Simple Summary
Canine hemangiosarcoma is an aggressive cancer with a short median survival time, commonly found in the spleen of older dogs. There are limited tests available to provide clinically useful prognostic information to guide therapies once a diagnosis is made. Predictors of survivability investigated in this case series included the application of a previously published tumor histological grading scheme, histological assessment of tumor cell atypia, clinical staging, and the level of CD 31 expression by tumor cells in 16 dogs diagnosed with splenic hemangiosarcoma. Medical records were reviewed, the time of death was obtained, and survival data were analyzed statistically. Histopathological grading, cellular atypia, and clinical staging of canine splenic hemangiosarcomas were not significantly associated with the median survival time of the study dogs. Strong expression of CD 31 by canine splenic hemangiosarcoma tumor cells was observed in dogs with short survival times. This study provides a basis for future work to further investigate the prognostic usefulness of CD 31 expression for determining the survivability of canine splenic hemangiosarcoma patients.
Abstract
Canine hemangiosarcoma is a common, highly fatal tumor of older dogs, and predictors of survivability may provide clinically useful information. The objectives of this case series were to determine if a previously published tumor histological grading scheme, the level of tumor cellular atypia, clinical staging, or the level of CD 31 expression were useful for predicting the survival time in dogs with splenic hemangiosarcoma. Canine splenic hemangiosarcomas from 16 dogs were histologically graded, clinically staged, and assessed for CD 31 expression. Medical records were reviewed, the date of death was obtained, and survival data were analyzed statistically. Histopathological grading and clinical staging of canine splenic hemangiosarcomas, and the expression of CD 31 by the tumor cells were not significantly associated with the median survival time of the dogs in this study. However, strong expression of CD 31 by canine splenic hemangiosarcoma tumor cells was observed in dogs with short survival times, which warrants further studies to evaluate the potential prognostic value of CD 31 expression for the survival of dogs with splenic hemangiosarcoma.
1. Introduction
Canine hemangiosarcoma (HSA) is an aggressive cancer commonly found in older dogs, and splenic HSA is the commonest form of the disease, representing approximately half of the HSA cases in dogs [1,2,3,4]. Median survival times (MST) of dogs treated with splenectomy alone is less than 3 months [3,5,6]. To date, the most prognostically useful factor for canine hemangiosarcoma is the clinical staging of the disease, with dogs at stage I having longer survival times compared with dogs at stages II and III [3,6,7,8,9], but the usefulness of clinical staging is somewhat disputed in the literature, [10,11,12] with the administration of chemotherapy post splenectomy generally accepted as the most useful factor to extend survival time [5,6,7,9].
Histological grading of canine HSA was first proposed by Ogilvie et al. 1996 [7] but is not routinely performed as there little data to support its prognostic value. However, tumors exhibiting increased mitotic counts may be associated with shortened survival times [13]. Immunohistochemistry (IHC) is not routinely required by veterinary pathologists to reach a diagnosis of canine splenic HSA but may be useful to identify poorly differentiated tumors or epithelioid variants of HSA using endothelial markers such as CD 31, Factor VIII, and von Willebrand Factor (vWF) [14,15,16,17]. CD 31, or platelet endothelial cell adhesion molecule-1 (PECAM), is an adhesion receptor expressed by endothelial cells involved in regulating apoptosis and maintaining vascular permeability and integrity [18,19]. Canine HSA tumor expression of these endothelial markers has not been specifically investigated for their usefulness in predicting post-splenectomy survival although dogs with less differentiated tumors may fail to express these markers [17,20,21]. Cellular atypia was assessed as per the scoring scheme of Maharani et al., 2018 [21].
In this study, clinical stage, histological grade, cellular atypia, and the expression level of CD 31 were determined in 16 dogs with splenic HSA, and their potential associations with the survival time of dogs post splenectomy were evaluated. In addition, the survival times of dogs were compared based on each individual histological feature of the published grading scheme of Ogilvie et al. 1996 [7] (Table 1), which included mitotic count and tumor differentiation.

Table 1.
Canine splenic hemangiosarcoma grading scheme from Ogilvie et al. 1996 [7].
2. Materials and Methods
2.1. Animals and Study Design
All canine splenic HSA cases from 2018 to 2021 were retrieved from the electronic medical record database of City University of Hong Kong’s Veterinary Diagnostic Laboratory (VDL). Dogs were included in this study based on the following selection criteria: splenectomized dogs with a histological confirmation of splenic HSA, successfully discharged from hospital following splenectomy as their sole source of treatment, with the time of death recorded. Dogs excluded from this study were those who received adjunct chemotherapy post splenectomy, which can introduce a confounding effect on survivability, as various treatment regimens are known to improve survival times [3,5,6,7,9], and we intended to examine the potential effect, if any, of CD 31 expression by tumor cells on post splenectomy survival times.
Splenectomized HSA dogs that died or were euthanized before hospital discharge and dogs whose clinical outcomes were lost to follow-up were also excluded from this study. Sixteen dogs met the eligibility criteria, and the patient’s sex, breed, and age at the time of splenic HSA diagnosis were recorded (Table 2). The survival time for each dog was determined from the clinical records and defined from the date of splenectomy to the date of death. Clinical staging at the time of splenectomy was performed for all dogs based on abdominal ultrasound, echocardiogram, and/or 3-view thoracic radiographs, as well as visual assessment of abdominal organs during surgery. The previously established three-tiered staging system (stages I–III) for canine patients with HSA was used, based on the size of the primary tumor, evidence of tumor rupture, and presence of regional lymph node and/or distant metastasis [22].

Table 2.
Demographic information at the time of splenectomy, clinical stage, cellular atypia score, histological grade, mitotic count, CD 31 intensity, and survival time post splenectomy for 16 dogs with splenic hemangiosarcoma.
2.2. Histology
Hematoxylin and eosin (H&E)-stained glass slides of splenic tissue, previously fixed in 10% neutral buffered formalin and embedded in paraffin cassettes, were retrieved for each dog. For each case, 5–7 slides were originally created to confirm a diagnosis of splenic HSA as recommended by Herman et al. 2019 [23]. For each dog, a single slide was identified as being most representative of the tumor cell population and this slide was chosen for histological grading and assessment of cellular atypia, with the matching paraffin block retrieved from storage for IHC staining.
Each H&E glass slide was graded as per Ogilvie et al. 1996 [7], summarized in Table 1. In brief, four histological parameters for neoplastic endothelial cells were assessed: cellular differentiation, nuclear pleomorphism, mitotic count, and percentage of necrosis. Cellular differentiation is related to the ability of neoplastic endothelial cells to form recognizable vessels. Nuclear pleomorphism equated to uniformity of nuclear size and shape between tumor cells and represented a measure of cellular atypia. Mitotic count was calculated by counting all mitotic figures in 10 high-power fields exhibiting the highest mitotic activity. As the microscope had an ocular field number of 22, the total area evaluated was 2.37 mm2; mitotic count/2.37 mm2. Tumor necrosis was histologically defined by abundant cell debris, loss of tissue architecture, and/or loss of H&E tinctorial staining intensity. Each factor combined to create a final score equating to the three-tiered histological grade. Grading was performed independently by the first (K.T.C.) and last author (J.R.S.). If grades differed, repeat grading was performed by both authors simultaneously, so that a consensus score was reached, which was then recorded as a data point. Cellular atypia was scored as per Maharani et al., 2018 [21]. Neoplastic endothelial cells were given a score of 1 if the tumor cells were spindle-shaped with an elongated nucleus (width less than 1 erythrocyte). Score 2 cells were spindle-shaped with a plump oval nucleus (width of approximately 1–2 erythrocytes). Score 3 cells were polygonal- or cuboidal-shaped with plump cytoplasm, and a large round or irregularly shaped nucleus in which three or more erythrocytes could easily fit.
2.3. Immunohistochemistry
CD 31 IHC was performed on the same tissue section used for grading, using the Bond-III Fully Automated IHC and ISH Staining System (Leica Biosystems, Newcastle, United Kingdom). A 4 µm section was cut from each paraffin block, deparaffinized with descending concentrations of alcohol and xylene, and rinsed in tap water followed by distilled water. Heat-induced epitope retrieval was performed using the BOND Epitope Retrieval Solution 2 (ER2) (EDTA, pH 9.0; Leica Biosystems, Newcastle, UK) at 100 °C for 30 min. Tissue samples were washed 3 times with Bond Wash Solution (Leica Biosystems, Newcastle, UK). For blocking, sections were incubated in hydrogen peroxide for 10 min at room temperature (RT), followed by 3 repeat washings with Bond wash solution. Sections were then incubated at RT with anti-CD 31 in a 1:30 working solution (Monoclonal Mouse Anti-Human CD 31, Endothelial Cell, Cide M0823, Agilent Technologies, Santa Clara, CA, USA) for 15 min, and sections were washed 3 times with the Bond Wash Solution. The 3,3’Diaminobenzidine (DAB) detection system was used as the chromagen. Sections were treated with a post primary solution for 8 min at RT, washed with Bond wash 3 times for 2 min each then treated with the polymer solution for 8 min, washed for 2 min in Bond wash 3 times, followed by 3 2-min washes with distilled water. The final steps were treatment with DAB solution for 10 min, followed by 3 washes with distilled water, then counterstaining with hematoxylin. For each case, a positive and negative control tissue sample was included. Positive controls represented paraffin-embedded tissues from previously diagnosed cases of canine vascular neoplasms, including hemangioma or well-differentiated HSA, prepared under the same staining protocol to ensure identical staining conditions. A negative control slide was produced with the same protocol on HSA tissue, with the exclusion of the primary anti-CD 31 antibody.
Tissue from one dog (dog 2), with a poorly differentiated splenic HSA, had vWF and microglia (Iba1) IHC performed on representative splenic tissue during the initial diagnostic workup of the case. For both of these antibodies, the same protocol was utilized as for CD 31 with the following exceptions. Iba1 (BIOCARE Medical, Pacheco, CA, USA) heat epitope antigen retrieval was performed for 30 min using Epitope Retrieval Solution 1 (ER1) (citrate, pH 6.0; Leica Biosystems, Newcastle, UK). Blocking with hydrogen peroxide was performed for 5 min at RT and the primary antibody was used as a 1:33 working solution incubated for 30 min at RT and vWF (DAKO Omnis, Agilent, Santa Clara, CA, USA) as a 1:200 working solution. Heat epitope antigen retrieval was performed for 20 min using ER2 (EDTA, pH 9.0; Leica Biosystems, Newcastle, UK). Blocking with hydrogen peroxide was performed for 10 min at RT.
Positive expression of CD 31 by tumor cells was characterized by either brown DAB staining of the cell membrane or both the cell membrane and cytoplasm. Expression was scored from 0 to 3 (0 = no brown staining, 1 = pale brown membranous staining with minimal/no cytoplasmic staining, 2 = coarse mid/dark brown stippling of the cytoplasm with or without membranous staining, 3 = diffuse dark brown cytoplasmic/membranous staining of tumor cells), based on the protocol of Maharani et al., 2018. [21]. Positive control tissues were included and endothelial cells near the tumor mass were utilized as internal positive controls.
2.4. Statistical Analysis
Due to the low number of dogs in this study and their imbalanced distribution within some categories of the predictor variables of interest, all predictors were dichotomized to enable more robust screening for potential statistical associations between each variable and the survival time as the outcome of interest (Table 3). Within each category of predictors, median survival time (MST) and the corresponding range were calculated and compared using the non-parametric “equality-of-median test” in Stata v17 (StataCorp, College Station, TX, USA). A significance level of 0.05 was considered in all statistical tests. Two Fisher’s exact tests were conducted to determine whether clinical stage and histological grade had any associations with the intensity of CD 31 expression by tumor cells.

Table 3.
Screening for potential associations between the median survival time (MST) and the dichotomized predictors of interest for the 16 study dogs with splenic hemangiosarcoma.
3. Results
3.1. Demographics
Demographic information, clinical staging, histological grading, intensity of CD 31 expression, and survival time of canine splenic HSA patients post splenectomy are summarized in Table 2. Three Labradors, three schnauzers, two corgis, two bichon frises, two poodles, and one each of beagle, golden retriever, and dachshund and one mongrel dog were included (Table 2). Twelve dogs were male and four were female. The age of dogs ranged from 8 to 14 years, with a median of 11 years. For all dogs, the survival time ranged from 5 to 201 days, with a median of 68 days (Figure 1; Table 2).
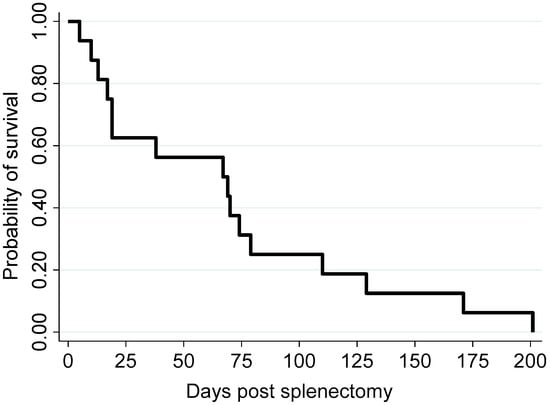
Figure 1.
Kaplan–Meier survival curve illustrating the survival probability of the 16 study dogs following splenectomy.
3.2. Clinical Staging
Clinical staging classified patients as follows: two dogs (dogs 2 and 8) were stage I with survival dates of 10 and 67 days, respectively, seven dogs (dogs 5, 9, 11, 12, 13, 15, 16) were stage II with survival dates between 19 and 201 days, and seven dogs (dogs 1, 3, 4, 6, 7, 10, 14) were stage III with survival dates between 5 and 129 days (Table 2). Eight dogs (dogs 2, 5, 6, 7, 8, 10, 12, 15) were euthanized, with the clinical reason recorded as poor quality of life with pallor and lethargy, and specifically for some dogs, severe anemia and thrombocytopenia (dog 2, stage I and dog 12, stage II), moderate anemia (dog 8, stage I), severe anemia (dog 15, stage II), a nonresectable liver mass (dog 10, stage III), and shock (dog 6, stage III). Two dogs (dogs 1 and 3, both stage III) were reported to have passed away by their owners, and details of death were not recorded for six dogs (dogs 4, 9, 11, 13, 14, 16).
3.3. Histological Grade and CD 31 Expression
There were seven grade 1 tumors (dogs 1, 8, 9, 10, 11, 12, 13), nine grade 2 tumors (dogs 2, 3, 4, 5, 6, 7, 14, 15, 16) and no grade 3 tumors (Table 2). Tumor cells from one dog (dog 2) failed to express CD 31, one dog (dog 6) had weak (intensity 1) CD 31 expression, six dogs (dogs 10, 11, 12, 13, 15, 16) had moderate (intensity 2), and eight dogs (dogs 1, 3, 4, 5, 7, 8, 9, 14) had strong (intensity 3) CD 31 expression (Table 2, Figure 2). Dogs with moderate CD 31 expression (intensity 2) tended to have the longest survival times irrespective of their histological grade, whilst dogs with no (intensity 0), weak (intensity 1), or strong (intensity 3) CD 31 expression tended to have the shortest survival times.
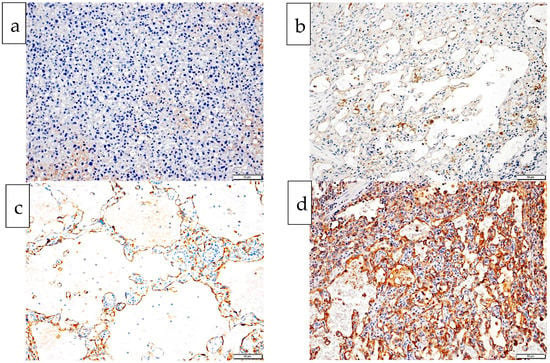
Figure 2.
Histological images from four dogs with splenic hemangiosarcoma exhibiting each of the four intensity levels of endothelial CD 31 expression (0 to 3). Chromogen is DAB, counterstained with hematoxylin. (a) Endothelial cells fail to express any CD 31. There is a small amount of patchy pale brown background staining: CD 31 intensity 0, dog 2. (b) Endothelial cell membrane is stained pale brown with minimal/no cytoplasmic staining: CD 31 intensity 1, dog 6. (c) Endothelial cell cytoplasm show coarse mid/dark brown stippling of the cytoplasm with or without membranous staining: CD 31 intensity 2, dog 13. (d) Endothelial cells show diffuse dark brown cytoplasmic/membranous staining: CD 31 intensity 3, dog 14.
3.4. Cellular Atypia and CD 31 Expression
Endothelial cells from four dogs scored 1 on cellular atypia, and either intensity 2 (dogs 10, 12, 16) or 3 (dog 7) for CD 31 expression. Endothelial cells from nine dogs scored 2 on cellular atypia and either scored 1 (dog 6), 2 (dogs 11, 13, 15), or 3 (dogs 1, 3, 8, 9, 14) for intensity of CD 31 expression. Endothelial cells from three dogs scored 3 on cellular atypia and either scored 3 (dogs 4, 5) or 0 (dog 2) for intensity of CD 31 expression.
Cellular atypia as per Maharani et al., 2018 [21]. Score 1: spindle-shaped cells with elongated nucleus (width less than 1 erythrocyte). Score 2: spindle-shaped cells with plump oval nucleus (width of approximately 1–2 erythrocytes). Score 3: polygonal- or cuboidal-shaped cells with plump cytoplasm and a large round nucleus in which three or more erythrocytes could easily fit.
Mitotic count. Total number of mitotic figures counted in 10 high power field (×40 objective) using an ocular with a field number of 22. Equates to total number of mitotic figures in 2.37 mm2.
CD 31 intensity. Intensity of CD 31 expression by tumor cells (brown coloration of tumor cells) on histopathology based on Maharani et al., 2018 [21]. (0 = no brown staining, 1 = pale brown membranous staining with minimal/no cytoplasmic staining, 2 = coarse mid/dark brown stippling of the cytoplasm with or without membranous staining, 3 = diffuse dark brown cytoplasmic/membranous staining of tumor cells).
Table 3 depicts the distribution of survival time (median and range) by the predictor variables of interest. None of the predictors had a statistically significant association with the survival time of all study dogs (all p-values > 0.1). MST for dogs with level 2 CD 31 expression intensity was 94.5 days compared with dogs with level 3 CD 31 expression, which was 28.5 days, indicating that dogs with tumor cells with level 3 (high) CD 31 expression (dogs 1, 3, 4, 5, 7, 8, 9, 14) had the lowest MST (Table 2). No statistically significant associations were observed between clinical stage, histological grade, or cellular atypia and the intensity of CD 31 expression in tumor cells of the 16 patients (p = 0.315 and p = 0.999, respectively).
4. Discussion
Clinical staging of 16 dogs diagnosed with splenic HSA, treated by splenectomy alone, showed no significant correlation with survival times. Histological grading of canine splenic HSA, using the scheme of Ogilvie et al. 1996 [7] and assessment of cellular atypia as per Maharani et al., 2018 [21] were not useful in predicting survival times. While dogs with tumor cells that strongly expressed CD 31 (CD 31 expression intensity of level 3) had shorter survival times compared to those with lower CD 31 intensity levels; this difference was not statistically significant. With respect to the descriptive nature of the current study (case series), sample selection process, and the limited number of cases, the results of statistical comparisons must be interpreted with caution and only considered as a “hypothesis generator” to be tested and validated in follow-up studies specifically designed to evaluate the prognostic value of the outlined predictors.
Study dogs included twelve males and four females, similar to the reported slight male predisposition for this disease [1,14,20,24]; however, the observed sex ratio (12/4) could have been a result of sample selection in this case series. The median age at diagnosis of splenic HSA in this study was 11 years, consistent with the reported age predisposition of canine splenic HSA [1,2,3,11,20]. The age of dogs in this study had a narrow distribution, so age was not expected to confound the analysis of survival times in our study.
The lack of correlation between the clinical stage of canine splenic HSA and survival time in this study is similar to previous reports [10,11,12], but is at odds with other studies [3,4,6,7,8,9,22,25], which may highlight the need for the further investigation of other, more reliable predictors of survival times. The MST of dogs in the current study was 68 days, similar to previously reported MST ranges of 19 to 86 days in dogs with splenic HSA treated by splenectomy alone [3,5,6,11]. None of the 16 dogs were alive one year after their diagnosis of splenic HSA. This finding was consistent with the short survival of dogs with visceral HSA (splenic HSA representing the majority of visceral cases), who typically die within a year despite treatment [3,7,8,13,26,27]. Half of the dogs in this study were euthanized (8/16 dogs; dogs 2, 5, 6, 7, 8, 10, 12, 15) because of poor quality of life post splenectomy, commonly associated with lethargy and anemia [4,12,22,28].
We did not observe any correlations between survival time and the individual histopathological features of neoplastic cells, including the mitotic count and degree of nuclear pleomorphism. These histopathological features have been reported to be associated with the survival time in dogs treated for canine HSA by splenectomy and chemotherapy [7,13]. However, post-splenectomy chemotherapy represents a confounding effect on survivability, as various treatment regimens are known to improve survival times [3,5,6,7,9].
Tumor cells from three dogs in this study exhibiting the most cellular atypia (score of 3) had survival times of 10, 17, and 19 days (dogs 2, 4, 5), shorter than the MST of 68 days for this study. This finding may suggest that increasing levels of cellular atypia could represent a negative prognostic indicator for dogs with splenic HSA. When cellular atypia was linked with CD 31 expression, no clear pattern was seen to support the finding of Maharani et al., 2018 [21], where loss of the intensity of CD 31 expression by tumor cells was seen with increasing cellular atypia. Eight dogs (dogs 1, 3, 4, 5, 7, 8, 9, 14) with level 3 CD 31 expression intensity had a median survival time of 28.5 days, compared with a median survival time of median 94.5 days for six dogs (dogs 10, 11, 12, 13, 15, 16) with level 2 CD 31 expression intensity. Normal canine endothelial cells in control tissues for this study expressed CD 31 at levels 2 and 3, similar to previous studies [20], so a negative correlation of level 3 (strong) expression of CD 31 by neoplastic splenic endothelial cells with survival time in dogs post splenectomy is an interesting and unexpected finding. Increased expression of CD 31 may impart properties to neoplastic endothelial cells, aiding malignant behavior due to its role as a regulator of endothelial apoptosis. Upregulation of various cellular receptors such as CD 44, HER-2, HER-2 in malignant canine mammary tumors [29] and gain of C-MYC function in canine prostatic carcinomas [30] have been linked to increased invasiveness and metastatic potential of these tumors. Perhaps maintenance of cellular receptors such as CD 31 at levels similar to nonneoplastic endothelial cells imparts a survival tactic by neoplastic cells. Therefore, the influence of both cellular atypia and CD 31 expression as potential prognostic indicators for dogs with splenic HSA warrants further testing in future, well-designed studies.
The loss or reduction in CD 31 expression by two dogs in our study (dogs 2 and 6) is also of interest, as each dog had very short survival times of 10 and 19 days, respectively, post splenectomy. Absent or weak expression of various differentiation markers by tumor cells is reported in different types of canine tumors where it can represent a negative prognostic finding with an increased risk of invasive and metastatic disease [30,31,32]. Maharani et al., 2018 [21] showed that pleomorphic visceral HSA cells from 17 dogs had decreased expression of CD 31 and vWF, and others have reported rare canine HSAs with loss of CD 31 expression [16,17,20] but expression of CD 31 has not been previously correlated with survival times. Venkataramani et al. 2018 [33] assessed CD 31 expression within tissue cultures derived from angiosarcomas, the human counterpart to animal hemangiosarcomas. They found that tumors expressing CD 31LOW patterns represented prognostically worse tumors when compared with tumors expressing CD 31HIGH. Therefore, maintenance or over-expression, as well as reduction or loss of cellular receptor expression such as CD 31, may both impart different growth advantages to neoplastic endothelial cells, but further studies are needed to determine their prognostic usefulness. In addition, correlating the success rate that chemotherapeutic agents extend the survival times of dogs with splenic HSA with tumor expression of CD 31 would be of interest, as CD 31LOW endothelial cells in human angiosarcomas has been linked to resistance to various chemotherapeutic agents, including doxorubicin, which is used in the treatment of canine HSA [33].
Although there were over 200 cases of splenic HSA cases in our database, the majority of dogs were excluded from our study due to the loss of clinical follow-up post splenectomy. Another limitation of our study, common to most studies investigating survival times in dogs with HSA, was the lack of postmortem data, so that the death of these dogs was assumed to be attributed solely to their clinical diagnosis of splenic HSA. A final limitation in this study involves problems with repeatability when applying the grading schemes discussed in this paper. There is a high likelihood of generating data with considerable inter-pathologist variation, especially when scoring the intensity of CD 31 expression by tumor cells, which may limit the ability to differentiate between CD 31 intensity levels 2 and 3.
5. Conclusions
Clinical staging, histological grading, cellular atypia, and individual histological criteria provided no prognostic information in terms of survivability post splenectomy for the 16 canine HSA patients studied in this case series, which may not be reflective of the general population due to the small number of cases and the case selection process. Over- or underexpression of CD 31 may provide useful information in predicting the survival time in dogs with HSA post splenectomy. Further studies with larger sample sizes are warranted to specifically evaluate the expressivity of CD 31 by the tumor cells, and its prognostic value, either from splenectomized patients or possibly using splenic aspirates. CD 31 expression could also be assessed to examine the role that the loss of this marker may have in chemotherapeutical treatment failures of canine splenic HSA.
Author Contributions
Conceptualization, K.-T.C. and J.R.S.; methodology, J.R.S. and K.-T.C.; software, O.N.; investigation, J.R.S. and K.-T.C.; Resources, J.R.S., K.-T.C. and O.N.; data curation, J.R.S., K.-T.C. and O.N.; writing—original draft preparation, K.-T.C.; writing—review and editing, J.R.S., K.-T.C. and O.N.; Supervision, J.R.S. and O.N.; Statistical analysis, O.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. This study presented data collected from veterinary disease investigation of privately owned dogs. IHC was funded by the corresponding author’s internal resources (J.S.).
Institutional Review Board Statement
Ethical review and approval were waived for this study because all animal samples were archived clinical materials used with permission.
Informed Consent Statement
Written informed consent for publication was obtained from the authorized agent of the owners for all privately owned dogs.
Data Availability Statement
All relevant data have been included in this submission. Additional data exist, incorporated as part of confidential medical records of the attending veterinary hospital.
Acknowledgments
We thank MUI Chi Keung for preparing all of the H&E-stained histology slides and performing immunohistochemistry for all dogs in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schultheiss, P.C. A retrospective study of visceral and nonvisceral hemangiosarcoma and hemangiomas in domestic animals. J. Vet. Diagn. Investig. 2004, 16, 522–526. [Google Scholar] [CrossRef]
- Prymak, C.; McKee, L.J.; Goldschmidt, M.; Glickman, L.T. Epidemiologic, clinical, pathologic, and prognostic characteristics of splenic hemangiosarcoma and splenic hematoma in dogs: 217 cases (1985). J. Am. Vet. Med. Assoc. 1988, 193, 706–712. [Google Scholar]
- Batschinski, K.; Nobre, A.; Vargas-Mendez, E.; Tedardi, M.V.; Cirillo, J.; Cestari, G.; Ubukata, R.; Dagli, M.L.Z. Canine visceral hemangiosarcoma treated with surgery alone or surgery and doxorubicin: 37 cases (2005–2014). Can. Vet. J. 2018, 59, 967–972. [Google Scholar]
- Griffin, M.A.; Culp, W.T.N.; Rebhun, R.B. Canine and feline haemangiosarcoma. Vet. Rec. 2021, 189, e585. [Google Scholar] [CrossRef]
- Kim, S.E.; Liptak, J.M.; Gall, T.T.; Monteith, G.J.; Woods, J.P. Epirubicin in the adjuvant treatment of splenic hemangiosarcoma in dogs: 59 cases (1997–2004). J. Am. Vet. Med. Assoc. 2007, 231, 1550–1557. [Google Scholar] [CrossRef] [PubMed]
- Wendelburg, K.M.; Price, L.L.; Burgess, K.E.; Lyons, J.A.; Lew, F.H.; Berg, J. Survival time of dogs with splenic hemangiosarcoma treated by splenectomy with or without adjuvant chemotherapy: 208 cases (2001–2012). J. Am. Vet. Med. Assoc. 2015, 247, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Ogilvie, G.K.; Powers, B.E.; Mallinckrodt, C.H.; Withrow, S.J. Surgery and Doxorubicin in Dogs with Hemangiosarcoma. J. Vet. Intern. Med. 1996, 10, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Hammer, A.S.; Couto, C.G.; Filppi, J.; Getzy, D.; Shank, K. Efficacy and Toxicity of VAC Chemotherapy (Vincristine, Doxorubicin, and Cyclophosphamide) in Dogs with Hemangiosarcoma. J. Vet. Intern. Med. 1991, 5, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Sorenmo, K.U.; Baez, J.L.; Clifford, C.A.; Mauldin, E.; Overley, B.; Skorupski, K.; Bachman, R.; Samluk, M.; Shofer, F. Efficacy and toxicity of a dose-intensified doxorubicin protocol in canine hemangiosarcoma. J. Vet. Intern. Med. 2004, 18, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.O.; Patnaik, A.K.; MacEwen, E.G. Canine hemangiosarcoma: Retrospective analysis of 104 cases. J. Am. Vet. Med. Assoc. 1985, 186, 56–58. [Google Scholar] [PubMed]
- Johnson, K.; Powers, B.; Withrow, S.; Sheetz, M.; Curtis, C.; Wrigley, R. Splenomegaly in dogs: Predictors of neoplasia and survival after splenectomy. J. Vet. Intern. Med. 1989, 3, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.A.; Moore, A.S.; Gliatto, J.M.; Ablin, L.A.; Berg, R.J.; Rand, W.M. Prognosis for dogs with stage I or II splenic hemangiosarcoma treated by splenectomy alone: 32 cases (1991–1993). J. Am. Anim. Hosp. Assoc. 1998, 34, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.S.; Rassnick, K.M.; Frimberger, A.E. Evaluation of clinical and histologic factors associated with survival time in dogs with stage II splenic hemangiosarcoma treated by splenectomy and adjuvant chemotherapy: 30 cases (2011–2014). J. Am. Vet. Med. Assoc. 2017, 251, 559–565. [Google Scholar] [CrossRef]
- Gamlem, H.; Nordstoga, K.; Arnesen, K. Canine vascular neoplasia--a population-based clinicopathologic study of 439 tumours and tumour-like lesions in 420 dogs. Apmis 2008, 116, 41–54. [Google Scholar] [CrossRef] [PubMed]
- von Beust, B.R.; Suter, M.M.; Summers, B.A. Factor VIII-related Antigen in Canine Endothelial Neoplasms: An Immunohistochemical Study. Vet. Pathol. 1988, 25, 251–255. [Google Scholar] [CrossRef]
- Ferrer, L.; Fondevila, D.; Rabanal, R.; Vilafranca, M. Immunohistochemical detection of CD31 antigen in normal and neoplastic canine endothelial cells. J. Comp. Pathol. 1995, 112, 319–326. [Google Scholar] [CrossRef]
- Gamlem, H.; Nordstoga, K. Canine vascular neoplasia—histologic classification and immunohistochemical analysis of 221 tumours and tumour-like lesions. Apmis 2008, 116, 19–40. [Google Scholar] [CrossRef]
- Tsuneki, M.; Madri, J.A. CD44 Regulation of Endothelial Cell Proliferation and Apoptosis via Modulation of CD31 and VE-cadherin Expression. J. Biol. Chem. 2014, 289, 5357–5370. [Google Scholar] [CrossRef]
- Privratsky, J.R.; Newman, P.J. PECAM-1: Regulator of endothelial junctional integrity. Cell Tissue Res. 2014, 355, 607–619. [Google Scholar] [CrossRef]
- Göritz, M.; Müller, K.; Krastel, D.; Staudacher, G.; Schmidt, P.; Kühn, M.; Nickel, R.; Schoon, H.-A. Canine Splenic Haemangiosarcoma: Influence of Metastases, Chemotherapy and Growth Pattern on Post-splenectomy Survival and Expression of Angiogenic Factors. J. Comp. Pathol. 2013, 149, 30–39. [Google Scholar] [CrossRef]
- Maharani, A.; Aoshima, K.; Onishi, S.; Gulay, K.C.M.; Kobayashi, A.; Kimura, T. Cellular atypia is negatively correlated with immunohistochemical reactivity of CD31 and vWF expression levels in canine hemangiosarcoma. J. Vet. Med. Sci. 2018, 80, 213–218. [Google Scholar] [CrossRef]
- Vail, D.M.; Thamm, D.H.; Liptak, J. Withrow and MacEwen’s Small Animal Clinical Oncology-E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Herman, E.J.; Stern, A.W.; Fox, R.J.; Dark, M.J. Understanding the Efficiency of Splenic Hemangiosarcoma Diagnosis Using Monte Carlo Simulations. Vet. Pathol. 2019, 56, 856–859. [Google Scholar] [CrossRef] [PubMed]
- Davies, O.; Taylor, A.J. Refining the “double two-thirds” rule: Genotype-based breed grouping and clinical presentation help predict the diagnosis of canine splenic mass lesions in 288 dogs. Vet. Comp. Oncol. 2020, 18, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Masyr, A.R.; Rendahl, A.K.; Winter, A.L.; Borgatti, A.; Modiano, J.F. Retrospective evaluation of thrombocytopenia and tumor stage as prognostic indicators in dogs with splenic hemangiosarcoma. J. Am. Vet. Med. Assoc. 2021, 258, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.A.; Mullin, C.M.; de Lorimier, L.P.; Burgess, K.E.; Risbon, R.E.; Fred, R.M., 3rd; Drobatz, K.; Clifford, C.A. Doxorubicin and deracoxib adjuvant therapy for canine splenic hemangiosarcoma: A pilot study. Can. Vet. J. 2013, 54, 237–242. [Google Scholar] [PubMed]
- Sorenmo, K.; Duda, L.; Barber, L.; Cronin, K.; Sammarco, C.; Usborne, A.; Goldschmidt, M.; Shofer, F. Canine hemangiosarcoma treated with standard chemotherapy and minocycline. J. Vet. Intern. Med. 2000, 14, 395–398. [Google Scholar] [CrossRef]
- Hammond, T.N.; Pesillo-Crosby, S.A. Prevalence of hemangiosarcoma in anemic dogs with a splenic mass and hemoperitoneum requiring a transfusion: 71 cases (2003–2005). J. Am. Vet. Med. Assoc. 2008, 232, 553–558. [Google Scholar] [CrossRef]
- Damasceno, K.A.; Ferreira, E.; Estrela-Lima, A.; Bosco, Y.; Silva, L.P.; Barros, A.L.; Bertagnolli, A.C.; Cassali, G.D. Relationship between the expression of Versican and EGFR, HER-2, HER-3 and CD44 in matrix-producing tumours in the canine mammary gland. Histol. Histopathol. 2016, 31, 675–688. [Google Scholar] [CrossRef]
- Fonseca-Alves, C.E.; Rodrigues, M.M.; de Moura, V.M.; Rogatto, S.R.; Laufer-Amorim, R. Alterations of C-MYC, NKX3.1, and E-cadherin expression in canine prostate carcinogenesis. Microsc. Res. Tech. 2013, 76, 1250–1256. [Google Scholar] [CrossRef]
- Klopfleisch, R.; Schütze, M.; Gruber, A. Loss of p27 expression in canine mammary tumors and their metastases. Res. Vet. Sci. 2010, 88, 300–303. [Google Scholar] [CrossRef]
- Gamba, C.D.O.; Damasceno, K.A.; Ferreira, I.C.; Rodrigues, M.A.; Gomes, D.; Alves, M.R.; Rocha, R.M.; Lima, A.E.; Ferreira, E.; Cassali, G.D. The investigation of transcriptional repression mediated by ZEB2 in canine invasive micropapillary carcinoma in mammary gland. PLoS ONE 2019, 14, e0209497. [Google Scholar] [CrossRef]
- Venkataramani, V.; Küffer, S.; Cheung, K.C.; Jiang, X.; Trümper, L.; Wulf, G.G.; Ströbel, P. CD31 Expression Determines Redox Status and Chemoresistance in Human Angiosarcomas. Clin. Cancer Res. 2018, 24, 460–473. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).