The Marginal Abatement Cost of Antimicrobials for Dairy Cow Mastitis: A Bioeconomic Optimization Perspective
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bioeconomic Modeling
2.2. Strategies Tested and Calibration
2.3. Farmer’s ALEA Marginal Abatement Cost
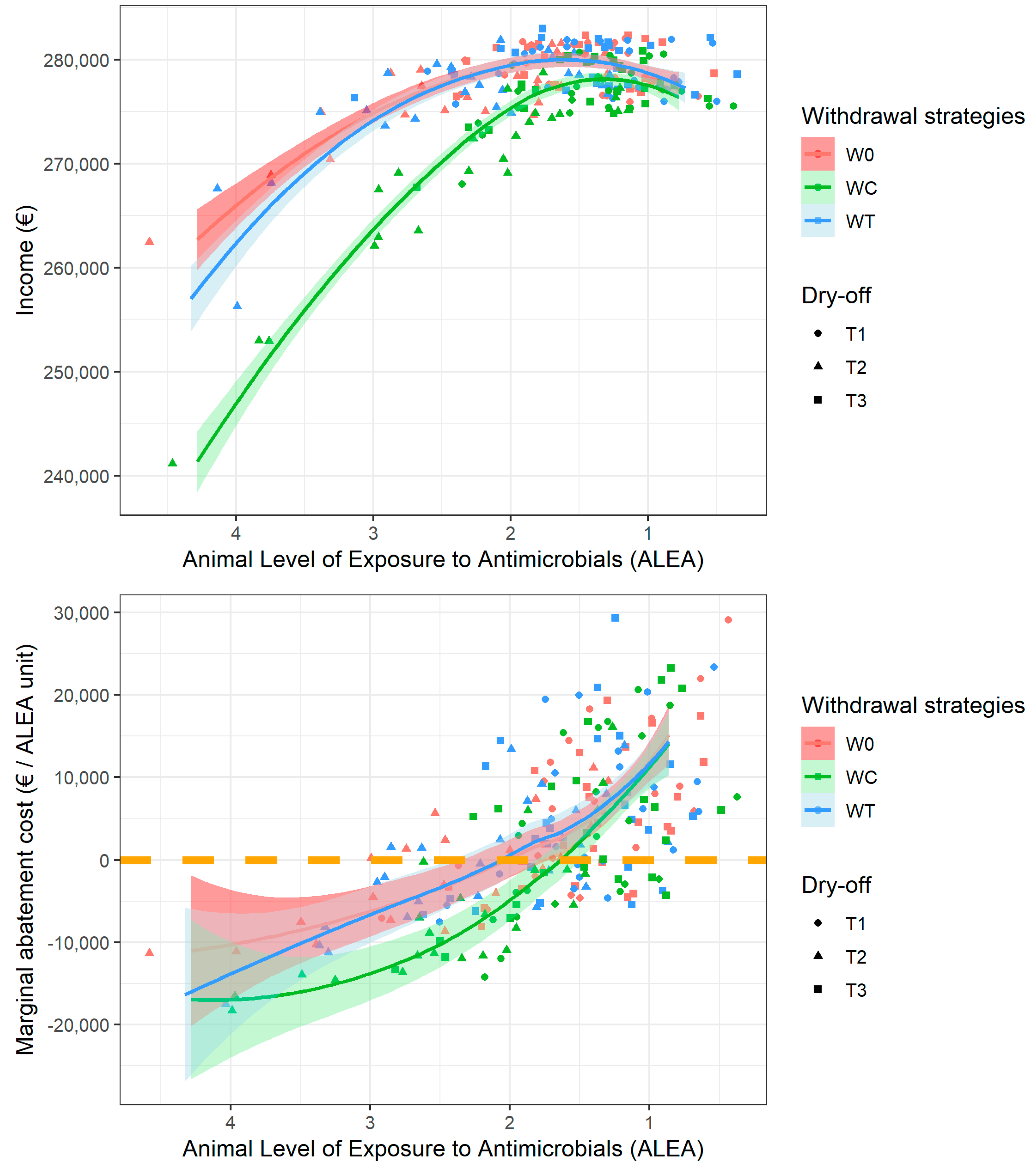
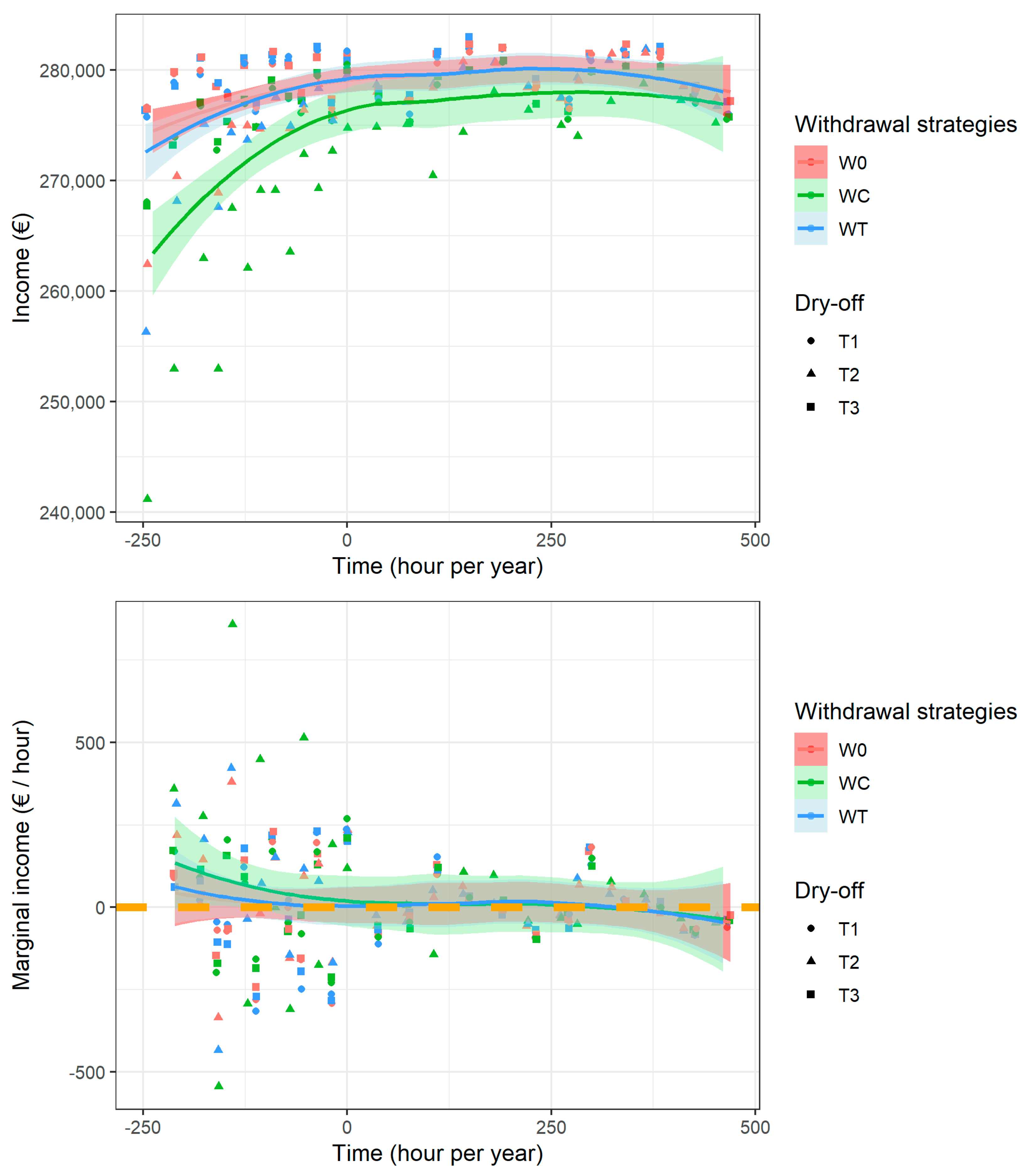
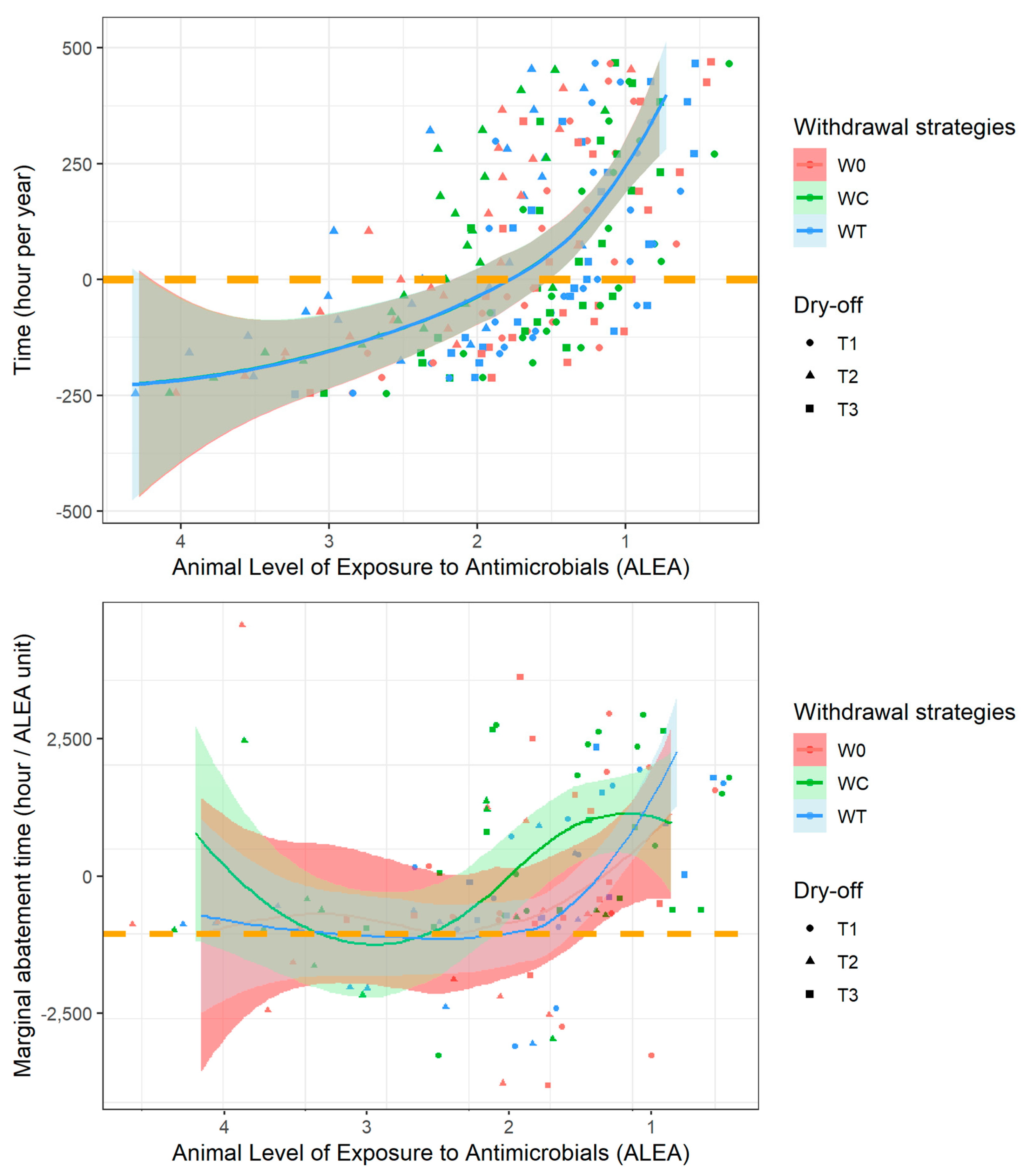
3. Results
3.1. Biological Impacts of Farmers’ Strategies
3.2. Bioeconomic Optimization to Define the Best Farmer Strategies
3.3. Marginal Abatement Curve Analysis
4. Discussion
4.1. Farmer Decision and MAC
4.2. Empirical Results and Public Policy Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raboisson, D.; Ferchiou, A.; Pinior, B.; Gautier, T.; Sans, P.; Lhermie, G. The Use of Meta-Analysis for the Measurement of Animal Disease Burden: Losses Due to Clinical Mastitis as an Example. Front. Vet. Sci. 2020, 7, 149. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ryan, C.; Barbano, D.M.; Galton, D.M.; Rudan, M.A.; Boor, K.J. Effects of Somatic Cell Count on Quality and Shelf-Life of Pasteurized Fluid Milk1. J. Dairy Sci. 2000, 83, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Urban, D.; Chevance, A.; Moulin, G. Surveillance Des Ventes de Médicaments Vétérinaires Contenant Des Antibiotiques En France En 2020; Anses, Agence nationale de sécurité sanitaire de l’alimentation, de l’environnement et du travail: Maisons-Alfort Cedex, France, 2021. [Google Scholar]
- Prouillac, C. Use of Antimicrobials in a French Veterinary Teaching Hospital: A Retrospective Study. Antibiot. Basel Switz. 2021, 10, 1369. [Google Scholar] [CrossRef] [PubMed]
- Vaarst, M.; Bennedsgaard, T.W.; Klaas, I.; Nissen, T.B.; Thamsborg, S.M.; Østergaard, S. Development and Daily Management of an Explicit Strategy of Nonuse of Antimicrobial Drugs in Twelve Danish Organic Dairy Herds. J. Dairy Sci. 2006, 89, 1842–1853. [Google Scholar] [CrossRef] [PubMed]
- Poizat, A.; Bonnet-Beaugrand, F.; Rault, A.; Fourichon, C.; Bareille, N. Antibiotic Use by Farmers to Control Mastitis as Influenced by Health Advice and Dairy Farming Systems. Prev. Vet. Med. 2017, 146, 61–72. [Google Scholar] [CrossRef]
- Ruegg, P.L. A 100-Year Review: Mastitis Detection, Management, and Prevention. J. Dairy Sci. 2017, 100, 10381–10397. [Google Scholar] [CrossRef]
- Lhermie, G.; Tauer, L.W.; Gröhn, Y.T. The Farm Cost of Decreasing Antimicrobial Use in Dairy Production. PLoS ONE 2018, 13, e0194832. [Google Scholar] [CrossRef]
- Lago, A.; Godden, S.M. Use of Rapid Culture Systems to Guide Clinical Mastitis Treatment Decisions. Mastitis 2018, 34, 389–412. [Google Scholar] [CrossRef]
- Kabera, F.; Roy, J.-P.; Afifi, M.; Godden, S.; Stryhn, H.; Sanchez, J.; Dufour, S. Comparing Blanket vs. Selective Dry Cow Treatment Approaches for Elimination and Prevention of Intramammary Infections during the Dry Period: A Systematic Review and Meta-Analysis. Front. Vet. Sci. 2021, 8, 688450. [Google Scholar] [CrossRef]
- Verbeke, J.; Piepers, S.; Supré, K.; De Vliegher, S. Pathogen-Specific Incidence Rate of Clinical Mastitis in Flemish Dairy Herds, Severity, and Association with Herd Hygiene. J. Dairy Sci. 2014, 97, 6926–6934. [Google Scholar] [CrossRef]
- Ferchiou, A.; Lhermie, G.; Raboisson, D. New Standards in Stochastic Simulations of Dairy Cow Disease Modelling: Bio-Economic Dynamic Optimization for Rational Health Management Decision-Making. Agric. Syst. 2021, 194, 103249. [Google Scholar] [CrossRef]
- Schreiner, D.A.; Ruegg, P.L. Relationship Between Udder and Leg Hygiene Scores and Subclinical Mastitis. J. Dairy Sci. 2003, 86, 3460–3465. [Google Scholar] [CrossRef]
- Hansson, H.; Szczensa-Rundberg, M.; Nielsen, C. Which Preventive Measures against Mastitis Can Increase the Technical Efficiency of Dairy Farms? Animal 2011, 5, 632–640. [Google Scholar] [CrossRef]
- Vasquez, A.K.; Nydam, D.V.; Foditsch, C.; Wieland, M.; Lynch, R.; Eicker, S.; Virkler, P.D. Use of a Culture-Independent on-Farm Algorithm to Guide the Use of Selective Dry-Cow Antibiotic Therapy. J. Dairy Sci. 2018, 101, 5345–5361. [Google Scholar] [CrossRef] [PubMed]
- Scherpenzeel, C.G.M.; den Uijl, I.E.M.; van Schaik, G.; Riekerink, R.G.M.O.; Hogeveen, H.; Lam, T.J.G.M. Effect of Different Scenarios for Selective Dry-Cow Therapy on Udder Health, Antimicrobial Usage, and Economics. J. Dairy Sci. 2016, 99, 3753–3764. [Google Scholar] [CrossRef]
- Winder, C.B.; Sargeant, J.M.; Kelton, D.F.; Leblanc, S.J.; Duffield, T.F.; Glanville, J.; Wood, H.; Churchill, K.J.; Dunn, J.; Bergevin, M.D.; et al. Comparative Efficacy of Blanket versus Selective Dry-Cow Therapy: A Systematic Review and Pairwise Meta-Analysis. Anim. Health Res. Rev. 2019, 20, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Rollin, E.; Dhuyvetter, K.C.; Overton, M.W. The Cost of Clinical Mastitis in the First 30 Days of Lactation: An Economic Modeling Tool. Prev. Vet. Med. 2015, 122, 257–264. [Google Scholar] [CrossRef]
- Stevens, M.; Piepers, S.; Supré, K.; Dewulf, J.; De Vliegher, S. Quantification of Antimicrobial Consumption in Adult Cattle on Dairy Herds in Flanders, Belgium, and Associations with Udder Health, Milk Quality, and Production Performance. J. Dairy Sci. 2016, 99, 2118–2130. [Google Scholar] [CrossRef]
- Scherpenzeel, C.G.M.; den Uijl, I.E.M.; van Schaik, G.; Olde Riekerink, R.G.M.; Keurentjes, J.M.; Lam, T.J.G.M. Evaluation of the Use of Dry Cow Antibiotics in Low Somatic Cell Count Cows. J. Dairy Sci. 2014, 97, 3606–3614. [Google Scholar] [CrossRef]
- Crispie, F.; Flynn, J.; Ross, R.P.; Hill, C.; Meaney, W.J. Dry Cow Therapy with a Non-Antibiotic Intramammary Teat Seal-A Review. Ir. Vet. J. 2004, 57, 412. [Google Scholar] [CrossRef]
- Moran, D. A Framework for Improved One Health Governance and Policy Making for Antimicrobial Use. BMJ Glob. Health 2019, 4, e001807. [Google Scholar] [CrossRef] [PubMed]
- Vliegher, S.D.; Ohnstad, I.; Piepers, S. Management and Prevention of Mastitis: A Multifactorial Approach with a Focus on Milking, Bedding and Data-Management. J. Integr. Agric. 2018, 17, 1214–1233. [Google Scholar] [CrossRef]
- Dufour, S.; Fréchette, A.; Barkema, H.W.; Mussell, A.; Scholl, D.T. Invited Review: Effect of Udder Health Management Practices on Herd Somatic Cell Count. J. Dairy Sci. 2011, 94, 563–579. [Google Scholar] [CrossRef]
- Gerber, M.; Dürr, S.; Bodmer, M. Reducing Antimicrobial Use by Implementing Evidence-Based, Management-Related Prevention Strategies in Dairy Cows in Switzerland. Front. Vet. Sci. 2021, 7, 611682. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.; Piepers, S.; De Vliegher, S. The Effect of Mastitis Management Input and Implementation of Mastitis Management on Udder Health, Milk Quality, and Antimicrobial Consumption in Dairy Herds. J. Dairy Sci. 2019, 102, 2401–2415. [Google Scholar] [CrossRef]
- van Soest, F.J.S.; Santman-Berends, I.M.G.A.; Lam, T.J.G.M.; Hogeveen, H. Failure and Preventive Costs of Mastitis on Dutch Dairy Farms. J. Dairy Sci. 2016, 99, 8365–8374. [Google Scholar] [CrossRef]
- Rushton, J. The Economics of Animal Health and Production; Cabi: Wallingford, UK, 2009; ISBN 1-84593-244-7. [Google Scholar]
- Lhermie, G.; Gröhn, Y.T.; Raboisson, D. Addressing Antimicrobial Resistance: An Overview of Priority Actions to Prevent Suboptimal Antimicrobial Use in Food-Animal Production. Front. Microbiol. 2017, 7, 2114. [Google Scholar] [CrossRef] [PubMed]
- Lhermie, G.; Verteramo Chiu, L.; Kaniyamattam, K.; Tauer, L.W.; Scott, H.M.; Gröhn, Y.T. Antimicrobial Policies in United States Beef Production: Choosing the Right Instruments to Reduce Antimicrobial Use and Resistance Under Structural and Market Constraints. Front. Vet. Sci. 2019, 6, 245. [Google Scholar] [CrossRef]
- Aarestrup, F.M. The Livestock Reservoir for Antimicrobial Resistance: A Personal View on Changing Patterns of Risks, Effects of Interventions and the Way Forward. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140085. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, C. Chapter 6-Costing Environmental Health Intervention. In Cost-Benefit Analysis of Environmental Health Interventions; Guerriero, C., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 111–127. ISBN 978-0-12-812885-5. [Google Scholar]
- Moran, D.; Lucas, A.; Barnes, A. Mitigation Win–Win. Nat. Clim. Change 2013, 3, 611–613. [Google Scholar] [CrossRef]
- Affognon, H.D. Economic Analysis of Trypanocide Use in Villages under Risk of Drug Resistance in West Africa. Ph.D. Thesis, Gottfried Wilhelm Leibniz Universität Hannover, Hannover, Germany, 2007. [Google Scholar]
- MacLeod, M.; Moran, D. Integrating Livestock Health Measures into Marginal Abatement Cost Curves. Rev. Sci. Tech.-Off. Int. Épizooties 2017, 36, 97–104. [Google Scholar] [CrossRef]
- Lhermie, G.; Tauer, L.W.; Gröhn, Y.T. An Assessment of the Economic Costs to the U.S. Dairy Market of Antimicrobial Use Restrictions. Prev. Vet. Med. 2018, 160, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Skjølstrup, N.K.; Lastein, D.B.; Jensen, C.S.; Vaarst, M. The Antimicrobial Landscape as Outlined by Danish Dairy Farmers. J. Dairy Sci. 2021, 104, 11147–11164. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.; Clift, C.; Schulze, K.; Sagan, A.; Nahrgang, S.; Mossialos, E. Averting the AMR Crisis: What Are the Avenues for Policy Action for Countries in Europe? European Observatory of Health Systems and Policies: Brussels, Belgium, 2019. [Google Scholar]
- Cobo-Angel, C.; LeBlanc, S.J.; Roche, S.M.; Ritter, C. A Focus Group Study of Canadian Dairy Farmers’ Attitudes and Social Referents on Antimicrobial Use and Antimicrobial Resistance. Front. Vet. Sci. 2021, 8, 645221. [Google Scholar] [CrossRef] [PubMed]
- Adam, C.J.M.; Fortané, N.; Ducrot, C.; Paul, M.C. Transition Pathways Toward the Prudent Use of Antimicrobials: The Case of Free-Range Broiler Farmers in France. Front. Vet. Sci. 2020, 7, 548483. [Google Scholar] [CrossRef]
- Raboisson, D.; Ferchiou, A.; Sans, P.; Lhermie, G.; Dervillé, M. The Economics of Antimicrobial Resistance in Veterinary Medicine: Optimizing Societal Benefits through Mesoeconomic Approaches from Public and Private Perspectives. One Health 2020, 10, 100145. [Google Scholar] [CrossRef]
- Van Arendonk, J.A.M. A model to estimate the performance, revenues and costs of dairy cows under different production and price situations. Agric. Syst. 1985, 16, 157–189. [Google Scholar] [CrossRef]
- Giordano, J.O.; Kalantari, A.S.; Fricke, P.M.; Wiltbank, M.C.; Cabrera, V.E. A daily herd Markov-chain model to study the reproductive and economic impact of reproductive programs combining timed artificial insemination and estrus detection. J. Dairy Sci. 2012, 95, 5442–5460. [Google Scholar] [CrossRef]
- Wood, P.D.P. Algebraic model of the lactation curve in cattle. Nature 1967, 216, 164–165. [Google Scholar] [CrossRef]
- Østergaard, S.; Chagunda, M.G.G.; Friggens, N.C.; Bennedsgaard, T.W.; Klaas, I.C. A Stochastic Model Simulating Pathogen-Specific Mastitis Control in a Dairy Herd. J. Dairy Sci. 2005, 88, 4243–4257. [Google Scholar] [CrossRef]
- Cha, E.; Kristensen, A.R.; Hertl, J.A.; Schukken, Y.H.; Tauer, L.W.; Welcome, F.L.; Gröhn, Y.T. Optimal insemination and replacement decisions to minimize the cost of pathogen-specific clinical mastitis in dairy cows. J. Dairy Sci. 2014, 97, 2101–2117. [Google Scholar] [CrossRef] [PubMed]
- Sprecher, D.J.; Hostetler, D.E.; Kaneene, J.B. A lameness scoring system that uses posture and gait to predict dairy cattle reproductive performance. Theriogenology 1997, 47, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Enting, H.; Kooij, D.; Dijkhuizen, A.A.; Huirne, R.B.M.; Noordhuizen-Stassen, E.N. Economic losses due to clinical lameness in dairy cattle. Livest. Prod. Sci. 1997, 49, 259–267. [Google Scholar] [CrossRef]
- Ostergaard, S.; Gröhn, Y.T. Effects of diseases on test day milk yield and body weight of dairy cows from Danish research herds. J. Dairy Sci. 1999, 82, 1188–1201. [Google Scholar] [CrossRef] [PubMed]
- Gröhn, Y.T.; Rajala-Schultz, P.J.; Allore, H.G.; DeLorenzo, M.A.; Hertl, J.A.; Galligan, D.T. Optimizing replacement of dairy cows: Modeling the effects of diseases. Prev. Vet. Med. 2003, 61, 27–43. [Google Scholar] [CrossRef]
- Østergaard, S.; Sørensen, J.T.; Houe, H. A stochastic model simulating milk fever in a dairy herd. Prev. Vet. Med. 2003, 58, 125–143. [Google Scholar] [CrossRef] [PubMed]
- Ettema, J.F.; Østergaard, S. Economic decision making on prevention and control of clinical lameness in Danish dairy herds. Livest. Sci. 2006, 102, 92–106. [Google Scholar] [CrossRef]
- Raboisson, D.; Mounié, M.; Maigné, E. Diseases, reproductive performance, and changes in milk production associated with subclinical ketosis in dairy cows: A meta-analysis and review. J. Dairy Sci. 2014, 97, 7547–7563. [Google Scholar] [CrossRef] [PubMed]
- Raboisson, D.; Mounié, M.; Khenifar, E.; Maigné, E. The economic impact of subclinical ketosis at the farm level: Tackling the challenge of over-estimation due to multiple interactions. Prev. Vet. Med. 2015, 122, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Mahnani, A.; Sadeghi-Sefidmazgi, A.; Cabrera, V.E. Consequences and economics of metritis in Iranian Holstein dairy farms. J. Dairy Sci. 2015, 98, 6048–6057. [Google Scholar] [CrossRef]
- Raboisson, D.; Barbier, M. Economic Synergy between Dry Cow Diet Improvement and Monensin Bolus Use to Prevent Subclinical Ketosis: An Experimental Demonstration Based on Available Literature. Front. Vet. Sci. 2017, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- de Haas, Y.; Barkema, H.W.; Veerkamp, R.F. The Effect of Pathogen-Specific Clinical Mastitis on the Lactation Curve for Somatic Cell Count. J. Dairy Sci. 2002, 85, 1314–1323. [Google Scholar] [CrossRef] [PubMed]
- Meadows, C.; Rajala-Schultz, P.J.; Frazer, G.S. A Spreadsheet-Based Model Demonstrating the Nonuniform Economic Effects of Varying Reproductive Performance in Ohio Dairy Herds. J. Dairy Sci. 2005, 88, 1244–1254. [Google Scholar] [CrossRef] [PubMed]
- Rutten, C.J.; Steeneveld, W.; Vernooij, J.C.M.; Huijps, K.; Nielen, M.; Hogeveen, H. A prognostic model to predict the success of artificial insemination in dairy cows based on readily available data. J. Dairy Sci. 2016, 99, 6764–6779. [Google Scholar] [CrossRef] [PubMed]
- Friggens, N.C.; Ingvartsen, K.L.; Emmans, G.C. Prediction of Body Lipid Change in Pregnancy and Lactation. J. Dairy Sci. 2004, 87, 988–1000. [Google Scholar] [CrossRef]
- Groenendaal, H.; Galligan, D.T.; Mulder, H.A. An Economic Spreadsheet Model to Determine Optimal Breeding and Replacement Decisions for Dairy Cattle. J. Dairy Sci. 2004, 87, 2146–2157. [Google Scholar] [CrossRef]
- De Vries, A. Economic Value of Pregnancy in Dairy Cattle. J. Dairy Sci. 2006, 89, 3876–3885. [Google Scholar] [CrossRef]
- Inchaisri, C.; Jorritsma, R.; Vos, P.L.A.M.; van der Weijden, G.C.; Hogeveen, H. Economic consequences of reproductive performance in dairy cattle. Theriogenology 2010, 74, 835–846. [Google Scholar] [CrossRef]
- Wathes, D.C.; Pollott, G.E.; Johnson, K.F.; Richardson, H.; Cooke, J.S. Heifer fertility and carry over consequences for life time production in dairy and beef cattle. Animal 2014, 8, 91–104. [Google Scholar] [CrossRef]
- Mohd Nor, N.; Steeneveld, W.; Mourits, M.C.M.; Hogeveen, H. The optimal number of heifer calves to be reared as dairy replacements. J. Dairy Sci. 2015, 98, 861–871. [Google Scholar] [CrossRef]
- Opsomer, G.; Coryn, M.; de Kruif, A. Measurement of Ovarian Cyclicity in the Post Partum Dairy Cow by Progesterone Analysis. Reprod. Domest. Anim. 1999, 34, 297–300. [Google Scholar] [CrossRef]
- de Vries, A. Economics of Delayed Replacement When Cow Performance is Seasonal. J. Dairy Sci. 2004, 87, 2947–2958. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.E.P.; Juchem, S.O.; Cerri, R.L.A.; Galvão, K.N.; Chebel, R.C.; Thatcher, W.W.; Dei, C.S.; Bilby, C.R. Effect of bST and Reproductive Management on Reproductive Performance of Holstein Dairy Cows. J. Dairy Sci. 2004, 87, 868–881. [Google Scholar] [CrossRef]
- Kristensen, E.; Østergaard, S.; Krogh, M.A.; Enevoldsen, C. Technical Indicators of Financial Performance in the Dairy Herd. J. Dairy Sci. 2008, 91, 620–631. [Google Scholar] [CrossRef]
- Cabrera, V.E. A large Markovian linear program to optimize replacement policies and dairy herd net income for diets and nitrogen excretion. J. Dairy Sci. 2010, 93, 394–406. [Google Scholar] [CrossRef]
- Ettema, J.; Østergaard, S.; Kristensen, A.R. Modelling the economic impact of three lameness causing diseases using herd and cow level evidence. Prev. Vet. Med. 2010, 95, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Inchaisri, C.; Jorritsma, R.; Vos, P.L.A.M.; van der Weijden, G.C.; Hogeveen, H. Analysis of the economically optimal voluntary waiting period for first insemination. J. Dairy Sci. 2011, 94, 3811–3823. [Google Scholar] [CrossRef]
- Kalantari, A.S.; Cabrera, V.E. The effect of reproductive performance on the dairy cattle herd value assessed by integrating a daily dynamic programming model with a daily Markov chain model. J. Dairy Sci. 2012, 95, 6160–6170. [Google Scholar] [CrossRef]
- Rutten, C.J.; Steeneveld, W.; Inchaisri, C.; Hogeveen, H. An ex ante analysis on the use of activity meters for automated estrus detection: To invest or not to invest? J. Dairy Sci. 2014, 97, 6869–6887. [Google Scholar] [CrossRef]
- Shahinfar, S.; Guenther, J.N.; David Page, C.; Kalantari, A.S.; Cabrera, V.E.; Fricke, P.M.; Weigel, K.A. Optimization of reproductive management programs using lift chart analysis and cost-sensitive evaluation of classification errors. J. Dairy Sci. 2015, 98, 3717–3728. [Google Scholar] [CrossRef]
- Sørensen, J.T.; Kristensen, E.S.; Thysen, I. A stochastic model simulating the dairy herd on a PC. Agric. Syst. 1992, 39, 177–200. [Google Scholar] [CrossRef]
- Dechow, C.D.; Goodling, R.C. Mortality, Culling by Sixty Days in Milk, and Production Profiles in High- and Low-Survival Pennsylvania Herds. J. Dairy Sci. 2008, 91, 4630–4639. [Google Scholar] [CrossRef] [PubMed]
- Korver, S. Efficiency of breeds for milk and beef production. In Proc. British Cattle Breeders Club Winter Conf., Cambridge; Academic Press: Cambridge, MA, USA, 1984. [Google Scholar]
- De Vries, A. Improved accuracy of a model to optimize breeding and replacement decisions for dairy cattle. In Proceedings of the Computers in Agriculture and Natural Resources, Orlando, FL, USA, 23–25 July 2006; p. 624. [Google Scholar]
- Margerison, J.; Downey, N. Guidelines for optimal dairy heifer rearing and herd performance. Calf Heifer Rearing Princ. Rearing Mod. Dairy Heifer Calf Calving Nottm. Univ. Press Nottm. 2005, 307–338. [Google Scholar]
- Raboisson, D.; Trillat, P.; Cahuzac, C. Failure of Passive Immune Transfer in Calves: A Meta-Analysis on the Consequences and Assessment of the Economic Impact. PloS ONE 2016, 11, e0150452. [Google Scholar] [CrossRef] [PubMed]
- Rio, O. Fréquences et Risques de Mortalité et Troubles de Santé des Veaux en Élevage Laitier; Thèse Méd. Vét.; École Nationale Vétérinaire de Nantes: Nantes, France, 1999. [Google Scholar]
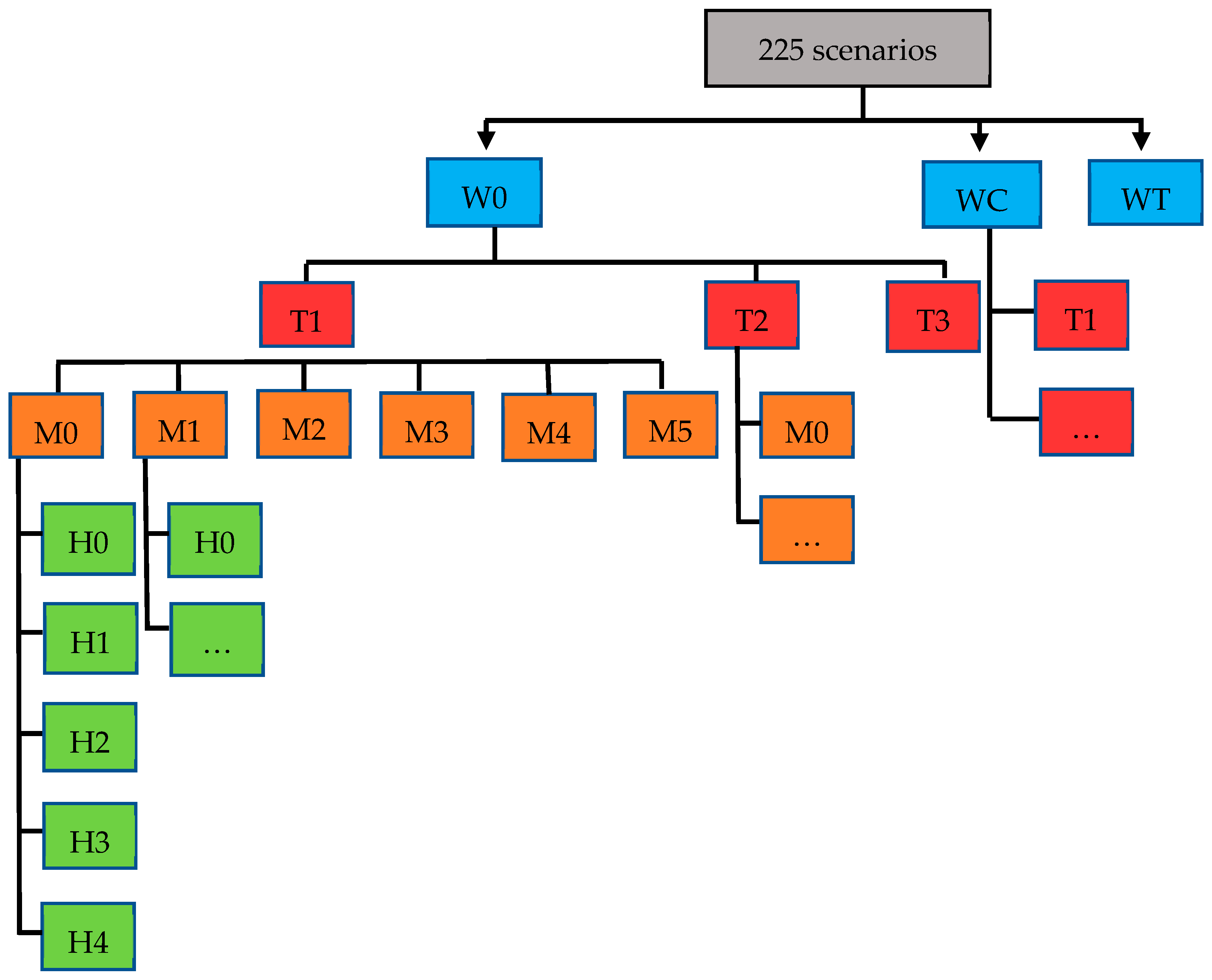
| Description | Declination as Impact | |
|---|---|---|
| W (milk withdrawal) | ||
| W0: no milk withdrawal | A cow’s milk is removed from the milk tank when it contains more than 10,000,000 SCC/mL of milk. | |
| WC: strict cow threshold (SCC) milk withdrawal strategy | A cow’s milk is removed from the milk tank when it contains more than 800,000 SCC/mL of milk. | |
| WT: mixed cow and tank threshold (SCC) milk withdrawal strategy | A cow’s milk is removed from the milk tank when it contains more than 800,000 SCC/mL of milk only if the milk tank is at more than 300,000 SCC/mL of milk. | |
| T (treatment at dry-off) | ||
| T1: common practice | Systematic antibiotic treatment at dry-off for all cows. | [19] |
| T2: simple selective antibiotic treatment | Selective antibiotic treatment at dry-off for cows that produced > 250,000 SCC/mL of milk in the previous month. | Relative risk = 2 for clinical mastitis up to 14 WIM in untreated cows (<250,000 SCC/mL of milk) compared to conventional treatment [20]. |
| T3: combined selective antibiotic treatment | Selective antibiotic treatment at dry-off of cows that produced > 250,000 SCC/mL of milk in the previous month and teat sealant for other cows. | Relative risk = 1 for cows treated with antibiotics at dry-off and for cows that received a teat sealant [21]. |
| H (housing hygiene) | ||
| H0 | Very good dairy housing hygiene (more straw for cows lactating and undergoing dry-off) and higher farmer time investment 2. | For lactating cows: 4–6 kg straw/cow/d + 12 s/cow; for cows undergoing dry-off: 5 kg straw/cow/d; relative risk 1 of clinical mastitis = 0.7. |
| H1 | Good hygienic measures (relatively more straw for lactating cows and more straw for cows undergoing dry-off) and lower farmer time investment 2. | For lactating cows: 3–5 kg straw/cow/d + 6 s/cow; for cows undergoing dry-off: 5 kg straw/cow/d; relative risk 1 of clinical mastitis = 0.8. |
| H2 | Average hygienic measures (lesser amount of straw) and the recommended farmer time investment. | For lactating cows: 2–3 kg straw/cow/d + recommended time; for dry-off cows: 3 kg straw/cow/d; relative risk 1 of clinical mastitis = 1. |
| H3 | Deteriorated hygienic measures (small amount of straw) and some farmer time savings 2. | Lactating: 1.5–3 kg straw/cow/d − 6 s/cow; dry-off: 1.5 kg straw/cow/d; relative risk 1 of clinical mastitis = 1.25. |
| H4 | Very deteriorated hygienic measures (small amount of straw) and increased farmer time savings 2. | Lactating: 1.5–3 kg straw/cow/d − 12 s/cow; 1.5 kg straw/cow/d; relative risk 1 of clinical mastitis = 1.5. |
| M (Milking parlor hygiene) | ||
| M0 | Very good hygienic measures, extra farmer time investment and increased consumable use 3. | 1 min/cow/d + EUR 0.0452; relative risk 1 of clinical mastitis = 0.7. |
| M1 | Good hygienic measures, extra farmer time investment and increased consumable use 3. | 30 s/cow/d + EUR 0.0226; relative risk 1 of clinical mastitis = 0.8. |
| M2 | Average hygienic measures, only the recommended farmer time investment and no increased consumable use 3. | Time and cost according to recommendations; relative risk 1 of clinical mastitis = 1. |
| M3 | Deteriorated hygienic measures, farmer time savings 2 and no increased consumable use 3. | −7 s/cow/d + EUR 0; relative risk 1 of clinical mastitis = 1.25. |
| M4 | Very deteriorated hygienic measures, increased farmer time savings 2 and no increased consumable use 3. | −15 s/cow/d + EUR 0; relative risk 1 of clinical mastitis = 1.5. |
| ALEA reduction constraints | ||||||
|---|---|---|---|---|---|---|
| 0% | 10% | 20% | 30% | 40% | ||
| Maximum extra time constraint. | 5 h/month | W0_T1_M2_H2 | WT_T3_M2_H1 | |||
| 10 h/month | WT_T3_M2_H0 | |||||
| 15 h/month | WT_T3_M1_H3 | |||||
| 20 h/month | WT_T3_M1_H1 | |||||
| 25 h/month | ||||||
| 30 h/month | W0_T3_M0_H3 | |||||
| 35 h/month | WT_T3_M0_H2 | WT_T3_M1_H1 | ||||
| 40 h/month | WT_T3_M0_H0 | |||||
| Unlimited | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferchiou, A.; Ndiaye, Y.; Mandour, M.A.; Herman, N.; Lhermie, G.; Raboisson, D. The Marginal Abatement Cost of Antimicrobials for Dairy Cow Mastitis: A Bioeconomic Optimization Perspective. Vet. Sci. 2023, 10, 92. https://doi.org/10.3390/vetsci10020092
Ferchiou A, Ndiaye Y, Mandour MA, Herman N, Lhermie G, Raboisson D. The Marginal Abatement Cost of Antimicrobials for Dairy Cow Mastitis: A Bioeconomic Optimization Perspective. Veterinary Sciences. 2023; 10(2):92. https://doi.org/10.3390/vetsci10020092
Chicago/Turabian StyleFerchiou, Ahmed, Youba Ndiaye, Mostafa A. Mandour, Nicolas Herman, Guillaume Lhermie, and Didier Raboisson. 2023. "The Marginal Abatement Cost of Antimicrobials for Dairy Cow Mastitis: A Bioeconomic Optimization Perspective" Veterinary Sciences 10, no. 2: 92. https://doi.org/10.3390/vetsci10020092
APA StyleFerchiou, A., Ndiaye, Y., Mandour, M. A., Herman, N., Lhermie, G., & Raboisson, D. (2023). The Marginal Abatement Cost of Antimicrobials for Dairy Cow Mastitis: A Bioeconomic Optimization Perspective. Veterinary Sciences, 10(2), 92. https://doi.org/10.3390/vetsci10020092






