Reduction in Rumen Tetracycline-Insensitive Bacteria during a Grain Challenge Using the Isoflavone Biochanin A
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Media and Culture Techniques
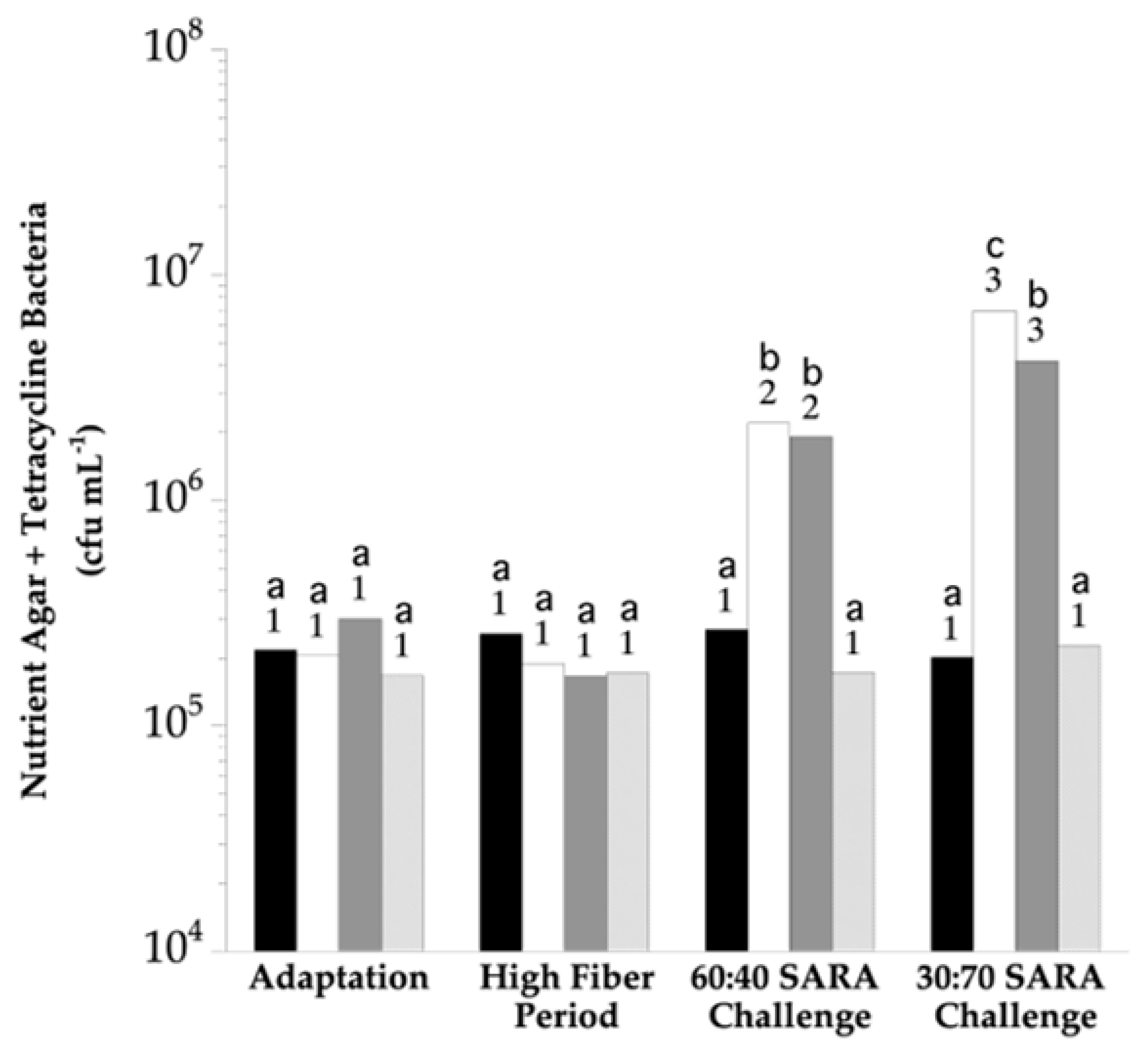
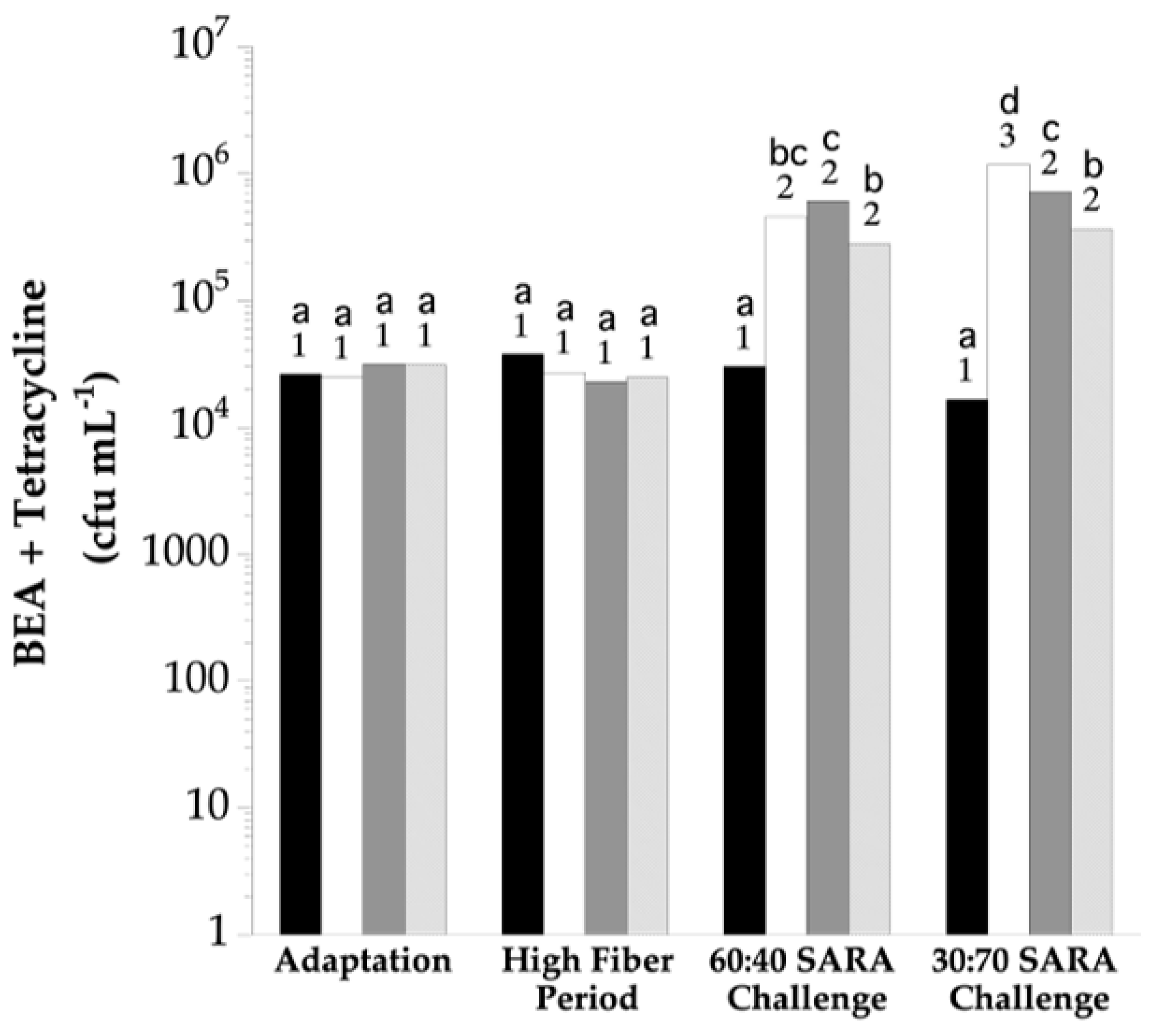
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Callaway, T.R.; Edrington, T.S.; Rychlik, J.L.; Genovese, K.J.; Poole, T.L.; Jung, Y.S.; Bischoff, K.M.; Anderson, R.C.; Nisbet, D.J. Ionophores: Their use as ruminant growth promotants and impact on food safety. Curr. Issues Intest. Microbiol. 2003, 4, 43–51. [Google Scholar]
- Russell, J.B.; Strobel, H.J. Effect of ionophores on ruminal fermentation. Appl. Environ. Microbiol. 1989, 55, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, L.O.; Fox, D.G.; Tylutki, T.P. Potential Environmental Benefits of Ionophores in Ruminant Diets. J. Environ. Qual. 2003, 32, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Allen, H.K.; Stanton, T.B. Altered Egos: Antibiotic Effects on Food Animal Microbiomes. Annu. Rev. Microbiol. 2014, 68, 297–315. [Google Scholar] [CrossRef] [PubMed]
- Economou, V.; Gousia, P. Agriculture and food animals as a source of antimicrobial-resistant bacteria. Infect. Drug Resist. 2015, 8, 49–61. [Google Scholar] [CrossRef]
- Doyle, M.E. Multidrug-Resistant Pathogens in the Food Supply. Foodborne Pathog. Dis. 2015, 12, 261–279. [Google Scholar] [CrossRef]
- Ceccarelli, I.; Bioletti, L.; Peparini, S.; Solomita, E.; Ricci, C.; Casini, I.; Miceli, E.; Aloisi, A.M. Estrogens and phytoestrogens in body functions. Neurosci. Biobehav. Rev. 2022, 132, 648–663. [Google Scholar] [CrossRef] [PubMed]
- Morel, C.; Stermitz, F.R.; Tegos, G.; Lewis, K. Isoflavones as Potentiators of Antibacterial Activity. J. Agric. Food Chem. 2003, 51, 5677–5679. [Google Scholar] [CrossRef]
- Lechner, D.; Gibbons, S.; Bucar, F. Plant phenolic compounds as ethidium bromide efflux inhibitors in Mycobacterium smegmatis. J. Antimicrob. Chemother. 2008, 62, 345–348. [Google Scholar] [CrossRef]
- Liu, G.; Liang, J.C.; Wang, X.L.; Li, Z.H.; Wang, W.; Guo, N.; Wu, X.P.; Shen, F.G.; Xing, M.X.; Liu, L.H.; et al. In vitro synergy of biochanin A and ciprofloxacin against clinical isolates of Staphylococcus aureus. Molecules 2011, 16, 6656–6666. [Google Scholar] [CrossRef]
- Lalouckova, K.; Mala, L.; Marsik, P.; Skrivanova, E. In vitro antibacterial effect of the methanolic extract of the Korean soybean fermented product doenjang against Staphylococcus aureus. Animals 2021, 11, 2319. [Google Scholar] [CrossRef]
- Lu, W.-J.; Huang, Y.-J.; Lin, H.-J.; Chang, C.-J.; Hsu, P.-H.; Ooi, G.-X.; Huang, M.-Y.; Lin, H.-T.V. Phenolic Compound Ethyl 3,4-Dihydroxybenzoate Retards Drug Efflux and Potentiates Antibiotic Activity. Antibiotics 2022, 11, 497. [Google Scholar] [CrossRef] [PubMed]
- Guz, N.R.; Stermitz, F.R.; Johnson, J.B.; Beeson, T.D.; Willen, S.; Hsiang, J.-F.; Lewis, K. Flavonolignan and flavone inhibitors of a Staphylococcus aureus multidrug resistance pump: Structure-activity relationships. J. Med. Chem. 2001, 44, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-Z.; He, X.-G.; Lindenmaier, M.; Yang, J.; Cleary, M.; Qiu, S.-X.; Cordell, G.A. LC-ESI-MS Study of the flavonoid glycoside malonates of red clover (Trifolium pratense). J. Agric. Food Chem. 2000, 48, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Flythe, M.; Kagan, I. Antimicrobial effect of red clover (Trifolium pratense) phenolic extract on the ruminal hyper ammonia-producing bacterium, clostridium sticklandii. Curr. Microbiol. 2010, 61, 125–131. [Google Scholar] [CrossRef]
- Flythe, M.D.; Harrison, B.; Kagan, I.A.; Klotz, J.L.; Gellin, G.L.; Goff, B.M.; Aiken, G.E. Antimicrobial activity of red clover (Trifolium pratense L.) extract on caprine hyper-ammonia-producing bacteria. Agric. Food Anal. Bacteriol. 2013, 3, 176–185. [Google Scholar]
- Harlow, B.E.; Flythe, M.D.; Kagan, I.A.; Aiken, G.E. Biochanin A (an isoflavone produced by red clover) promotes weight gain of steers grazed in mixed grass pastures and fed dried-distillers’ grains. Crop Sci. 2017, 57, 506–514. [Google Scholar] [CrossRef]
- Melchior, E.A.; Smith, J.K.; Schneider, L.G.; Mulliniks, J.T.; Bates, G.E.; Flythe, M.D.; Klotz, J.L.; Ji, H.; Goodman, J.P.; Lee, A.R.; et al. Effects of endophyte-infected tall fescue seed and red clover isoflavones on rumen microbial populations and physiological parameters of beef cattle1,2. Transl. Anim. Sci. 2018, 3, 315–328. [Google Scholar] [CrossRef]
- Harlow, B.E.; Flythe, M.D.; Kagan, I.A.; Goodman, J.P.; Klotz, J.L.; Aiken, G.E. Isoflavone supplementation, via red clover hay, alters the rumen microbial community and promotes weight gain of steers grazing mixed grass pastures. PLoS ONE 2020, 15, e0229200. [Google Scholar] [CrossRef]
- Ault-Seay, T.B.; Melchior-Tiffany, E.A.; Clemmons, B.A.; Cordero, J.F.; Bates, G.E.; Flythe, M.D.; Klotz, J.L.; Ji, H.; Goodman, J.P.; McLean, K.J.; et al. Rumen and serum metabolomes in response to endophyte-infected tall fescue seed and isoflavone supplementation in beef steers. Toxins 2020, 12, 744. [Google Scholar] [CrossRef]
- Auffret, M.D.; Dewhurst, R.J.; Duthie, C.A.; Rooke, J.A.; John Wallace, R.; Freeman, T.C.; Stewart, R.; Watson, M.; Roehe, R. The rumen microbiome as a reservoir of antimicrobial resistance and pathogenicity genes is directly affected by diet in beef cattle. Microbiome 2017, 5, 159. [Google Scholar] [CrossRef] [PubMed]
- Federation of Animal Science Societies. Guide for the Care and Use of Agricultural Animals in Research and Teaching, 3rd ed.; Federation of Animal Science Societies: Champaign, IL, USA, 2010. [Google Scholar]
- Harlow, B.E.; Flythe, M.D.; Klotz, J.L.; Harmon, D.L.; Aiken, G.E. Effect of biochanin a on the rumen microbial community of holstein steers consuming a high fiber diet and subjected to a subacute acidosis challenge. PLoS ONE 2021, 16, e0253754. [Google Scholar] [CrossRef] [PubMed]
- Baquero, F.; Alvarez-Ortega, C.; Martinez, J.L. Ecology and evolution of antibiotic resistance. Environ. Microbiol. Rep. 2009, 1, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Atlas, R.M. Handbook of Microbiological Media, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Paine, T.F.; Collins, H.S.; Finland, M. Bacteriologic studies on aureomycin. J. Bacteriol. 1948, 56, 489–497. [Google Scholar] [CrossRef]
- Nagaraja, T.G.; Titgemeyer, E.C. Ruminal acidosis in beef cattle: The current microbiological and nutritional outlook. J. Dairy Sci. 2007, 90, E17–E38. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Dai, C.; Shen, Z.; Tang, Q.; Wang, H.; Zhai, B.; Zhao, L.; Hao, Z. Mechanism of synergy between tetracycline and quercetin against antibiotic resistant Escherichia coli. Front. Microbiol. 2019, 10, 2536. [Google Scholar] [CrossRef]
- Harmon, D.L. Grand challenge in animal nutrition. Front. Anim. Sci. 2020, 1, 621638. [Google Scholar] [CrossRef]
- Harlow, B.E.; Flythe, M.D.; Aiken, G.E. Effect of biochanin A on corn grain (Zea mays) fermentation by bovine rumen amylolytic bacteria. J. Appl. Microbiol. 2017, 122, 870–880. [Google Scholar] [CrossRef]
- Aiken, G.E.; Flythe, M.D.; Kagan, I.A.; Ji, H.; Bush, L.P. Mitigation of ergot vasoconstriction by clover isoflavones in goats (Capra hircus). Front. Vet. Sci. 2016, 3, 17. [Google Scholar] [CrossRef]
- Harlow, B.E.; Flythe, M.D.; Goodman, J.P.; Ji, H.; Aiken, G.E. Isoflavone containing legumes mitigate ergot alkaloid-induced vasoconstriction in goats (Capra hircus). Animals 2022, 12, 750. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flythe, M.D.; Davis, B.E.; Kagan, I.A. Reduction in Rumen Tetracycline-Insensitive Bacteria during a Grain Challenge Using the Isoflavone Biochanin A. Vet. Sci. 2023, 10, 273. https://doi.org/10.3390/vetsci10040273
Flythe MD, Davis BE, Kagan IA. Reduction in Rumen Tetracycline-Insensitive Bacteria during a Grain Challenge Using the Isoflavone Biochanin A. Veterinary Sciences. 2023; 10(4):273. https://doi.org/10.3390/vetsci10040273
Chicago/Turabian StyleFlythe, Michael D., Brittany E. Davis, and Isabelle A. Kagan. 2023. "Reduction in Rumen Tetracycline-Insensitive Bacteria during a Grain Challenge Using the Isoflavone Biochanin A" Veterinary Sciences 10, no. 4: 273. https://doi.org/10.3390/vetsci10040273
APA StyleFlythe, M. D., Davis, B. E., & Kagan, I. A. (2023). Reduction in Rumen Tetracycline-Insensitive Bacteria during a Grain Challenge Using the Isoflavone Biochanin A. Veterinary Sciences, 10(4), 273. https://doi.org/10.3390/vetsci10040273





