The Use of Ovarian Fluid as Natural Fertilization Medium for Cryopreserved Semen in Mediterranean Brown Trout: The Effects on Sperm Swimming Performance
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Collection of Samples
2.2. Experimental Design
2.3. Statistical Analysis
3. Results
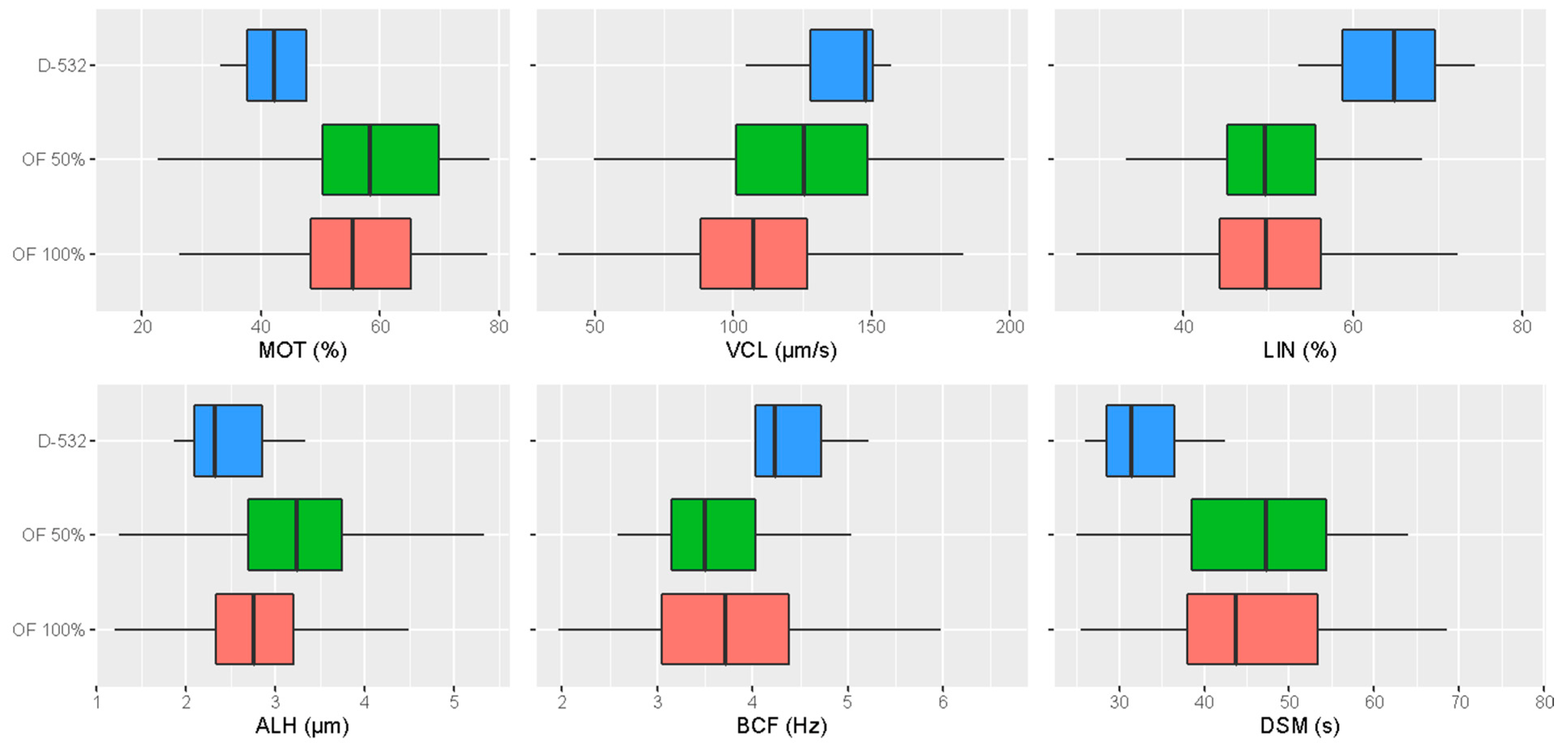
3.1. Comparison of the Post-Thaw Sperm Motility Parameters among the Different Activation Media
3.2. Differences between OF 100% and OF 50%: The Effect of Males, Females and Male–Female Interaction
3.3. Correlation among the Post-Thaw Sperm Motility Parameters Activated in Ovarian Fluid and Control Activation Solution
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
 .
.Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roldan, E.R.S.; Garde, J. Biotecnología de la reproducción y conservación de especies en peligro de extinción. In Los Retos Medioambientales del Siglo XXI; Gomendio, M., Ed.; La Conservación de la Biodiversidad en España, Fundación BBVA: Bilbao, Spain, 2004; pp. 283–307. [Google Scholar]
- Martínez-Páramo, S.; Pérez-Cerezales, S.; Gómez-Romano, F.; Blanco, G.; Sánchez, J.A.; Herráez, M.P. Cryobanking as tool for conservation of biodiversity: Effect of brown trout sperm cryopreservation on the male genetic potential. Theriogenology 2009, 71, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Páramo, S.; Horváth, A.; Labbé, C.; Zhang, T.; Robles, V.; Herráez, P.; Suquet, M.; Adams, S.; Viveiros, A.; Tiersch, T.R.; et al. Cryobanking of aquatic species. Aquaculture 2017, 472, 156–177. [Google Scholar] [CrossRef] [PubMed]
- Rusco, G.; Di Iorio, M.; Iampietro, R.; Esposito, S.; Gibertoni, P.P.; Penserini, M.; Roncarati, A.; Iaffaldano, N. A Simple and Efficient Semen Cryopreservation Method to Increase the Genetic Variability of Endangered Mediterranean Brown Trout Inhabiting Molise Rivers. Animals 2020, 10, 403. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, M.; Rusco, G.; Esposito, S.; D’Andrea, M.; Roncarati, A.; Iaffaldano, N. The role of semen cryobanks for protecting endangered native salmonids: Advantages and perspectives as outlined by the LIFE Nat.Sal.Mo. project on Mediterranean brown trout (Molise region—Italy). Front. Mar. Sci. 2023, 9, 1075498. [Google Scholar] [CrossRef]
- Alavi, S.M.H.; Cosson, J. Sperm motility in fishes. I. Effects of temperature and pH: A review. Cell Biol. Intern. 2005, 29, 101–110. [Google Scholar] [CrossRef]
- Alavi, S.M.H.; Cosson, J. Sperm motility in fishes. II. Effects of ions and osmolality: A review. Cell Biol. Intern. 2006, 30, 1–14. [Google Scholar] [CrossRef]
- Wilcox, K.W.; Stoss, J.; Donaldson, E.M. Broken eggs as a cause of infertility of coho salmon gametes. Aquaculture 1984, 40, 77–87. [Google Scholar] [CrossRef]
- Billard, R. Artificial insemination and gamete management in fish. Mar. Freshw. Behav. Physiol. 1988, 14, 3–21. [Google Scholar]
- Billard, R. Reproduction in rainbow trout: Sex differentiation, dynamics of gametogenesis, biology and preservatic of gametes. Aquaculture 1992, 100, 263–298. [Google Scholar] [CrossRef]
- Goetz, F.W.; Coffman, M.A. Storage of unfertilized eggs of rainbow trout (Oncorhynchus mykiss) in artificial media. Aquaculture 2000, 184, 267–276. [Google Scholar] [CrossRef]
- Dietrich, G.J.; Wojtczak, M.; Słowińska, M.; Dobosz, S.; Kuźmiński, H.; Ciereszko, A. Broken eggs decrease pH of rainbow trout (Oncorhynchus mykiss) ovarian fluid. Aquaculture 2007, 273, 748–751. [Google Scholar] [CrossRef]
- Hugunin, H.A.; Parsons, J.E.; Nagler, J.J. The influence of coelomic fluid on in vitro fertilization success in rainbow trout (Oncorhynchus mykiss). Aquaculture 2008, 281, 155–157. [Google Scholar] [CrossRef]
- Hatef, A.; Niksirat, H.; Alavi, S.M.H. Composition of ovarian fluid in endangered Caspian brown trout, Salmo trutta caspius, and its effects on spermatozoa motility and fertilizing ability compared to freshwater and a saline medium. Fish Physiol. Biochem. 2009, 35, 695–700. [Google Scholar] [CrossRef]
- Łuczynski, M.J.; Nowosad, J.; Łuczynska, J.; Kucharczyk, D. Effect of Application of Different Activation Media on Fertilization and Embryo Survival of Northern Pike, (Esox lucius) under Hatchery Conditions. Animals 2022, 12, 1022. [Google Scholar] [CrossRef]
- Rusco, G.; Di Iorio, M.; Iampietro, R.; Roncarati, A.; Esposito, S.; Iaffaldano, N. Cryobank of Mediterranean Brown Trout Semen: Evaluation of the Use of Frozen Semen up to Six Hours Post-Collection. Fishes 2021, 6, 26. [Google Scholar] [CrossRef]
- Turner, E.; Montgomerie, R. Ovarian fluid enhances sperm movement in Arctic charr. J. Fish Biol. 2002, 60, 1570–1579. [Google Scholar] [CrossRef]
- Urbach, D.; Folstad, I.; Rudolfsen, G. Effects of ovarian fluid on sperm velocity in Arctic charr (Salvelinus alpinus). Behav. Ecol. Sociobiol. 2005, 57, 438–444. [Google Scholar] [CrossRef]
- Rosengrave, P.; Gemmell, N.J.; Metcalf, V.; McBride, K.; Montgomerie, R. A mechanism for cryptic female choice in chinook salmon. Behav. Ecol. 2008, 19, 1178–1185. [Google Scholar] [CrossRef]
- Rosengrave, P.; Taylor, H.; Montgomerie, R.; Metcalf, V.; McBride, K.; Gemmell, N.J. Chemical composition of seminal and ovarian fluids of chinook salmon (Oncorhynchus tshawytscha) and their effects on sperm motility traits. Comp. Biochem. Physiol. Part A 2009, 152, 123–129. [Google Scholar] [CrossRef]
- Rosengrave, P.; Montgomerie, R.; Gemmell, N. Cryptic female choice enhances fertilization success and embryo survival in chinook salmon. Proc. R. Soc. 2016, 283, 20160001. [Google Scholar] [CrossRef]
- Dietrich, G.J.; Wojtczak, M.; Słowińska, M.; Dobosz, S.; Kuźmiński, H.; Ciereszko, A. Effects of ovarian fluid on motility characteristics of rainbow trout (Oncorhynchus mykiss Walbaum) spermatozoa. J. Appl. Ichthyol. 2008, 24, 503–507. [Google Scholar] [CrossRef]
- Diogo, P.; Soares, F.; Dinis, M.T.; Cabrita, E. The influence of ovarian fluid on Solea senegalensis sperm motility. J. Appl. Ichthyol. 2010, 26, 690–695. [Google Scholar] [CrossRef]
- Butts, I.A.E.; Johnson, K.; Wilson, C.C.; Pitcher, T.E. Ovarian fluid enhances sperm velocity based on relatedness in lake trout, Salvelinus namaycush. Theriogenology 2012, 78, 2105–2109. [Google Scholar] [CrossRef] [PubMed]
- Butts, I.A.E.; Prokopchuk, G.; Kašpar, V.; Cosson, J.; Pitcher, T.E. Ovarian fluid impacts flagellar beating and biomechanical metrics of sperm between alternative reproductive tactics. J. Exp. Biol. 2017, 220, 2210–2217. [Google Scholar] [CrossRef]
- Galvano, P.M.; Johnson, K.; Wilson, C.C.; Pitcher, T.E.; Butts, I.A. Ovarian fluid influences sperm performance in lake trout, Salvelinus namaycush. Reprod. Biol. 2013, 13, 172–175. [Google Scholar] [CrossRef]
- Alonzo, S.H.; Stiver, K.A.; Marsh-Rollo, S.E. Ovarian fluid allows directional cryptic female choice despite external fertilization. Nat. Commun. 2016, 16, 12452. [Google Scholar] [CrossRef]
- Zadmajid, V.; Myers, J.N.; Sørensen, S.R.; Butts, I.A.E. Ovarian fluid and its impacts on spermatozoa performance in fish: A Review. Theriogenology 2019, 132, 144–152. [Google Scholar] [CrossRef]
- Poli, F.; Immler, S.; Gasparini, C. Effects of ovarian fluid on sperm traits and its implications for cryptic female choice in zebrafish. Behav. Ecol. 2019, 30, 1298–1305. [Google Scholar] [CrossRef]
- Purchase, C.F.; Rooke, A.C. Freezing ovarian fluid does not alter how it affects fish sperm swimming performance: Creating a cryptic female choice ‘spice rack’ for use in split-ejaculate experimentation. J. Fish Biol. 2020, 96, 693–699. [Google Scholar] [CrossRef]
- Wilson-Leedy, J.G.; Ingermann, R.L. Development of a novel CASA system based on open source software for characterization of zebrafish sperm motility parameters. Theriogenology 2007, 67, 661–672. [Google Scholar] [CrossRef]
- Cosson, J. Frenetic activation of fish spermatozoa flagella entails short-term motility, portending their precocious decadence. J. Fish Biol. 2010, 76, 240–279. [Google Scholar] [CrossRef]
- Dietrich, G.J.; Kowalski, R.; Wojtczak, M.; Dobosz, S.; Goryczko, K.; Ciereszko, A. Motility parameters of rainbow trout (Oncorhynchus mykiss) spermatozoa in relation to sequential collection of milt, time of post-mortem storage and anesthesia. Fish Physiol. Biochem. 2005, 31, 1–9. [Google Scholar] [CrossRef]
- Wojtczak, M.; Dietrich, G.J.; Słowińska, M.; Dobosz, S.; Kuźmiński, H.; Ciereszko, A. Ovarian fluid pH enhances motility parameters of rainbow trout (Oncorhynchus mykiss) spermatozoa. Aquaculture 2007, 270, 259–264. [Google Scholar] [CrossRef]
- Beirão, J.; Purchase, C.F.; Wringe, B.F.; Fleming, I.A. Wild Atlantic cod sperm motility is negatively affected by ovarian fluid of farmed females. Aquacult. Environ. Interact. 2014, 5, 61–70. [Google Scholar] [CrossRef]
- İnanan, B.E.; Öğretmen, F. Determination of differences in the biochemical properties of sperm activating and non-activating ovarian fluids and their influences on sperm motility in rainbow trout (Oncorhynchus mykiss). Aquaculture 2015, 448, 539–544. [Google Scholar] [CrossRef]
- Hamano, S. On the spermatozoa agglutinating agents of the dog salmon and rainbow trout eggs. Bull. Jpn. Soc. Sci. Fish. 1961, 27, 225–231. [Google Scholar] [CrossRef]
- Nomura, M. Studies on reproduction of rainbow trout, Salmo gairdneri with special reference to egg taking. VI. The activities of spermatozoa in different diluents and preservation of semen. Bull. Jpn. Soc. Sci. Fish. 1964, 30, 723–733. [Google Scholar] [CrossRef]
- Billard, R. A new technique of artificial insemination for salmonids using a sperm diluent. Fisheries 1977, 2, 24–25. [Google Scholar]
- Aegerter, S.; Jalabert, B. Effects of post-ovulatory oocyte ageing and temperature on egg quality and on the occurrence of triploid fry in rainbow trout, Oncorhynchus mykiss. Aquaculture 2004, 231, 59–71. [Google Scholar] [CrossRef]
- Billard, R.; Petit, J.; Jalabert, B.; Szollosi, D. Artificial insemination in trout using a sperm diluant. In The Early Life History of Fish; Blaxter, J.H.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1974; pp. 715–723. [Google Scholar]
- Billard, R. Effects of coelomic and seminal fluids and various saline diluents on the fertilizing ability of spermatozoa in the rainbow trout, Salmo gairdneri. J. Reprod. Fert. 1983, 68, 77–84. [Google Scholar] [CrossRef]
- Billard, R.; Cosson, M.P. Sperm motility in rainbow trout Parasalmo mykiss: Effect of pH and temperature. Reprod. Biol. Endocrinol. 1988, 161–167. [Google Scholar]
- Lahnsteiner, F.; Weismann, T.; Patzner, R. Composition of the ovarian fluid in 4 salmonid species: Oncorhynchus mykiss, Salmo trutta f lacustris, Saivelinus alpinus and Hucho hucho. Reprod. Nutr. Dev. 1995, 35, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Kholodnyy, V.; Gadêlha, H.; Cosson, J.; Boryshpolets, S. How do freshwater fish sperm find the egg? The physicochemical factors guiding the gamete encounters of externally fertilizing freshwater fish. Rev. Aquac. 2020, 12, 1165–1192. [Google Scholar] [CrossRef]
- Gage, M.J.; Macfarlane, C.P.; Yeates, S.; Ward, R.G.; Searle, J.B.; Parker, G.A. Spermatozoal traits and sperm competition in Atlantic salmon: Relative sperm velocity is the primary determinant of fertilization success. Curr. Biol. 2004, 14, 44–47. [Google Scholar] [CrossRef]
- Figueroa, E.; Lee-Estevez, M.; Valdebenito, I.; Watanabe, I.; Oliveira, R.P.S.; Romero, J.; Castillo, R.L.; Farías, J.G. Effects of cryopreservation on mitochondrial function and sperm quality in fish. Aquaculture 2019, 511, 634190. [Google Scholar] [CrossRef]
- Lorenzoni, M.; Carosi, A.; Giovannotti, M.; La Porta, G.; Splendiani, A.; Barucchi, V.C. Ecology and conservation of the Mediterranean trout in the central Apennines (Italy). J. Limnol. 2019, 78, 1. [Google Scholar] [CrossRef]
- D’Agaro, E.; Gibertoni, P.; Marroni, F.; Messina, M.; Tibaldi, E.; Esposito, S. Genetic and Phenotypic Characteristics of the Salmo trutta Complex in Italy. Appl. Sci. 2022, 12, 3219. [Google Scholar] [CrossRef]
- Polgar, G.; Iaia, M.; Righi, T.; Volta, P. The Italian Alpine and Subalpine trouts: Taxonomy, Evolution, and Conservation. Biology 2022, 11, 576. [Google Scholar] [CrossRef]

| Treatments | |||
|---|---|---|---|
| Sperm Traits | D-532 | OF 50% | OF 100% |
| MOT (%) | 43.04 ± 13.59 b | 57.49 ± 14.00 a | 55.56 ± 13.83 ab |
| VCL (µm/s) | 138.01 ± 18.74 a | 122.29 ± 32.01 a | 107.72± 27.69 b |
| LIN (%) | 64.23 ± 7.79 a | 50.00 ± 8.55 b | 49.98 ± 9.90 b |
| ALH (µm) | 2.49 ± 0.57 b | 3.21 ± 0.80 a | 2.84 ± 0.70 b |
| BCF (Hz) | 4.28 ± 0.72 | 3.71 ± 0.84 | 3.72 ± 0.94 |
| DSM (s) | 32.86 ± 6.33 b | 46.39 ± 9.60 a | 45.98 ± 10.84 a |
| Random Effects | MOT | VCL | ALH | |||
|---|---|---|---|---|---|---|
| p | AIC | p | AIC | p | AIC | |
| Male | *** | −154.41 | *** | 1308.1 | ** | 320.14 |
| Female | * | −129.15 | 1346.2 | ** | 320.5 | |
| Male–Female | *** | −216.94 | *** | 1329.2 | *** | 309.83 |
| Fixed effect | ||||||
| Treatment (OF 100% vs. OF 50%) | * | *** | *** | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusco, G.; Di Iorio, M.; Esposito, S.; Antenucci, E.; Roncarati, A.; Iaffaldano, N. The Use of Ovarian Fluid as Natural Fertilization Medium for Cryopreserved Semen in Mediterranean Brown Trout: The Effects on Sperm Swimming Performance. Vet. Sci. 2023, 10, 219. https://doi.org/10.3390/vetsci10030219
Rusco G, Di Iorio M, Esposito S, Antenucci E, Roncarati A, Iaffaldano N. The Use of Ovarian Fluid as Natural Fertilization Medium for Cryopreserved Semen in Mediterranean Brown Trout: The Effects on Sperm Swimming Performance. Veterinary Sciences. 2023; 10(3):219. https://doi.org/10.3390/vetsci10030219
Chicago/Turabian StyleRusco, Giusy, Michele Di Iorio, Stefano Esposito, Emanuele Antenucci, Alessandra Roncarati, and Nicolaia Iaffaldano. 2023. "The Use of Ovarian Fluid as Natural Fertilization Medium for Cryopreserved Semen in Mediterranean Brown Trout: The Effects on Sperm Swimming Performance" Veterinary Sciences 10, no. 3: 219. https://doi.org/10.3390/vetsci10030219
APA StyleRusco, G., Di Iorio, M., Esposito, S., Antenucci, E., Roncarati, A., & Iaffaldano, N. (2023). The Use of Ovarian Fluid as Natural Fertilization Medium for Cryopreserved Semen in Mediterranean Brown Trout: The Effects on Sperm Swimming Performance. Veterinary Sciences, 10(3), 219. https://doi.org/10.3390/vetsci10030219






