Figure 1.
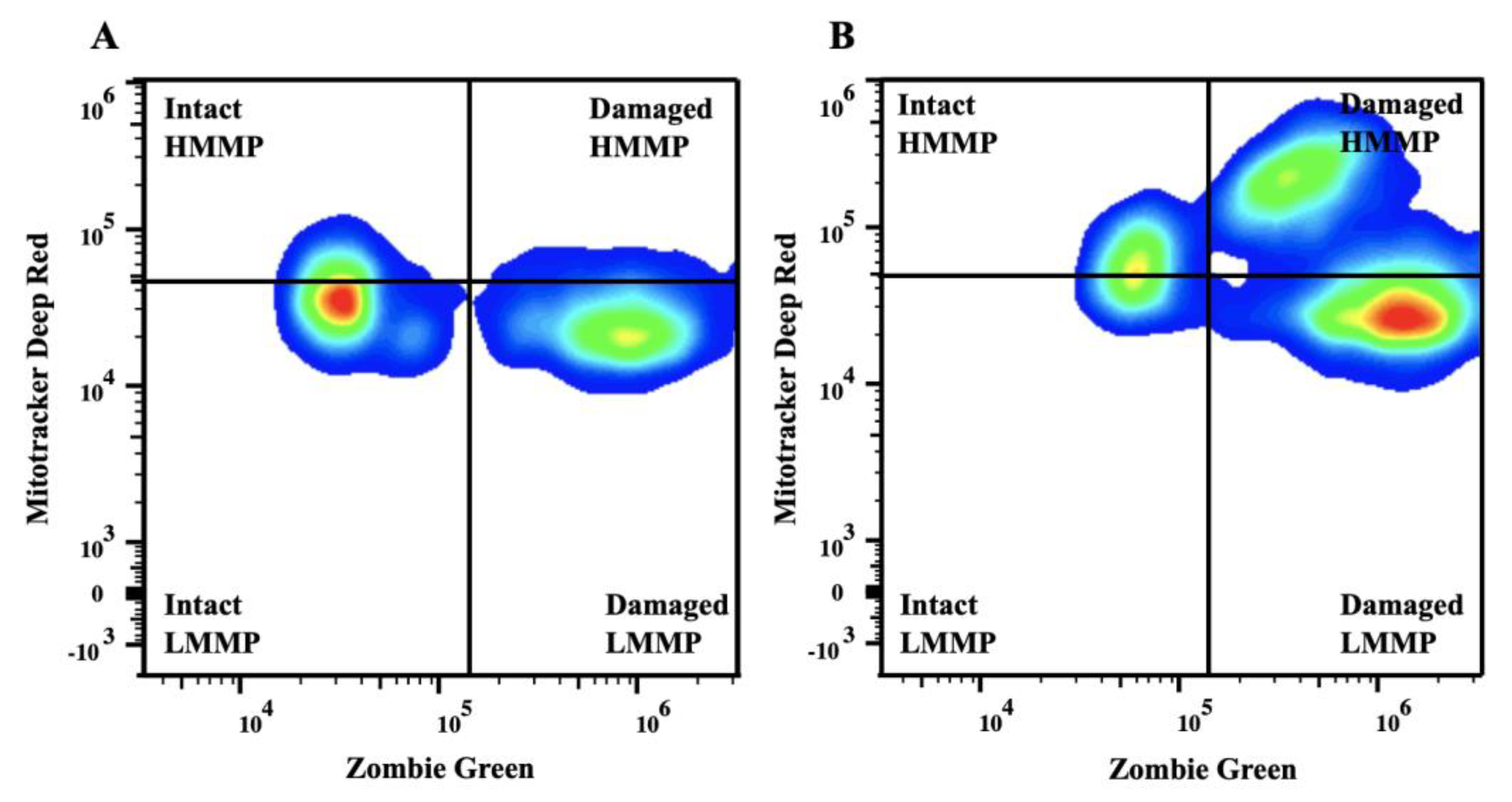
Representative flow cytometry plots of semen collected from a breeding inactive stallion before (A) and after (B) freezing. Sperm were stained with Zombie Green and MitoTracker Deep Red. The upper quadrants represent intact (left) and damaged (right) sperm with high mitochondrial membrane potential. The lower quadrants represent intact (left) and damaged (right) sperm with low mitochondrial membrane potential. Abbreviations: Intact, sperm with intact plasma membrane; HMMP, intact sperm with high mitochondrial membrane potential; LMMP, intact sperm with low mitochondrial membrane potential.
Figure 1.
Representative flow cytometry plots of semen collected from a breeding inactive stallion before (A) and after (B) freezing. Sperm were stained with Zombie Green and MitoTracker Deep Red. The upper quadrants represent intact (left) and damaged (right) sperm with high mitochondrial membrane potential. The lower quadrants represent intact (left) and damaged (right) sperm with low mitochondrial membrane potential. Abbreviations: Intact, sperm with intact plasma membrane; HMMP, intact sperm with high mitochondrial membrane potential; LMMP, intact sperm with low mitochondrial membrane potential.
Figure 2.
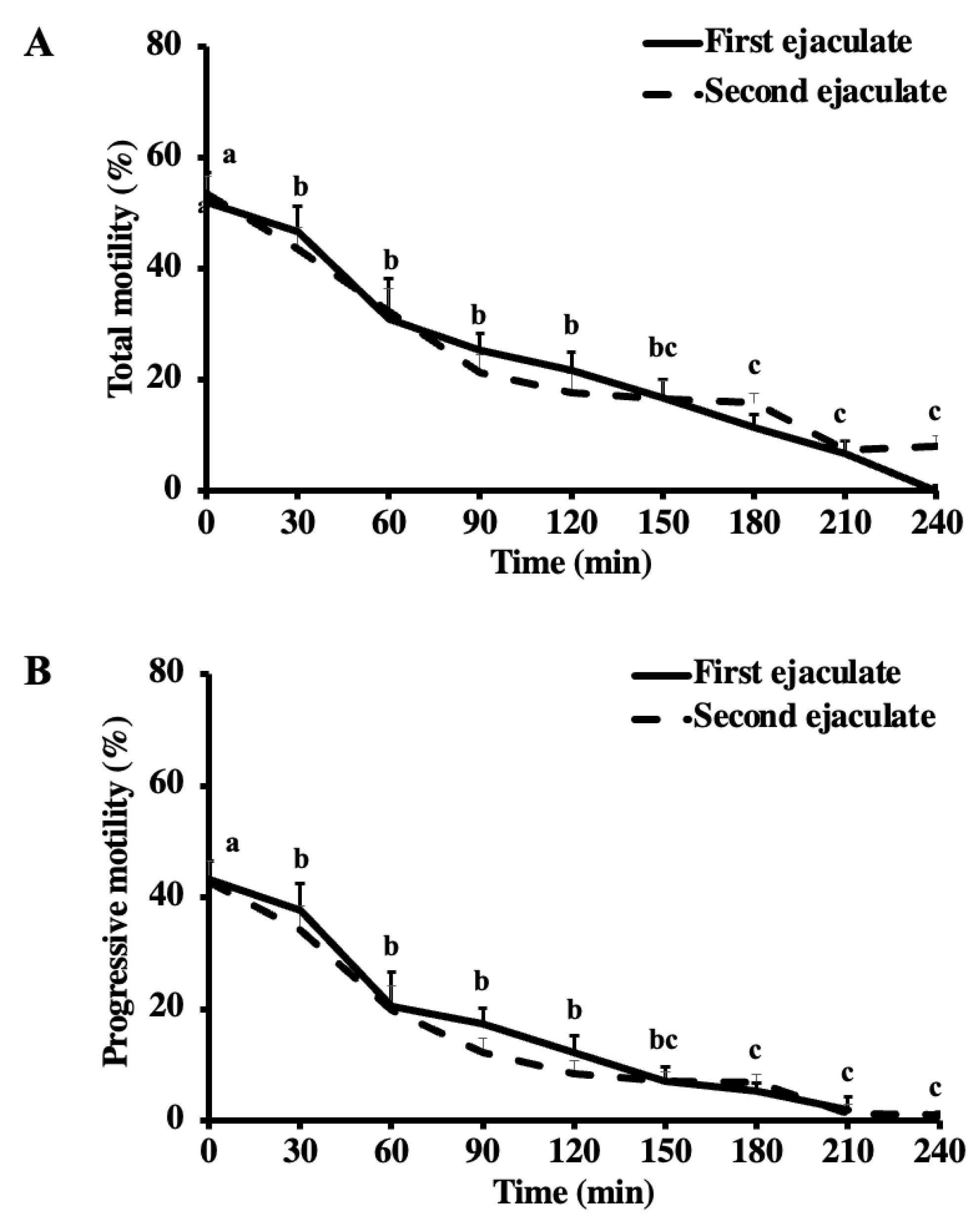
Total (A) and progressive (B) motility of the first and second ejaculates from breeding inactive stallions (n = 20) during the thermal longevity test. The frozen-thawed semen was incubated at 37 °C for 240 min. Different superscripts (abc) denote a difference between columns (p < 0.05).
Figure 2.
Total (A) and progressive (B) motility of the first and second ejaculates from breeding inactive stallions (n = 20) during the thermal longevity test. The frozen-thawed semen was incubated at 37 °C for 240 min. Different superscripts (abc) denote a difference between columns (p < 0.05).
Figure 3.
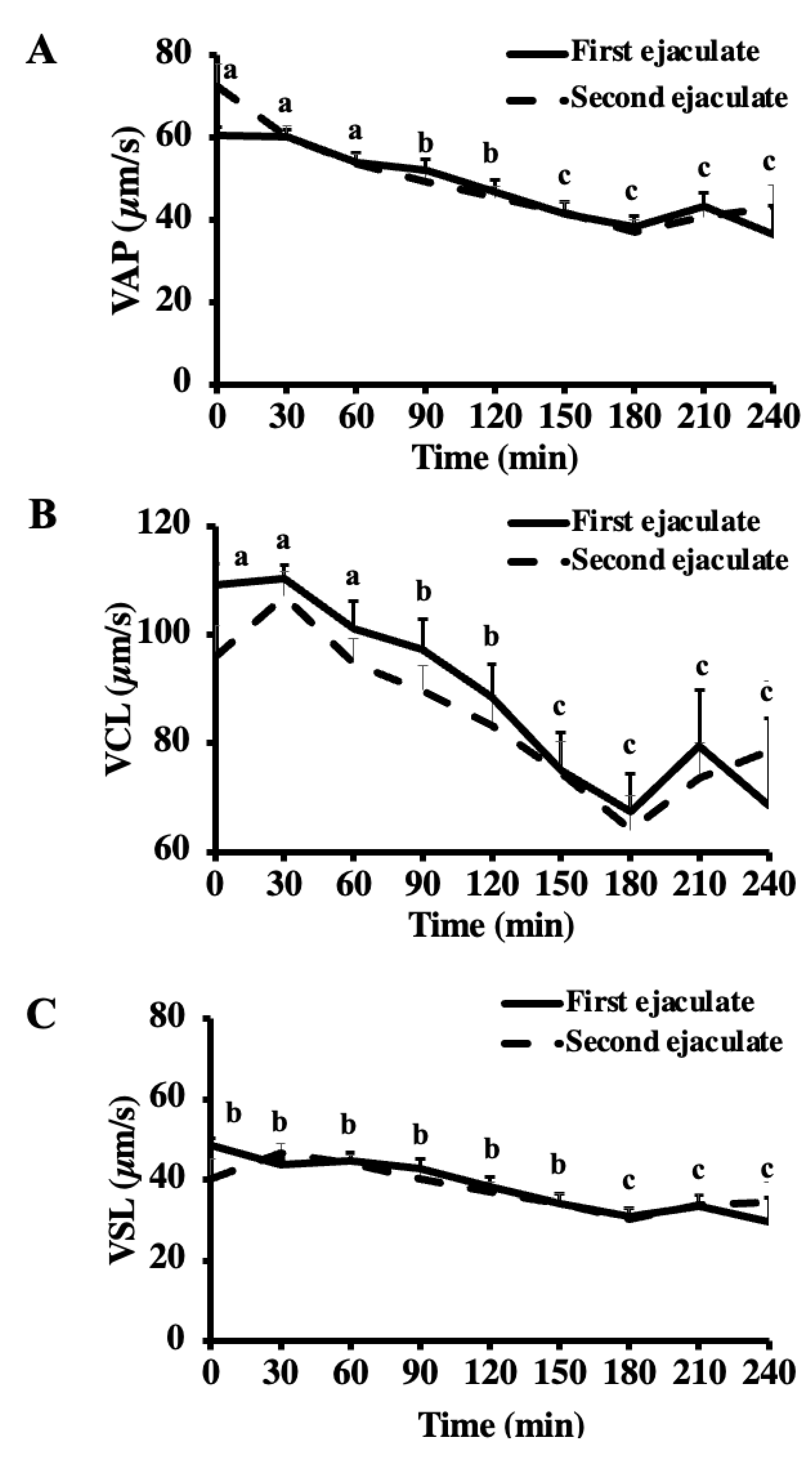
Velocity parameters ((A) VAP; (B) VCL; (C) VSL) of the first and second ejaculates collected from breeding inactive stallions (n = 20). The frozen-thawed semen was incubated at 37 °C for 240 min. Different superscripts (abc) denote a difference between columns (p < 0.05).
Figure 3.
Velocity parameters ((A) VAP; (B) VCL; (C) VSL) of the first and second ejaculates collected from breeding inactive stallions (n = 20). The frozen-thawed semen was incubated at 37 °C for 240 min. Different superscripts (abc) denote a difference between columns (p < 0.05).
Figure 4.
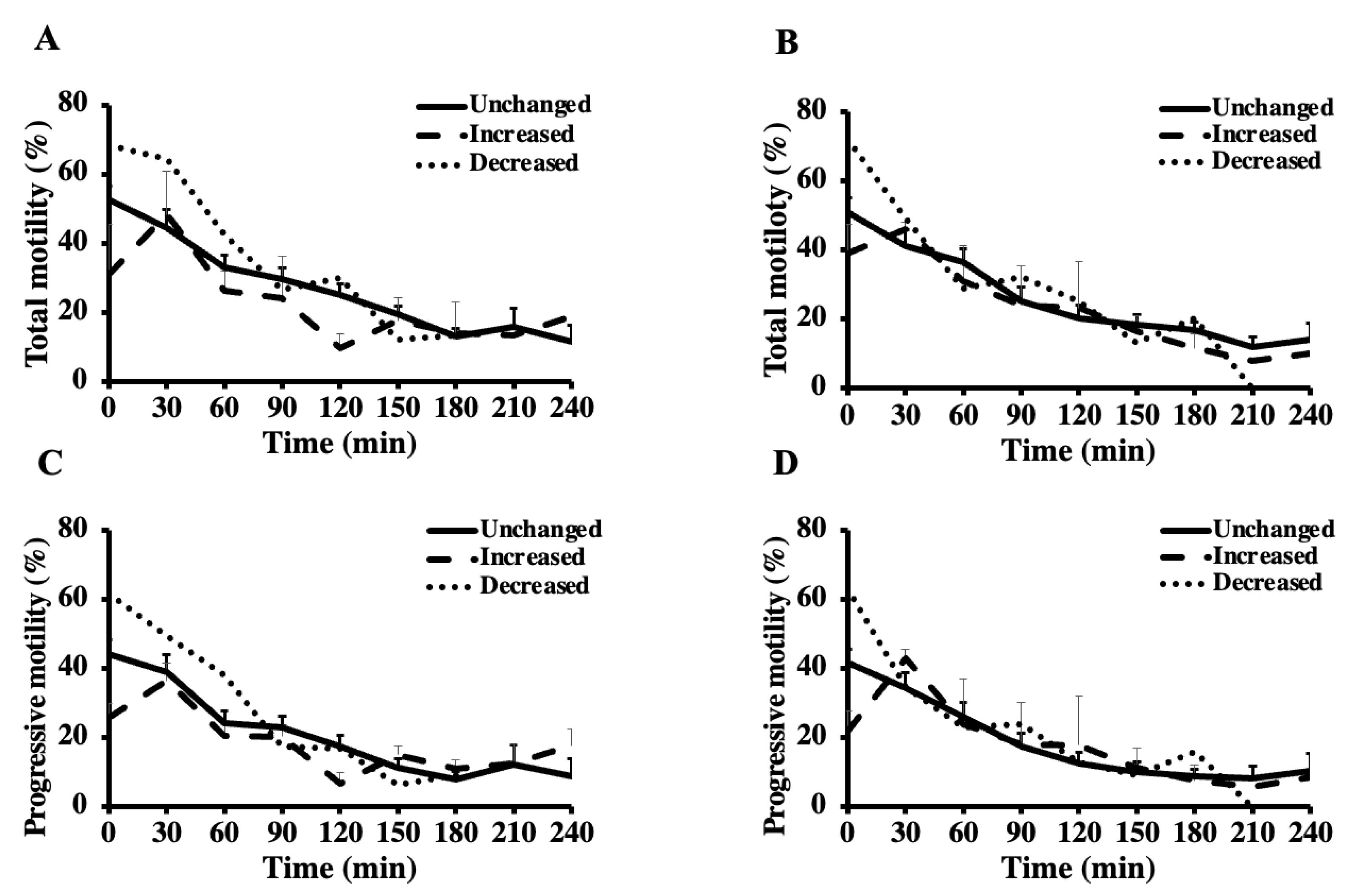
Total (A,B) and progressive (C,D) motility of the first (A,C) and second (B,D) ejaculates from breeding inactive stallions (n = 20) during a thermal longevity test. The frozen-thawed semen was incubated at 37 °C for 240 min. Based on the motility parameters between the first and second ejaculates, sixteen stallions were classified as unchanged (i.e., ≤10% change), three stallions as increased (i.e., improvement > 10%) group, and one stallion as decreased (i.e., reduction > 10%).
Figure 4.
Total (A,B) and progressive (C,D) motility of the first (A,C) and second (B,D) ejaculates from breeding inactive stallions (n = 20) during a thermal longevity test. The frozen-thawed semen was incubated at 37 °C for 240 min. Based on the motility parameters between the first and second ejaculates, sixteen stallions were classified as unchanged (i.e., ≤10% change), three stallions as increased (i.e., improvement > 10%) group, and one stallion as decreased (i.e., reduction > 10%).
Table 1.
Signalment, history, and reason for the presentation of the 20 breeding inactive stallions.
Table 1.
Signalment, history, and reason for the presentation of the 20 breeding inactive stallions.
| Stallion | Breed | Age (Years) | Pertinent Breeding History | Reason for Presentation |
|---|
| 1 | Quarter Horse | 9 | Low sperm count, seminal plasma toxicity | Washout collections |
| 2 | Quarter Horse | 13 | Fertile | Washout collections |
| 3 | Quarter Horse | 11 | Fertile | Washout collections |
| 4 | Quarter Horse | 23 | Used for live cover | Semen freezing |
| 5 | Paint Horse | 5 | Maiden | Training to collect semen |
| 6 | Quarter Horse | 15 | Fertile | Washout collections |
| 7 | Standardbred | 11 | Fertile | Washout collections |
| 8 | Standardbred | 22 | Fertile | Washout collections |
| 9 | Paint Horse | 14 | Low sperm count, seminal plasma toxicity | Washout collections |
| 10 | Quarter Horse | 7 | Fertile | |
| 11 | Standardbred | 11 | Sperm accumulator, seminal plasma toxicity | Washout collections |
| 12 | Standardbred | 15 | Fertile | Washout collections |
| 13 | Standardbred | 4 | Low fertility and inconsistent breeder | Washout collections |
| 14 | Standardbred | 6 | Fertile | Washout collections |
| 15 | Standardbred | 9 | Fertile | Washout collections |
| 16 | Arabian | 21 | Fertile | Washout collections |
| 17 | Arabian | 19 | Fertile | Washout collections |
| 18 | Quarter Horse | 13 | Fertile | Washout collections |
| 19 | Andalusian | 8 | Unknown | Semen shipping |
| 20 | Standardbred | 14 | Fertile | Semen shipping |
Table 2.
Gel-free semen volume, sperm concentration, and total number of sperm of raw semen from the first (n = 20) and second ejaculates (n = 20) of breeding inactive stallions (n = 20).
Table 2.
Gel-free semen volume, sperm concentration, and total number of sperm of raw semen from the first (n = 20) and second ejaculates (n = 20) of breeding inactive stallions (n = 20).
| | First Ejaculate | Second Ejaculate |
|---|
| | Mean ± SEM | CI-95% | Mean ± SEM | CI-95% |
|---|
| Gel-free volume (mL) | 83.5 ± 8.1 a | 66.5–100.5 | 68.8 ± 5.0 b | 58.3–79.4 |
| Sperm concentration (million/mL) | 245 ± 33.8 a | 174.4–316.1 | 146.0 ± 28.6 b | 86.1–205.7 |
| Total number of sperm (billion) | 17.8 ± 2.5 a | 12.3–23.1 | 10.3 ± 3.9 b | 4.0–16.0 |
Table 3.
Gel-free semen volume, sperm concentration, and total number of sperm of raw semen from the first and second ejaculates of breeding inactive stallions (n = 20). Based on the motility parameters between the first and second ejaculates, sixteen stallions were classified as unchanged (i.e., ≤10% change), three stallions as increased (i.e., improvement > 10%) group, and one stallion as decreased (i.e., reduction > 10%).
Table 3.
Gel-free semen volume, sperm concentration, and total number of sperm of raw semen from the first and second ejaculates of breeding inactive stallions (n = 20). Based on the motility parameters between the first and second ejaculates, sixteen stallions were classified as unchanged (i.e., ≤10% change), three stallions as increased (i.e., improvement > 10%) group, and one stallion as decreased (i.e., reduction > 10%).
| | Unchanged (n = 16) | Increased (n = 3) | Decreased (n = 1) |
|---|
| | First Ejaculate | Second Ejaculate | First Ejaculate | Second Ejaculate | First Ejaculate | Second Ejaculate |
|---|
| | Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | | |
|---|
| Gel-free volume (mL) | 79.3 ± 7.8 | 67.5 ± 5.3 | 124.0 ± 19.9 | 62.0 ± 12.5 | 30 | 37 |
| Sperm concentration (million/mL) | 255.7 ± 40 | 150.7 ± 35 | 162.0 ± 31 | 117.0 ± 28 | 328 | 154 |
| Total number of sperm (billion) | 17.6 ± 3.0 | 11.0 ± 3.6 | 21.3 ± 6.4 | 7.9 ± 3.4 | 9.8 | 5.6 |
Table 4.
Sperm morphology of the first and second ejaculates of the season collected from 20 breeding inactive stallions.
Table 4.
Sperm morphology of the first and second ejaculates of the season collected from 20 breeding inactive stallions.
| | First Ejaculate | Second Ejaculate |
|---|
| | Mean ± SEM | CI-95% | Mean ± SEM | CI-95% |
|---|
| Normal (%) | 62.7 ± 4.5 | 52.9–72.5 | 67.6 ± 4.6 | 57.9–77.2 |
| Proximal droplet (%) | 13.4 ± 4.2 | 5.4–22.7 | 12.9 ± 3.8 | 3.9–18.6 |
| Distal droplet (%) | 12.3 ± 2.5 | 1.8–13.8 | 5.0 ± 1.5 | 0.9–7.7 |
| Tailless (%) | 2.8 ± 0.6 | 0.7–3.4 | 1.3 ± 0.4 | 0.3–2.2 |
| Simple bent (%) | 5.5 ± 1.4 | 2.8–9.5 | 6.7 ± 1.4 | 3.1–9.2 |
| Strongly folded (%) | 1.7 ± 0.4 | 1.1–3.5 | 2.1 ± 0.6 | 1.2–5.4 |
| Midpiece defects (%) | 0.8 ± 0.4 | 1–1.8 | 0.9 ± 0.4 | 1–1.6 |
Table 5.
Sperm morphology of the first and second ejaculates of the season collected from 20 breeding inactive stallions. Based on the motility parameters between the first and second ejaculates, sixteen stallions were classified as unchanged (i.e., ≤10% change), three stallions as increased (i.e., improvement > 10%), and one stallion as decreased (i.e., reduction > 10%).
Table 5.
Sperm morphology of the first and second ejaculates of the season collected from 20 breeding inactive stallions. Based on the motility parameters between the first and second ejaculates, sixteen stallions were classified as unchanged (i.e., ≤10% change), three stallions as increased (i.e., improvement > 10%), and one stallion as decreased (i.e., reduction > 10%).
| | Unchanged (n = 16) | Increased (n = 3) | Decreased (n = 1) |
|---|
| | First Ejaculate | Second Ejaculate | First Ejaculate | Second Ejaculate | First Ejaculate | Second Ejaculate |
|---|
| | Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | | |
|---|
| Normal (%) | 63.4 ± 5.4 | 69.8 ± 4.8 | 58.3 ±13.3 | 52.0 ± 3.0 | 67 | 37 |
| Proximal droplet (%) | 14.7 ± 4.0 | 10.4 ± 3.1 | 5.6 ± 3.3 | 9.5 ± 4.5 | 22 | 154 |
| Distal droplet (%) | 10.4 ± 3.5 | 8.3 ± 3.5 | 22.3 ± 11.6 | 29.5 ± 9.5 | 6 | 5.6 |
| Tailless (%) | 3.4 ± 1.1 | 2.2 ± 3.5 | 1.0 ± 0.6 | 2.5 ± 1.5 | 1 | 2 |
| Simple bent (%) | 5.6 ± 1.5 | 5.5 ± 1.3 | 7.0 ± 3.2 | 5.0 ± 5.0 | 0 | 0 |
| Strongly folded (%) | 1.9 ± 0.5 | 2.7 ± 0.9 | 3.7 ± 2.3 | 8.0 ± 3.0 | 3 | 0 |
| Midpiece defects (%) | 0.6 ± 0.3 | 0.9 ± 0.4 | 2.0 ± 1.5 | 0.5 ± 0.5 | 0 | 3 |
Table 6.
Qualitative comparison of the first and second ejaculates collected from breeding inactive stallions (n = 20).
Table 6.
Qualitative comparison of the first and second ejaculates collected from breeding inactive stallions (n = 20).
| | First Ejaculate | Second Ejaculate |
|---|
| | Mean ± SEM | CI-95% | Mean ± SEM | CI-95% |
|---|
| TM (%) | 65.4 ± 5.6 | 53.5–77.2 | 67.8 ± 3.3 | 60.7–74.9 |
| PM (%) | 58.9 ± 5.4 | 47.4–70.3 | 61.7 ± 3.2 | 54.8–68.6 |
| VCL (μm/s) | 147.8 ± 4.0 | 140.4–156.8 | 151.4 ± 4.5 | 141.3–159.5 |
| VSL (μm/s) | 69.7 ± 3.5 | 62.3–76.2 | 75.3 ± 3.5 | 73.9–3.5 |
| VAP (μm/s) | 87.2 ± 3.9 | 79.3–94.7 | 91.9 ± 3.5 | 83.0–98.1 |
| PMI (%) | 68.0 ± 6.1 | 53.7–82.3 | 66.5 ± 5.6 | 53.9–79.1 |
| HMMP (%) | 40.5 ± 2.5 | 34.9–46.1 | 41.3 ± 4.0 | 32.1–50.5 |
Table 7.
Qualitative comparison of the first and second ejaculates collected from breeding inactive stallions (n = 20). Based on the motility parameters between the first and second ejaculates, sixteen stallions were classified as unchanged (i.e., ≤10% change), three stallions as increased (i.e., improvement >10%), and one stallion as decreased (i.e., reduction >10%).
Table 7.
Qualitative comparison of the first and second ejaculates collected from breeding inactive stallions (n = 20). Based on the motility parameters between the first and second ejaculates, sixteen stallions were classified as unchanged (i.e., ≤10% change), three stallions as increased (i.e., improvement >10%), and one stallion as decreased (i.e., reduction >10%).
| | Unchanged (n = 16) | Increased (n = 3) | Decreased (n = 1) |
|---|
| | First Ejaculate | Second Ejaculate | First Ejaculate | Second Ejaculate | First Ejaculate | Second Ejaculate |
|---|
| | Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | | |
|---|
| TM (%) | 71.3 ± 5.3 | 71.0 ± 4.3 | 32.2 ± 11.1 | 59.0 ± 5.9 | 86.6 | 74.3 |
| PM (%) | 64.0 ± 5.0 | 64.0 ± 4.2 | 26.4 ± 9.5 | 54.6 ± 6.4 | 82.2 | 69.0 |
| VCL (μm/s) | 145.7 ± 4.7 | 150.7 ± 5.2 | 143.9 ± 8.3 | 140.6 ± 0.8 | 164.4 | 162.9 |
| VSL (μm/s) | 67.7 ± 4.4 | 74.4 ± 4.3 | 66.2 ± 10.8 | 72.1 ± 9.5 | 81.1 | 81.4 |
| VAP (μm/s) | 85.4 ± 4.5 | 91.5 ± 4.1 | 83.7 ± 13.9 | 86.4 ± 11.7 | 100.0 | 97.4 |
Table 8.
Motility parameters of the first and second ejaculates of the season collected from breeding inactive stallions (n = 20) after 0, 24, and 48 h of cooling.
Table 8.
Motility parameters of the first and second ejaculates of the season collected from breeding inactive stallions (n = 20) after 0, 24, and 48 h of cooling.
| | | Non-Centrifuged | Cushion-Centrifuged |
|---|
| | Time (h) | First Ejaculate | Second Ejaculate | First Ejaculate | Second Ejaculate |
|---|
| TM (%) | 0 | 65.4 ± 5.6 a | 67.8 ± 3.3 a | 63.0 ± 5.1 a | 62.7 ± 3.9 a |
| 24 | 50.3 ± 7.1 b | 48.1 ± 5.6 b | 56.7 ± 5.5 b | 59.8 ± 5.0 b |
| 48 | 41.4 ± 7.0 b | 33.4 ± 4.3 b | 51.4 ± 5.3 b | 59.9 ± 5.4 b |
| PM (%) | 0 | 59.4 ± 5.6 a | 62.8 ± 3.6 a | 55.4 ± 5.0 a | 53.6 ± 4.6 a |
| 24 | 43.3 ± 7.3 b | 39.5 ± 5.3 b | 50.4 ± 5.3 b | 52.3 ± 4.9 b |
| 48 | 35.5 ± 7.1 b | 26.4 ± 4.0 b | 44.6 ± 5.3 b | 52.7 ± 5.7 b |
| VAP (μm/s) | 0 | 87.2 ± 3.9 a | 91.9 ± 3.5 a | 72.8 ± 5.5 a | 90.3 ± 4.7 a |
| 24 | 70.9 ± 5.2 ab | 76.0 ± 6.5 ab | 72.5 ± 4.5 ab | 72.5 ± 4.2 ab |
| 48 | 62.2 ± 6.9 b | 71.1 ± 3.5 b | 68.1 ± 4.1 b | 70.4 ± 3.7 b |
| VCL (μm/s) | 0 | 147.8 ± 4.0 a | 151.4 ± 4.5 a | 135.0 ± 8.8 a | 140.6 ± 7.6 a |
| 24 | 122.9 ± 8.3 ab | 132.6 ± 9.9 ab | 132.1 ± 5.8 ab | 127.7 ± 6.2 ab |
| 48 | 114.7 ± 12.8 b | 136.7 ± 6.2 b | 125.6 ± 4.5 b | 122.7 ± 4.7 b |
| VSL (μm/s) | 0 | 69.7 ± 3.5 a | 75.3 ± 3.5 a | 56.7 ± 4.2 a | 61.7 ± 3.9 a |
| 24 | 58.6 ± 4.0 ab | 62.5 ± 5.9 ab | 59.6 ± 4.1 ab | 59.4 ± 3.6 ab |
| 48 | 49.5 ± 5.3 b | 53.8 ± 2.5 b | 56.1 ± 3.5 b | 58.5 ± 3.4 b |
Table 9.
Percentage of sperm with an intact membrane (PMI) and high mitochondrial membrane potential (HMMP) from the first and second ejaculates of the season collected from breeding inactive stallions (n = 20) after 0, 24, and 48 h of cooling.
Table 9.
Percentage of sperm with an intact membrane (PMI) and high mitochondrial membrane potential (HMMP) from the first and second ejaculates of the season collected from breeding inactive stallions (n = 20) after 0, 24, and 48 h of cooling.
| | | Non-Centrifuged | Cushion-Centrifuged |
|---|
| | Time (h) | First Ejaculate | Second Ejaculate | First Ejaculate | Second Ejaculate |
|---|
| PMI (%) | 0 | 66.3 ± 5.6 | 66.3 ± 6.2 | 71.5 ± 3.7 | 69.8 ± 5.0 |
| 24 | 53.9 ± 6.4 | 53.5 ± 9.9 | 66.9 ± 5.5 | 63.5 ± 6.5 |
| 48 | 53.6 ± 8.9 | 52.0 ± 10.7 | 67.7 ± 5.6 | 69.9 ± 7.2 |
| HMMP (%) | 0 | 38.8 ± 2.5 | 41.3 ± 4.0 | 42.0 ± 2.9 | 39.4 ± 3.8 |
| 24 | 40.6 ± 4.3 | 36.0 ± 3.0 | 45.7 ± 9.9 | 40.5 ± 6.7 |
| 48 | 50.7 ± 10.0 | 46.0 ± 4.3 | 42.5 ± 3.1 | 38.7 ± 6.3 |
Table 10.
Motility parameters of the first and second ejaculates of the season collected from breeding inactive stallions (n = 20) after 0, 24, and 48 h of cooling. Based on the motility parameters between the first and second ejaculates, sixteen stallions were classified as unchanged (i.e., ≤10% change), three stallions as increased (i.e., improvement > 10%) group, and one stallion as decreased (i.e., reduction > 10%).
Table 10.
Motility parameters of the first and second ejaculates of the season collected from breeding inactive stallions (n = 20) after 0, 24, and 48 h of cooling. Based on the motility parameters between the first and second ejaculates, sixteen stallions were classified as unchanged (i.e., ≤10% change), three stallions as increased (i.e., improvement > 10%) group, and one stallion as decreased (i.e., reduction > 10%).
| | | Unchanged (n = 16) | Increased (n = 3) | Decreased (n = 1) |
|---|
| | Time (h) | First Ejaculate | Second Ejaculate | First Ejaculate | Second Ejaculate | First Ejaculate | Second Ejaculate |
|---|
| TM (%) | 0 | 71.3 ± 5.3 | 71.0 ± 4.3 | 32.3 ± 11.1 | 59.0 ± 5.9 | 86.6 | 74.3 |
| 24 | 53.9 ± 6.9 | 51.1 ± 5.2 | 23.2 ± 13.5 | 27.2 ± 11.5 | 88.2 | 77.1 |
| 48 | 43.0 ± 8.2 | 35.2 ± 4.3 | 31.3 ± 12.2 | 13.9 ± 9.8 | 61.4 | 74.3 |
| PM (%) | 0 | 64.0 ± 5.0 | 64.0 ± 4.2 | 26.4 ± 9.5 | 54.6 ± 6.4 | 82.2 | 69.0 |
| 24 | 46.5 ± 7.5 | 42.9 ± 5.0 | 17.7 ± 11.2 | 18.7 ± 10.7 | 80.3 | 61.5 |
| 48 | 37.1 ± 8.5 | 28.0 ± 4.2 | 25.5 ± 9.9 | 8.9 ± 7.8 | 53.1 | 97.4 |
| VAP (μm/s) | 0 | 85.4 ± 4.5 | 91.6 ± 4.1 | 83.7 ± 13.9 | 86.4 ± 11.7 | 100.0 | 40.2 |
| 24 | 71.2 ± 5.8 | 80.9 ± 7.2 | 60.9 ± 19.9 | 49.2 ± 12.5 | 86.7 | 70.5 |
| 48 | 138.8 ± 8.2 | 71.1 ± 4.5 | 81.8 ± 12.8 | 73.1 ± 2.8 | 65.3 | 97.4 |
| VCL (μm/s) | 0 | 145.7 ± 4.7 | 150.7 ± 5.2 | 143.9 ± 8.3 | 140.6 ± 0.8 | 164.4 | 67.2 |
| 24 | 121.7 ± 8.5 | 139.6 ± 10.5 | 114.1 ± 41.7 | 91.8 ± 34.2 | 154.4 | 130.1 |
| 48 | 63.1 ± 15.5 | 135.3 ± 7.9 | 151.6 ± 9.9 | 142.8 ± 1.0 | 126.2 | 162.9 |
| VSL (μm/s) | 0 | 67.7 ± 4.4 | 74.4 ± 4.3 | 66.2 ± 10.8 | 72.1 ± 9.5 | 81.1 | 33.6 |
| 24 | 59.1 ± 4.5 | 67.2 ± 6.5 | 50.7 ± 16.8 | 39.5 ± 9.5 | 68.3 | 52.7 |
| 48 | 63.1 ± 6.7 | 54.1 ± 3.1 | 61.3 ± 6.8 | 55.0 ± 1.5 | 51.0 | 81.4 |
Table 11.
Motility parameters of the first and second ejaculates of the season collected from breeding inactive stallions (n = 20) after 0, 24, and 48 h of cooling. The ejaculate was processed via cushion-centrifugation before cooling. Based on the motility parameters between the first and second ejaculates, sixteen stallions were classified as unchanged (i.e., ≤10% change), three stallions as increased (i.e., improvement > 10%) group, and one stallion as decreased (i.e., reduction > 10%).
Table 11.
Motility parameters of the first and second ejaculates of the season collected from breeding inactive stallions (n = 20) after 0, 24, and 48 h of cooling. The ejaculate was processed via cushion-centrifugation before cooling. Based on the motility parameters between the first and second ejaculates, sixteen stallions were classified as unchanged (i.e., ≤10% change), three stallions as increased (i.e., improvement > 10%) group, and one stallion as decreased (i.e., reduction > 10%).
| | | Unchanged (n = 16) | Increased (n = 3) | Decreased (n = 1) |
|---|
| | Time (h) | First Ejaculate | Second Ejaculate | First Ejaculate | Second Ejaculate | First Ejaculate | Second Ejaculate |
|---|
| TM (%) | 0 | 67.3 ± 4.1 | 63.7 ± 3.9 | 38.6 ± 14.5 | 54.8 ± 10.3 | 85.9 | 86.3 |
| 24 | 61.2 ± 4.3 | 60.3 ± 5.8 | 29.8 ± 10.8 | 55.4 ± 9.0 | 88.3 | 76.8 |
| 48 | 54.8 ± 5.3 | 63.4 ± 5.4 | 32.7 ± 9.1 | 29.5 ± 5.2 | 65.9 | 77.9 |
| PM (%) | 0 | 59.0 ± 4.1 | 53.9 ± 4.9 | 32.6 ± 13.8 | 48.9 ± 11.0 | 80.9 | 80.7 |
| 24 | 54.7 ± 4.6 | 52.4 ± 5.7 | 24.3 ± 9.7 | 47.1 ± 7.4 | 80.7 | 73.7 |
| 48 | 47.6 ± 5.6 | 56.4 ± 5.9 | 25.4 ± 6.8 | 22.3 ± 0.9 | 60.3 | 68.4 |
| VAP (μm/s) | 0 | 73.0 ± 6.3 | 75.6 ± 5.6 | 78.3 ± 19.2 | 77.4 ± 12.9 | 83.8 | 91.7 |
| 24 | 70.7 ± 5.6 | 72.3 ± 5.3 | 77.2 ± 10.0 | 71.3 ± 4.0 | 80.3 | 77.1 |
| 48 | 68.3 ± 5.5 | 70.8 ± 4.4 | 63.72.0 | 61.0 ± 2.4 | 75.3 | 83.8 |
| VCL (μm/s) | 0 | 134.3 ± 10.1 | 138.9 ± 9.2 | 135.1 ± 17.0 | 134.8 ± 8.0 | 159.9 | 171.1 |
| 24 | 127.0 ± 6.7 | 125.8 ± 7.5 | 149.8 ± 9.0 | 133.4 ± 11.3 | 147.8 | 139.1 |
| 48 | 125.1 ± 5.8 | 122.9 ± 5.3 | 123.1 ± 7.4 | 109.2 ± 4.7 | 134.7 | 146.7 |
| VSL (μm/s) | 0 | 57.0 ± 4.9 | 60.8 ± 4.6 | 62.2 ± 14.7 | 61.3 ± 12.2 | 62.6 | 72.2 |
| 24 | 59.7 ± 5.4 | 59.6 ± 4.5 | 59.4 ± 3.1 | 56.6 ± 4.8 | 59.9 | 63.4 |
| 48 | 56.5 ± 4.7 | 59.6 ± 4.0 | 51.3 ± 1.4 | 47.6 ± 2.4 | 61.9 | 67.1 |
Table 12.
Motility parameters and percentage of sperm with intact plasma membrane (PMI) and high mitochondrial membrane potential (HMMP) of the first and second ejaculates of the season collected from breeding inactive stallions (n = 20) before and after freezing.
Table 12.
Motility parameters and percentage of sperm with intact plasma membrane (PMI) and high mitochondrial membrane potential (HMMP) of the first and second ejaculates of the season collected from breeding inactive stallions (n = 20) before and after freezing.
| | First Ejaculate | Second Ejaculate |
|---|
| | Before Freezing | After Freezing | Before Freezing | After Freezing |
|---|
| TM (%) | 64.6 ± 3.7 a | 51.7 ± 3.7 b | 65.6 ± 3.2 a | 50.4 ± 3.7 b |
| PM (%) | 57.0 ± 5.1 a | 44.4 ± 3.7 b | 56.9 ± 3.8 a | 39.8 ± 3.8 b |
| VAP (μm/s) | 72.8 ± 4.4 a | 60.6 ± 2.1 b | 80.2 ± 4.0 a | 72.5 ± 5.5 b |
| VCL (μm/s) | 135.6 ± 7.2 a | 109.1 ± 3.9 b | 142.4 ± 6.5 a | 95.9 ± 5.8 b |
| VSL (μm/s) | 56.7 ± 3.6 a | 48.6 ± 1.6 b | 64.6 ± 3.4 a | 40.3 ± 4.8 b |
| PMI (%) | 77.2 ± 4.0 a | 31.7 ± 4.3 b | 70.5 ± 4.6 a | 34.0 ± 5.0 b |
| HMMP (%) | 41.6 ± 2.8 | 45.9 ± 2.6 | 41.2 ± 3.7 | 53.3 ± 0.9 |
Table 13.
Motility parameters of the first and second ejaculates of the season collected from breeding inactive stallions (n = 20) before and after freezing. Based on the motility parameters between the first and second ejaculates, sixteen stallions were classified as unchanged (i.e., ≤10% change), three stallions as increased (i.e., improvement > 10%) group, and one stallion as decreased (i.e., reduction > 10%).
Table 13.
Motility parameters of the first and second ejaculates of the season collected from breeding inactive stallions (n = 20) before and after freezing. Based on the motility parameters between the first and second ejaculates, sixteen stallions were classified as unchanged (i.e., ≤10% change), three stallions as increased (i.e., improvement > 10%) group, and one stallion as decreased (i.e., reduction > 10%).
| First Ejaculate |
| | Unchanged (n = 16) | Increased (n = 3) | Decreased (n = 1) |
| | Before Freezing | After Freezing | Before Freezing | After Freezing | Before Freezing | After Freezing |
| TM (%) | 67.2 ± 3.8 | 52.7 ± 4.0 | 35.2 ± 13.6 | 31.0 ± 14.5 | 85.9 | 68.4 |
| PM (%) | 59.1 ± 3.8 | 44.2 ± 4.1 | 29.3 ± 13.3 | 25.6 ± 16.0 | 80.9 | 61.8 |
| VAP (μm/s) | 73.0 ± 5.0 | 57.2 ± 2.2 | 61.2 ± 1.5 | 62.3 ± 1.8 | 83.8 | 71.0 |
| VCL (μm/s) | 135.1 ± 8.0 | 103.2 ± 4.6 | 118.6 ± 1.5 | 118.8 ± 2.6 | 159.9 | 122.1 |
| VSL (μm/s) | 57.0 ± 4.0 | 45.6 ± 1.5 | 49.5 ± 1.0 | 50.5 ± 0.6 | 62.6 | 53.4 |
| Second Ejaculate |
| | Unchanged (n = 16) | Increased (n = 3) | Decreased (n = 1) |
| | Before Freezing | After Freezing | Before Freezing | After Freezing | Before Freezing | After Freezing |
| TM (%) | 66.9 ± 3.1 | 51.2 ± 4.1 | 49.8 ± 5.5 | 39.0 ± 8.4 | 86.3 | 72.3 |
| PM (%) | 57.3 ± 4.1 | 41.7 ± 3.9 | 43.9 ± 6.3 | 21.8 ± 6.0 | 80.7 | 63.2 |
| VAP (μm/s) | 78.5 ± 4.8 | 72.5 ± 6.1 | 77.4 ± 12.9 | 72.8 ± 20.2 | 91.7 | 71.5 |
| VCL (μm/s) | 141.5 ± 8.0 | 97.2 ± 6.3 | 134.6 ± 8.0 | 79.2± 16.0 | 171.1 | 125.0 |
| VSL (μm/s) | 63.3 ± 3.9 | 41.6 ± 5.4 | 61.3 ± 12.2 | 28.4 ± 13.8 | 72.2 | 54.6 |