16S genes were positive to all isolates, allowing us to conclude that all of them were P. aeruginosa, as expected. The genes blaKPC, blaCTX, blaSHV, blaSmp, blaTEM, blaOXA, blaImp, blaPER, and blaVim were tested to verify genotypic resistance to β-lactams. In terms of genotypic resistance, the blaKPC gene tested positive for all 6 isolates tested, while all the remaining genes tested negative. These results are in line with the assay carried out by Neyestanaki et al., where no blaCTX or blaSmp was detected in any of the isolates, although blaKPC was also not detected [36]. Other assays have reported isolates carrying the blaTEM, blaOXA and blaPER genes, as well as isolates carrying the blaImp and blaVim genes [36,38]. Several studies have investigated the presence of resistance genes in Pseudomonas aeruginosa isolated from dogs, and some of them have reported the absence of certain genes. For example, one study found that the genes for metallo-β-lactamase enzymes (blaImp, blaVim, and blaNDM) were not detected in any of the P. aeruginosa isolates from dogs, indicating that these strains were unlikely to be resistant to carbapenem antibiotics [54]. These findings are encouraging and suggest that there may be P. aeruginosa strains in dogs that are not as resistant to antibiotics as some human isolates. However, it is important to note that resistance patterns can vary between bacterial strains and geographic regions, and that continuous monitoring of antibiotic resistance in veterinary medicine is crucial to ensuring the effective treatment of infections in dogs and to preventing the spread of antibiotic-resistant strains [55]. The establishment of the European Antimicrobial Resistance Surveillance Network for Veterinary Pathogens (EARS-Vet) in 2005 was a critical advancement in monitoring veterinary practices. The primary objective of this network is to create a uniform approach to AMR surveillance in veterinary pathogens throughout Europe. As such, the EARS-Vet initiative represents a significant step towards comprehending the prevalence and dissemination of AMR in veterinary medicine [56]. For the remaining resistance genes, no isolates were detected. In contrast, a study by Poonsuk et al. investigated the prevalence of antibiotic resistance genes in 60 P. aeruginosa strains isolated from canine and feline infections. None of the isolates were found to contain aph(3′)-IIb, ant(2”)-Ia and aac(6′)-IIb [57]. This study was in line with the results of our study.
All of the isolates showed the presence of the oprD gene for this porin, which is consistent with the findings of Haenni et al.’s study, where 11 out of 12 isolates had an amplified oprD gene and did not undergo any mutations [58]. The significance of investigating the oprD gene in P. aeruginosa among dogs is due to its role in producing a porin that governs the entry of antibiotics into bacterial cells. In the absence or mutation of this gene, the bacteria can become immune to specific antibiotics, resulting in complications in treating infections [59,60]. In dogs, P. aeruginosa infections can be particularly challenging to manage as they can cause a range of serious diseases, including skin infections, urinary tract infections, pneumonia, and sepsis [61]. The use of antibiotics is often necessary to treat these infections, but antibiotic-resistant P. aeruginosa is becoming increasingly common, making it essential to understand the mechanisms underlying this resistance. By studying the oprD gene in this bacterium, researchers can identify strains that are more likely to be resistant to antibiotics and develop more effective treatment strategies [59].
By studying the virulence genes in P. aeruginosa, researchers can identify the specific genes that are responsible for causing disease in dogs. This information can be used to identify risk factors for infection, improve infection control practices, and develop new therapies that target specific virulence factors. Investigating the virulence genes in P. aeruginosa is essential for comprehending the pathogenesis of infections in dogs and mitigating the possibility of infection and transmission to other animals and humans. All of the isolates in this study showed amplified virulence genes, including exoS, exoA, exoT, exoY, aprA, algD, and plcH (Figure 1). Moreover, 81% of the isolates also had the toxA virulence gene amplified. The studies conducted on canine ocular infection strains revealed that more than 81% of the isolates had the virulence gene exoS, while over 92% had the virulence gene exoY, and 96% had the virulence gene exoT [62]. Similarly, in other studies on the detection of genes exoS, aprA, plcH, toxA, and lasB, all isolates showed amplification of aprA, plcH, and lasB genes, while 87.5% of the isolates had the virulence gene exoS and 91.7% had the virulence gene toxA [55]. These findings are consistent with our results. The detection of these virulence genes in P. aeruginosa is not surprising given the bacterium’s well-established reputation for possessing a wide range of virulence factors. These factors play a critical role in its remarkable adaptability and infectious potential. The exoS, exoT and exoY genes encode for an effector protein that inhibits the host’s immune response, while exoA encodes for the protein exotoxin A in order to cause tissue damage and inhibit protein synthesis in host cells [48,49]. The aprA gene encodes for the protein alkaline protease, and plcH gene encodes for the phospholipase C enzyme; these are involved in tissue degradation and can also contribute to the bacterium’s ability to evade the host immune response [44]. The algD gene encodes for an enzyme that synthesizes a polysaccharide called alginate, an important component of the biofilm produced by P. aeruginosa [43]. The plcH gene encodes for the phospholipase C enzyme involved in the degradation of host cell membranes and can also contribute to the bacterium’s ability to evade the host immune response [43].
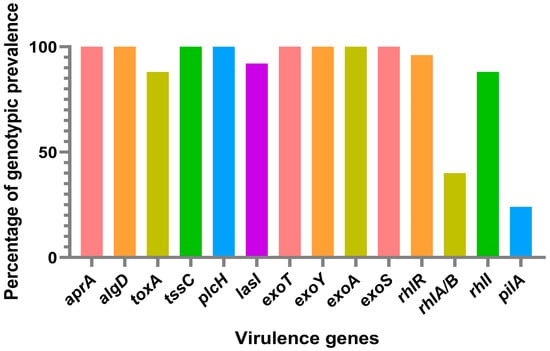
Figure 1.
The percentage of each virulence gene found within the P. aeruginosa isolates derived from dogs at the INNO Veterinary Laboratory, Braga, Portugal, between November 2021 and December 2021.
The rhII gene, which encodes for a protein involved in the regulation of the bacteria’s iron uptake system, was present in a majority of the isolates. This was the case in approximately 81.5% of the isolates. The rhIA/B gene, which encodes for enzymes involved in the biosynthesis of the O-antigen of the lipopolysaccharide, was also highly prevalent, occurring in 92.6% of the isaoltes. The pilA gene, which encodes for the structural component of type IV pili, was present in a lower percentage of the isolates, with just a 40.7% presence rate. The absence of the virulence genes lasR, lasA, rhIR, and pilB suggests that the P. aeruginosa isolates from dogs in this study may not pose a significant risk of causing severe infections. LasR and LasA are known to be involved in quorum sensing and the production of virulence factors, respectively [43,44]. RhIR is involved in the regulation of the bacteria’s response to iron starvation, while PilB is involved in the assembly of type IV pili, which are important for bacterial motility and adhesion [43]. The absence of these genes in the isolates suggests that they may be less virulent and less able to colonize and infect their host. However, P. aeruginosa is known for having a highly mutatable genome, which means that it can undergo genetic changes that may result in the acquisition of new virulence mechanisms. This bacterial species has been shown to have a remarkable ability to adapt to different environments, including the host’s tissues, where it can cause infections [2]. Therefore, even though the results of this study suggest that the P. aeruginosa isolates from dogs may not pose a significant risk of causing severe infections due to the absence of certain virulence genes, it is important to note that these bacteria are highly adaptable and can acquire new mechanisms for infection [63]. Further studies will be necessary to investigate the molecular mechanisms involved in the pathogenesis of P. aeruginosa infections in dogs and to monitor the evolution of the bacteria in response to selective pressures. Based on a literature review, there seem to be limited data available on the prevalence of the virulence genes lasR, lasA, rhIR, and pilB in P. aeruginosa isolates specifically from dogs, highlighting the importance of our study. However, there are some studies that have investigated the prevalence of these genes in P. aeruginosa isolates from other sources, such as humans and the environment. A study conducted by Yumi Park and Sun Hoe Koo investigated the prevalence of carbapenem-resistant P. aeruginosa (CRPA) in patients with urinary tract infections (UTIs), and also examined the molecular characteristics and virulence factors of the isolated strains. In terms of the prevalence of virulence genes, the study found that the genes lasR, lasA, and rhIR were present in a significant proportion of the CRPA isolates. Specifically, the lasR gene was detected in 96.7% of the isolates, the lasA gene was detected in 93.3% of the isolates, and the rhIR gene was detected in 86.7% of the isolates [64]. Another study conducted by O’Connor et al. investigated the prevalence of virulence genes in 90 environmental P. aeruginosa. They authors found that the lasR and lasA genes were present in 50% and 56% of the isolates, respectively. The rhIR gene was present in all isolates, while the pilB gene was present in 92% of the isolates [65].
The microtiter assay is the most commonly used method for the analysis of biofilm biomass due to its accuracy and reproducibility [66,67]. Biofilm formation ability was considered as positive at a cut-off level of 0.240. We determined cut-off arbitrarily using the negative control (culture medium, 0.058) plus tree standard deviations (0.01). Levels of biofilm production were established based on the following classification criteria: weak biofilm formers: 0.240 < A570 ≤ 0.481 (2 × negative controls); moderate biofilm formers: 0.481 < A570 ≤ 0.962 (4 × negative controls); strong biofilm formers: A570 > 0.962. All isolates appeared to be weak biofilm producers. This produces an interesting result because in humans, the production of biofilms is typically strong. A study by Płókarz et al. investigated the prevalence of virulence factor genes and biofilm-forming ability in P. aeruginosa isolates from dogs and cats [68]. The study aimed to identify potential biomarkers to predict biofilm formation ability and guide treatment decisions. The study found that 90.6% of P. aeruginosa isolates from dogs and 86.4% from cats were capable of biofilm formation. The most prevalent virulence factor gene in both species was ppyR, followed by pslA, fliC, nan1, and pelA. Additionally, the presence of the fliC gene was significantly associated with biofilm-forming ability in dogs, while the absence of the nan1gene was significantly associated with biofilm-forming ability in cats. These findings suggest that the detection of specific virulence genes may serve as useful biomarkers for predicting biofilm-forming ability in P. aeruginosa infections in dogs and cats. This information could potentially guide treatment decisions and improve clinical outcomes for affected animals [68]. Another study by Pye et al. evaluated the biofilm-forming capacity of P. aeruginosa isolated from canine ears and its impact on antimicrobial susceptibility. The hypothesis was that biofilm-forming capacity is common among P. aeruginosa isolates causing otitis in dogs, and that biofilm-embedded bacteria would have a higher minimal inhibitory concentration (MIC) than planktonic bacteria [69]. The findings of the study revealed that 33 out of the total isolates, equivalent to 40%, were categorized as biofilm producers. Moreover, the biofilm MICs for all four antimicrobials were significantly higher than the MICs for planktonic bacteria (p < 0.05), suggesting that biofilm-embedded bacteria are more resistant to these drugs. The study’s conclusions suggest that biofilm production is common among P. aeruginosa isolates causing otitis in dogs and that it may play a role in the pathogenesis of the disease. The higher MICs for biofilm-embedded bacteria also suggest that treatment with neomycin, polymyxin B, gentamicin, or enrofloxacin may be less effective in treating chronic otitis caused by P. aeruginosa. Thereby, dogs can serve as a model for human infections; by studying P. aeruginosa biofilms in dogs, researchers can gain a better understanding of how these structures contribute to infection and develop more effective treatments [68,70].
4. Conclusions
This study investigated the resistance patterns of 27 clinical isolates of P. aeruginosa to various antimicrobial agents used in both human and veterinary medicine. The results showed varying degrees of resistance to different antibiotics, with resistance to β-lactam antimicrobials being the most common. The study also highlighted the importance of understanding P. aeruginosa resistance patterns in different populations and regions and emphasized the need for the responsible use of antibiotics and strict adherence to infection control measures to prevent the emergence and spread of multidrug-resistant strains of P. aeruginosa. This study’s results align with previous research from various global locations indicating that P. aeruginosa resistance patterns differ based on the population, geography, and antibiotic usage. Monitoring the antimicrobial resistance patterns of P. aeruginosa is crucial in order to minimize public health problems worldwide. Surveillance of this pathogen is necessary to prevent the emergence and spread of multidrug-resistant strains, which can pose significant challenges in clinical settings. By monitoring this pathogen, we can take proactive measures to protect public health and combat antimicrobial resistance.
Author Contributions
Conceptualization, T.d.S.; methodology, T.d.S.; investigation, T.d.S.; resources A.G., A.S., R.L. and N.A.; data curation, T.d.S.; writing—original draft preparation, T.d.S.; supervision, P.P., M.H. and G.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FCT (Fundação para a Ciência e a Tecnologia) related to PhD grant, through the reference DFA/BD/5332/2020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work was supported by the projects UIDB/00772/2020 and LA/P/0059/2020 funded by the Portuguese Foundation for Science and Technology (FCT).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas aeruginosa: Pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct. Target. Ther. 2022, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, T.; Hebraud, M.; Dapkevicius, M.; Maltez, L.; Pereira, J.E.; Capita, R.; Alonso-Calleja, C.; Igrejas, G.; Poeta, P. Genomic and Metabolic Characteristics of the Pathogenicity in Pseudomonas aeruginosa. Int. J. Mol. Sci. 2021, 22, 12892. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Gdynia, A.; Tielen, P.; Rosenau, F.; Jaeger, K.E. The autotransporter esterase EstA of Pseudomonas aeruginosa is required for rhamnolipid production, cell motility, and biofilm formation. J. Bacteriol. 2007, 189, 6695–6703. [Google Scholar] [CrossRef] [PubMed]
- Rybtke, M.; Berthelsen, J.; Yang, L.; Hoiby, N.; Givskov, M.; Tolker-Nielsen, T. The LapG protein plays a role in Pseudomonas aeruginosa biofilm formation by controlling the presence of the CdrA adhesin on the cell surface. Microbiologyopen 2015, 4, 917–930. [Google Scholar] [CrossRef]
- Llor, C.; Bjerrum, L. Background for different use of antibiotics in different countries. Clin. Infect. Dis. 2005, 40, 333. [Google Scholar] [CrossRef]
- Taylor, P.K.; Yeung, A.T.; Hancock, R.E. Antibiotic resistance in Pseudomonas aeruginosa biofilms: Towards the development of novel anti-biofilm therapies. J. Biotechnol. 2014, 191, 121–130. [Google Scholar] [CrossRef]
- Hoiby, N.; Ciofu, O.; Johansen, H.K.; Song, Z.J.; Moser, C.; Jensen, P.O.; Molin, S.; Givskov, M.; Tolker-Nielsen, T.; Bjarnsholt, T. The clinical impact of bacterial biofilms. Int. J. Oral Sci. 2011, 3, 55–65. [Google Scholar] [CrossRef]
- Ma, Q.; Zhai, Y.; Schneider, J.C.; Ramseier, T.M.; Saier, M.H., Jr. Protein secretion systems of Pseudomonas aeruginosa and P fluorescens. Biochim. Biophys. Acta (BBA)-Biomembr. 2003, 1611, 223–233. [Google Scholar] [CrossRef]
- Manago, A.; Becker, K.A.; Carpinteiro, A.; Wilker, B.; Soddemann, M.; Seitz, A.P.; Edwards, M.J.; Grassme, H.; Szabo, I.; Gulbins, E. Pseudomonas aeruginosa pyocyanin induces neutrophil death via mitochondrial reactive oxygen species and mitochondrial acid sphingomyelinase. Antioxid. Redox Signal. 2015, 22, 1097–1110. [Google Scholar] [CrossRef]
- Hassett, D.J.; Charniga, L.; Bean, K.; Ohman, D.E.; Cohen, M.S. Response of Pseudomonas aeruginosa to pyocyanin: Mechanisms of resistance, antioxidant defenses, and demonstration of a manganese-cofactored superoxide dismutase. Infect. Immun. 1992, 60, 328–336. [Google Scholar] [CrossRef]
- Bleves, S.; Viarre, V.; Salacha, R.; Michel, G.P.; Filloux, A.; Voulhoux, R. Protein secretion systems in Pseudomonas aeruginosa: A wealth of pathogenic weapons. Int. J. Med. Microbiol. 2010, 300, 534–543. [Google Scholar] [CrossRef] [PubMed]
- DeBritto, S.; Gajbar, T.D.; Satapute, P.; Sundaram, L.; Lakshmikantha, R.Y.; Jogaiah, S.; Ito, S.I. Isolation and characterization of nutrient dependent pyocyanin from Pseudomonas aeruginosa and its dye and agrochemical properties. Sci. Rep. 2020, 10, 1542. [Google Scholar] [CrossRef] [PubMed]
- Dear, J.D. Bacterial Pneumonia in Dogs and Cats: An Update. Vet. Clin. Small Anim. Pract. 2020, 50, 447–465. [Google Scholar] [CrossRef]
- van Knapen, F.; Overgaauw, P. Dogs and Transmission of Infection to Man,“Respected Member of the Family?”. In Zoonoses-Infections Affecting Humans and Animals: Focus on Public Health Aspects; Springer: Dordrecht, The Netherlands, 2015; pp. 575–585. [Google Scholar]
- Haenni, M.; Hocquet, D.; Ponsin, C.; Cholley, P.; Guyeux, C.; Madec, J.Y.; Bertrand, X. Population structure and antimicrobial susceptibility of Pseudomonas aeruginosa from animal infections in France. BMC Vet. Res. 2015, 11, 9. [Google Scholar] [CrossRef]
- Sabath, L.D.; Jago, M.; Abraham, E.P. Cephalosporinase and penicillinase activities of a beta-lactamase from Pseudomonas pyocyanea. Biochem. J. 1965, 96, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Kimura, N.; Mima, T.; Mizushima, T.; Tsuchiya, T. Roles of MexXY- and MexAB-multidrug efflux pumps in intrinsic multidrug resistance of Pseudomonas aeruginosa PAO1. J. Gen. Appl. Microbiol. 2001, 47, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Johnson, N.V.; Kreamer, N.N.K.; Barnes, S.W.; Walker, J.R.; Woods, A.L.; Six, D.A.; Dean, C.R. Defects in Efflux (oprM), beta-Lactamase (ampC), and Lipopolysaccharide Transport (lptE) Genes Mediate Antibiotic Hypersusceptibility of Pseudomonas aeruginosa Strain Z61. Antimicrob. Agents Chemother. 2019, 63, e00784-19. [Google Scholar] [CrossRef] [PubMed]
- Sindeldecker, D.; Stoodley, P. The many antibiotic resistance and tolerance strategies of Pseudomonas aeruginosa. Biofilm 2021, 3, 100056. [Google Scholar] [CrossRef]
- Poole, K. Aminoglycoside resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2005, 49, 479–487. [Google Scholar] [CrossRef]
- Meletis, G.; Exindari, M.; Vavatsi, N.; Sofianou, D.; Diza, E. Mechanisms responsible for the emergence of carbapenem resistance in Pseudomonas aeruginosa. Hippokratia 2012, 16, 303–307. [Google Scholar]
- Jeannot, K.; Hagart, K.; Dortet, L.; Kostrzewa, M.; Filloux, A.; Plesiat, P.; Larrouy-Maumus, G. Detection of Colistin Resistance in Pseudomonas aeruginosa Using the MALDIxin Test on the Routine MALDI Biotyper Sirius Mass Spectrometer. Front. Microbiol. 2021, 12, 725383. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.W.; Han, M.L.; Zhao, J.; Zhu, Y.; Rao, G.; Forrest, A.; Song, J.; Kaye, K.S.; Hertzog, P.; Purcell, A.; et al. Synergistic Combination of Polymyxin B and Enrofloxacin Induced Metabolic Perturbations in Extensive Drug-Resistant Pseudomonas aeruginosa. Front. Pharmacol. 2019, 10, 1146. [Google Scholar] [CrossRef] [PubMed]
- Karruli, A.; Catalini, C.; D’Amore, C.; Foglia, F.; Mari, F.; Harxhi, A.; Galdiero, M.; Durante-Mangoni, E. Evidence-Based Treatment of Pseudomonas aeruginosa Infections: A Critical Reappraisal. Antibiotics 2023, 12, 399. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Animal Health and Welfare (AHAW); Nielsen, S.S.; Bicout, D.J.; Calistri, P.; Canali, E.; Drewe, J.A.; Garin-Bastuji, B.; Gonzales Rojas, J.L.; Gortazar, C.; Herskin, M.; et al. Assessment of listing and categorisation of animal diseases within the framework of the Animal Health Law (Regulation (EU) No 2016/429): Antimicrobial-resistant Pseudomonas aeruginosa in dogs and cats. EFSA J. 2022, 20, e07310. [Google Scholar] [CrossRef]
- Guardabassi, L.; Schwarz, S.; Lloyd, D.H. Pet animals as reservoirs of antimicrobial-resistant bacteria. J. Antimicrob. Chemother. 2004, 54, 321–332. [Google Scholar] [CrossRef]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef]
- O’Toole, G.A. Microtiter dish biofilm formation assay. J. Vis. Exp. 2011, e2437. [Google Scholar] [CrossRef]
- Dashti, A.A.; Jadaon, M.M.; Abdulsamad, A.M.; Dashti, H.M. Heat treatment of bacteria: A simple method of DNA extraction for molecular techniques. Kuwait Med. J. 2009, 41, 117–122. [Google Scholar]
- Amoon, R.H.; Abdallha, A.H.; Sharif, A.O.; Moglad, E.H.; Altyb, H.N.; Elzaki, S.G.; Salih, M.A. Molecular characterization of Pseudomonas aeruginosa isolates from Sudanese patients: A cross-sectional study. F1000Research 2018, 7, 1135. [Google Scholar] [CrossRef]
- Belaaouaj, A.; Lapoumeroulie, C.; Caniça, M.M.; Vedel, G.; Névot, P.; Krishnamoorthy, R.; Paul, G. Nucleotide sequences of the genes coding for the TEM-like β-lactamases IRT-1 and IRT-2 (formerly called TRI-1 and TRI-2). FEMS Microbiol. Lett. 1994, 120, 75–80. [Google Scholar]
- Steward, C.D.; Rasheed, J.K.; Hubert, S.K.; Biddle, J.W.; Raney, P.M.; Anderson, G.J.; Williams, P.P.; Brittain, K.L.; Oliver, A.; McGowan, J.E., Jr. Characterization of clinical isolates of Klebsiella pneumoniae from 19 laboratories using the National Committee for Clinical Laboratory Standards extended-spectrum β-lactamase detection methods. J. Clin. Microbiol. 2001, 39, 2864–2872. [Google Scholar] [CrossRef]
- Pagani, L.; Dell’Amico, E.; Migliavacca, R.; D’Andrea, M.M.; Giacobone, E.; Amicosante, G.; Romero, E.; Rossolini, G.M. Multiple CTX-M-type extended-spectrum β-lactamases in nosocomial isolates of Enterobacteriaceae from a hospital in northern Italy. J. Clin. Microbiol. 2003, 41, 4264–4269. [Google Scholar] [CrossRef]
- Weldhagen, G.F.; Poirel, L.; Nordmann, P. Ambler class A extended-spectrum β-lactamases in Pseudomonas aeruginosa: Novel developments and clinical impact. Antimicrob. Agents Chemother. 2003, 47, 2385–2392. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.S.; Kim, K.; Huh, J.Y.; Jung, B.; Kang, M.S.; Hong, S.G. Multiplex PCR for rapid detection of genes encoding class A carbapenemases. Ann. Lab. Med. 2012, 32, 359–361. [Google Scholar] [CrossRef] [PubMed]
- Neyestanaki, D.K.; Mirsalehian, A.; Rezagholizadeh, F.; Jabalameli, F.; Taherikalani, M.; Emaneini, M. Determination of extended spectrum beta-lactamases, metallo-beta-lactamases and AmpC-beta-lactamases among carbapenem resistant Pseudomonas aeruginosa isolated from burn patients. Burns 2014, 40, 1556–1561. [Google Scholar] [CrossRef]
- Ellington, M.J.; Kistler, J.; Livermore, D.M.; Woodford, N. Multiplex PCR for rapid detection of genes encoding acquired metallo-β-lactamases. J. Antimicrob. Chemother. 2007, 59, 321–322. [Google Scholar] [CrossRef]
- Amudhan, M.S.; Sekar, U.; Kamalanathan, A.; Balaraman, S. blaIMP and blaVIM mediated carbapenem resistance in Pseudomonas and Acinetobacter species in India. J. Infect. Dev. Ctries. 2012, 6, 757–762. [Google Scholar] [CrossRef]
- Franco, M.R.G.; Caiaffa-Filho, H.H.; Burattini, M.N.; Rossi, F. Metallo-beta-lactamases among imipenem-resistant Pseudomonas aeruginosa in a Brazilian university hospital. Clinics 2010, 65, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Van De Klundert, J.; Vliegenthart, J. Nomenclature of aminoglycoside resistance genes: A comment. Antimicrob. Agents Chemother. 1993, 37, 927–928. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, J.; Ju, Z.; Chang, W.; Sun, S. Molecular characterization of antimicrobial resistance in Escherichia coli from rabbit farms in Tai’an, China. BioMed Res. Int. 2018, 2018, 8607647. [Google Scholar] [CrossRef] [PubMed]
- Terzi, H.A.; Kulah, C.; Atasoy, A.R.; Ciftci, I.H. Investigation of OprD porin protein levels in carbapenem-resistant Pseudomonas aeruginosa isolates. Jundishapur J. Microbiol. 2015, 8, e25952. [Google Scholar]
- Fazeli, N.; Momtaz, H. Virulence gene profiles of multidrug-resistant Pseudomonas aeruginosa isolated from Iranian hospital infections. Iran. Red Crescent Med. J. 2014, 16, e15722. [Google Scholar] [CrossRef] [PubMed]
- Petit, S.M.-C.; Lavenir, R.; Colinon-Dupuich, C.; Boukerb, A.M.; Cholley, P.; Bertrand, X.; Freney, J.; Doléans-Jordheim, A.; Nazaret, S.; Laurent, F. Lagooning of wastewaters favors dissemination of clinically relevant Pseudomonas aeruginosa. Res. Microbiol. 2013, 164, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Khattab, M.; Nour, M.; ElSheshtawy, N.M. Genetic identification of Pseudomonas aeruginosa virulence genes among different isolates. J. Microb. Biochem. Technol. 2015, 7, 274–277. [Google Scholar]
- Zhang, L.; Hinz, A.J.; Nadeau, J.-P.; Mah, T.-F. Pseudomonas aeruginosa tssC1 links type VI secretion and biofilm-specific antibiotic resistance. J. Bacteriol. 2011, 193, 5510–5513. [Google Scholar] [CrossRef] [PubMed]
- Schaber, J.A.; Carty, N.L.; McDonald, N.A.; Graham, E.D.; Cheluvappa, R.; Griswold, J.A.; Hamood, A.N. Analysis of quorum sensing-deficient clinical isolates of Pseudomonas aeruginosa. J. Med. Microbiol. 2004, 53, 841–853. [Google Scholar] [CrossRef]
- Faraji, F.; Mahzounieh, M.; Ebrahimi, A.; Fallah, F.; Teymournejad, O.; Lajevardi, B. Molecular detection of virulence genes in Pseudomonas aeruginosa isolated from children with Cystic Fibrosis and burn wounds in Iran. Microb. Pathog. 2016, 99, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, T.; Allmond, L.R.; Sawa, T.; Wiener-Kronish, J.P. Single-nucleotide-polymorphism mapping of the Pseudomonas aeruginosa type III secretion toxins for development of a diagnostic multiplex PCR system. J. Clin. Microbiol. 2003, 41, 3526–3531. [Google Scholar] [CrossRef]
- Zhu, H.; Bandara, R.; Conibear, T.C.; Thuruthyil, S.J.; Rice, S.A.; Kjelleberg, S.; Givskov, M.; Willcox, M.D. Pseudomonas aeruginosa with lasI quorum-sensing deficiency during corneal infection. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1897–1903. [Google Scholar] [CrossRef]
- MSD Animal Health Portugal. 2023. Available online: https://www.msd-animal-health.pt/ (accessed on 20 March 2023).
- Harada, K.; Arima, S.; Niina, A.; Kataoka, Y.; Takahashi, T. Characterization of Pseudomonas aeruginosa isolates from dogs and cats in Japan: Current status of antimicrobial resistance and prevailing resistance mechanisms. Microbiol. Immunol. 2012, 56, 123–127. [Google Scholar] [CrossRef]
- Shahini, N.; Shahini, N.; Ala, S. Determining of resistance and sensitivity of Pseudomonas aeruginosa in Iran in 2010–2011. Res. Pharm. Sci. 2012, 7, 884. [Google Scholar]
- Kocsis, B.; Gulyas, D.; Szabo, D. Diversity and Distribution of Resistance Markers in Pseudomonas aeruginosa International High-Risk Clones. Microorganisms 2021, 9, 359. [Google Scholar] [CrossRef] [PubMed]
- Hattab, J.; Mosca, F.; Di, C.E.F.; Aste, G.; Marruchella, G.; Guardiani, P.; Tiscar, P.G. Occurrence, antimicrobial susceptibility, and pathogenic factors of Pseudomonas aeruginosa in canine clinical samples. Vet. World 2021, 14, 978. [Google Scholar] [CrossRef] [PubMed]
- Mader, R.; Damborg, P.; Amat, J.-P.; Bengtsson, B.; Bourély, C.; Broens, E.M.; Busani, L.; Crespo-Robledo, P.; Filippitzi, M.-E.; Fitzgerald, W. Building the European antimicrobial resistance surveillance network in veterinary medicine (EARS-Vet). Eurosurveillance 2021, 26, 2001359. [Google Scholar] [CrossRef]
- Poonsuk, K.; Chuanchuen, R. Contribution of the MexXY multidrug efflux pump and other chromosomal mechanisms on aminoglycoside resistance in Pseudomonas aeruginosa isolates from canine and feline infections. J. Vet. Med. Sci. 2012, 74, 1575–1582. [Google Scholar] [CrossRef] [PubMed]
- Haenni, M.; Bour, M.; Châtre, P.; Madec, J.-Y.; Plésiat, P.; Jeannot, K. Resistance of animal strains of Pseudomonas aeruginosa to carbapenems. Front. Microbiol. 2017, 8, 1847. [Google Scholar] [CrossRef]
- Li, H.; Luo, Y.-F.; Williams, B.J.; Blackwell, T.S.; Xie, C.-M. Structure and function of OprD protein in Pseudomonas aeruginosa: From antibiotic resistance to novel therapies. Int. J. Med. Microbiol. 2012, 302, 63–68. [Google Scholar] [CrossRef]
- Tamber, S.; Ochs, M.M.; Hancock, R.E. Role of the novel OprD family of porins in nutrient uptake in Pseudomonas aeruginosa. J. Bacteriol. 2006, 188, 45–54. [Google Scholar] [CrossRef]
- Davies, J.C.; Alton, E.; Simbo, A.; Murphy, R.; Seth, I.; Williams, K.; Somerville, M.; Jolly, L.; Morant, S.; Guest, C. Training dogs to differentiate Pseudomonas aeruginosa from other cystic fibrosis bacterial pathogens: Not to be sniffed at? Eur. Respir. J. 2019, 54, 1900970. [Google Scholar] [CrossRef]
- Ledbetter, E.C.; Mun, J.J.; Kowbel, D.; Fleiszig, S.M. Pathogenic phenotype and genotype of Pseudomonas aeruginosa isolates from spontaneous canine ocular infections. Investig. Ophthalmol. Vis. Sci. 2009, 50, 729–736. [Google Scholar] [CrossRef]
- Pottier, M.; Castagnet, S.; Gravey, F.; Leduc, G.; Sévin, C.; Petry, S.; Giard, J.-C.; Le Hello, S.; Léon, A. Antimicrobial Resistance and Genetic Diversity of Pseudomonas aeruginosa Strains Isolated from Equine and Other Veterinary Samples. Pathogens 2022, 12, 64. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Koo, S.H. Epidemiology, molecular characteristics, and virulence factors of carbapenem-resistant Pseudomonas aeruginosa isolated from patients with urinary tract infections. Infect. Drug Resist. 2022, 15, 141–151. [Google Scholar] [CrossRef]
- O’Connor, K.; Zhao, C.Y.; Diggle, S.P. Frequency of quorum sensing mutations in Pseudomonas aeruginosa strains isolated from different environments. bioRxiv 2021. [Google Scholar] [CrossRef] [PubMed]
- Abidi, S.H.; Sherwani, S.K.; Siddiqui, T.R.; Bashir, A.; Kazmi, S.U. Drug resistance profile and biofilm forming potential of Pseudomonas aeruginosa isolated from contact lenses in Karachi-Pakistan. BMC Ophthalmol. 2013, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Musken, M.; Di Fiore, S.; Romling, U.; Haussler, S. A 96-well-plate-based optical method for the quantitative and qualitative evaluation of Pseudomonas aeruginosa biofilm formation and its application to susceptibility testing. Nat. Protoc. 2010, 5, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Plokarz, D.; Czopowicz, M.; Bierowiec, K.; Rypula, K. Virulence Genes as Markers for Pseudomonas aeruginosa Biofilm Formation in Dogs and Cats. Animals 2022, 12, 422. [Google Scholar] [CrossRef]
- Pye, C.C.; Yu, A.A.; Weese, J.S. Evaluation of biofilm production by Pseudomonas aeruginosa from canine ears and the impact of biofilm on antimicrobial susceptibility in vitro. Vet. Dermatol. 2013, 24, 446-e99. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, P.; Wang, S.; Li, X.; Peng, L.; Fang, R.; Xiong, J.; Li, H.; Mei, C.; Gao, J. Pseudomonas aeruginosa biofilm dispersion by the mouse antimicrobial peptide CRAMP. Vet. Res. 2022, 53, 80. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).