Analysis of the Prevalence of Canine Splenic Mass Lesions in Republic of Korea via Histopathological Diagnosis with Immunohistochemistry
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Sample Collection
2.3. Histopathological Evaluation
2.4. Immunohistochemistry
3. Results
3.1. Patient Information
3.2. Histopathological Evaluation and Immunohistochemistry
3.2.1. Nodular Hyperplasia
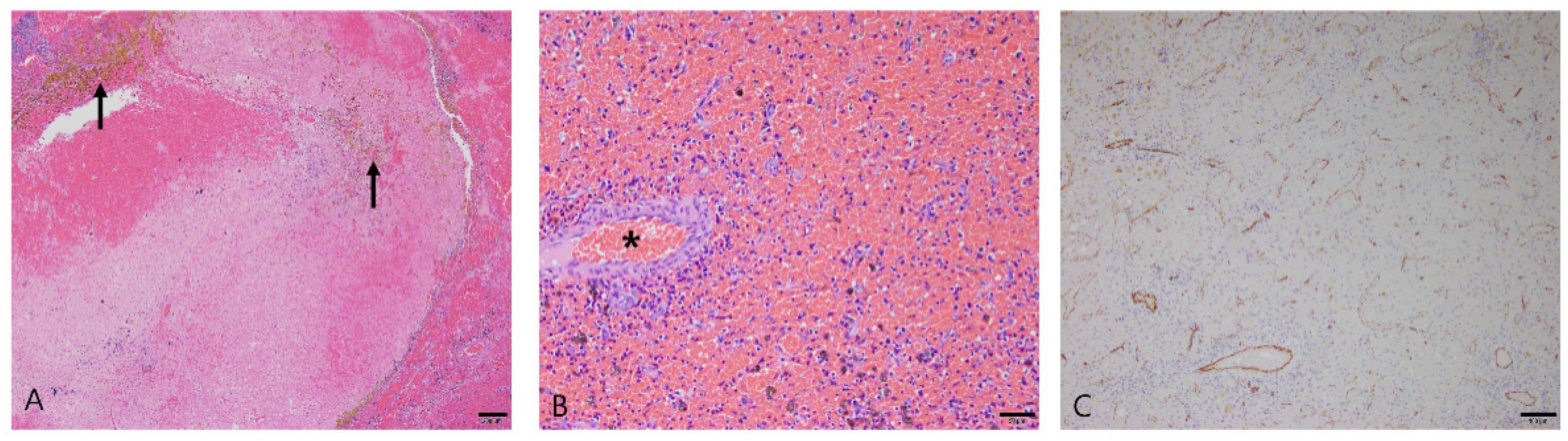
3.2.2. Hematoma
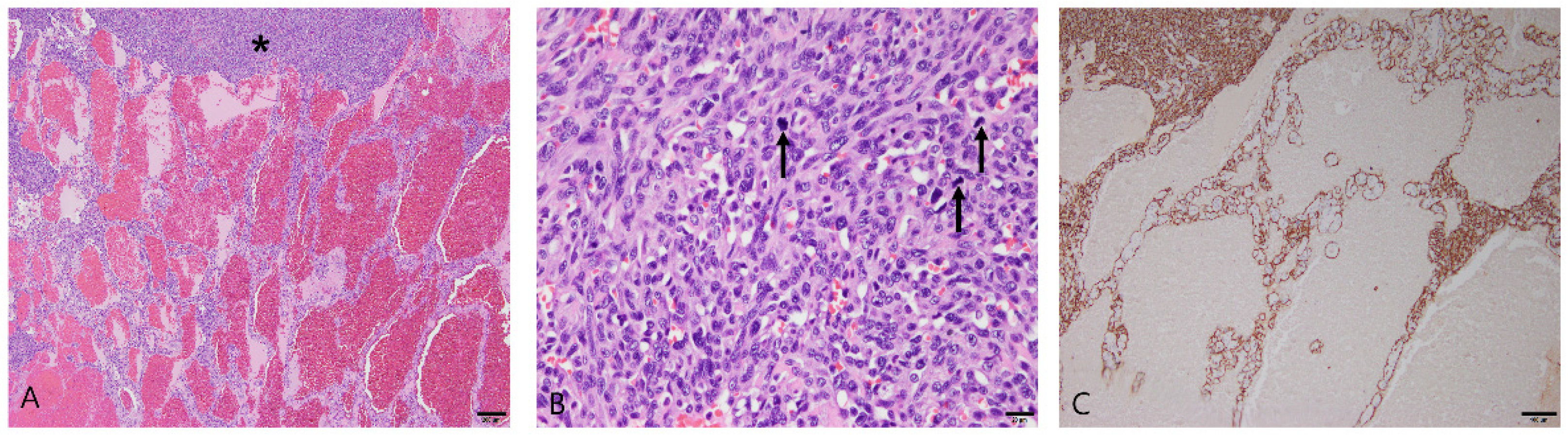
3.2.3. Splenic Hemangiosarcoma
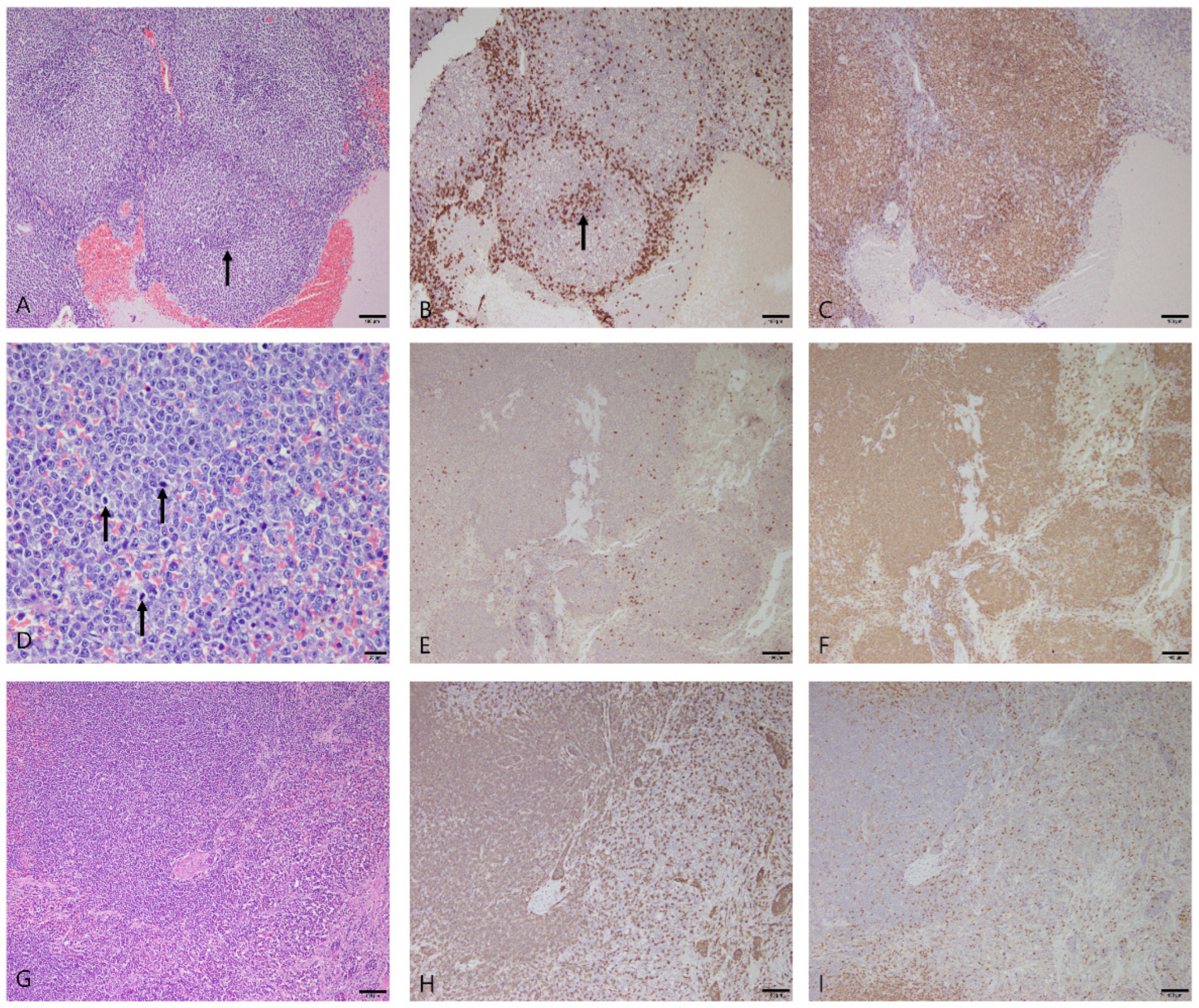
3.2.4. Splenic Lymphoma
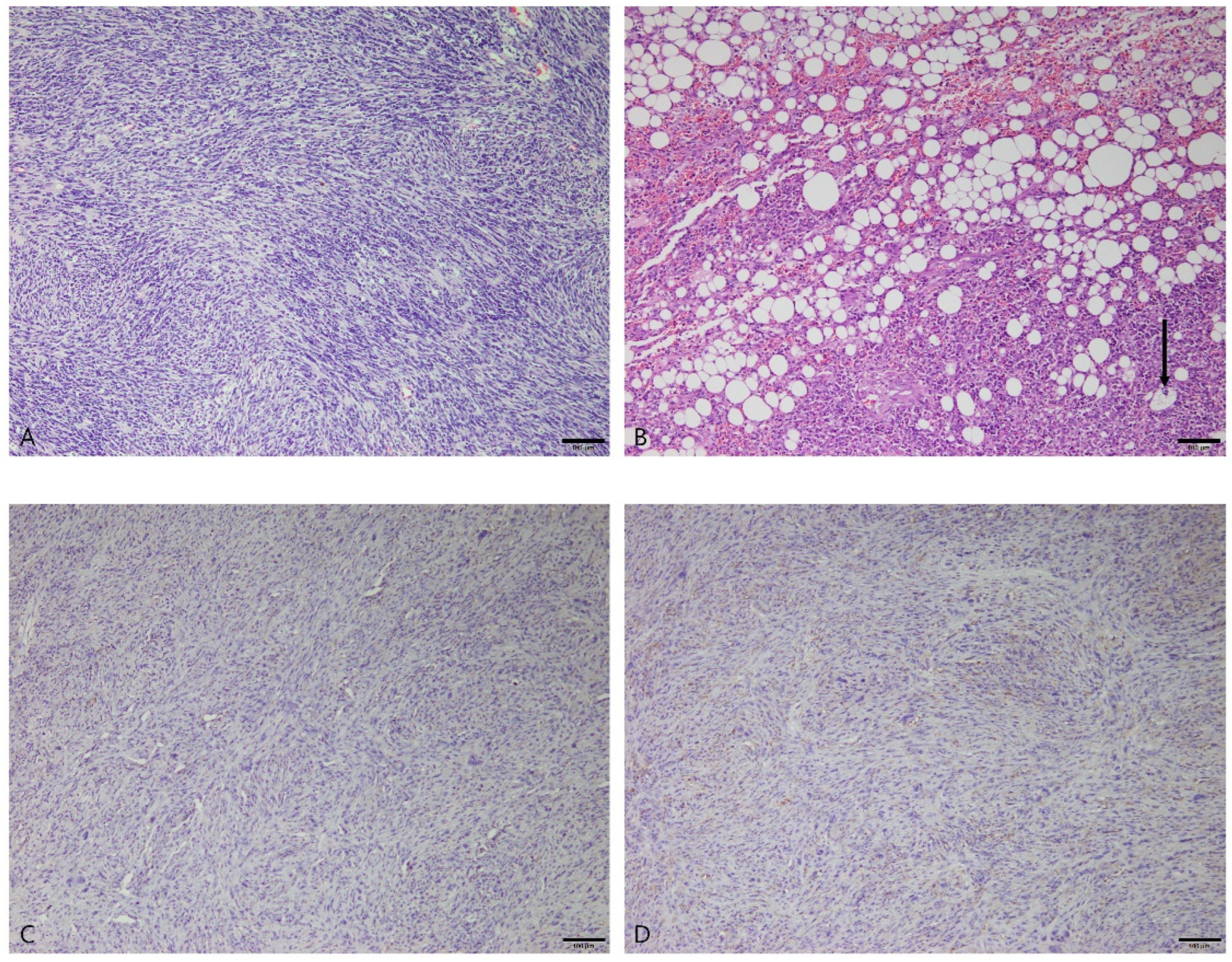
3.2.5. Stromal Sarcomas
3.2.6. Myelolipoma
3.2.7. Mast Cell Tumor
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaltreider, H.B.; Barth, E.; Pellegrini, C. The Effect of Splenectomy on the Appearance of Specific Antibody-Forming Cells in Lungs of Dogs after Intravenous Immunization with Sheep Erythrocytes. Exp. Lung Res. 1981, 2, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Christensen, N.I.; Canfield, P.J.; Martin, P.A.; Krockenberger, M.B.; Spielman, D.S.; Bosward, K.L. Cytopathological and Histopathological Diagnosis of Canine Splenic Disorders. Aust. Vet. J. 2009, 87, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Ballegeer, E.A.; Forrest, L.J.; Dickinson, R.M.; Schutten, M.M.; Delaney, F.A.; Young, K.M. Correlation of Ultrasonographic Appearance of Lesions and Cytologic and Histologic Diagnoses in Splenic Aspirates from Dogs and Cats: 32 Cases (2002–2005). J. Am. Vet. Med. Assoc. 2007, 230, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Day, M.J.; Lucke, V.M.; Pearson, H. A Review of Pathological Diagnoses Made from 87 Canine Splenic Biopsies. J. Small Anim. Pract. 1995, 36, 426–433. [Google Scholar] [CrossRef]
- Morrison, W.B.; DeNicola, D.B. Advantages and Disadvantages of Cytology and Histopathology for the Diagnosis of Cancer. Semin. Vet. Med. Surg. Small Anim. 1993, 8, 222–227. [Google Scholar]
- Lee, M.; Park, J.; Choi, H.; Lee, H.; Jeong, S.M. Presurgical Assessment of Splenic Tumors in Dogs: A Retrospective Study of 57 Cases (2012–2017). J. Vet. Sci. 2018, 19, 827–834. [Google Scholar] [CrossRef]
- Brown, N.O.; Patnaik, A.K.; MacEwen, E.G. Canine Hemangiosarcoma: Retrospective Analysis of 104 Cases. J. Am. Vet. Med. Assoc. 1985, 186, 56–58. [Google Scholar]
- van Stee, L.L.; Boston, S.E.; Singh, A.; Romanelli, G.; Rubio-Guzman, A.; Scase, T.J. Outcome and Prognostic Factors for Canine Splenic Lymphoma Treated by Splenectomy (1995–2011). Vet. Surg. 2015, 44, 976–982. [Google Scholar] [CrossRef]
- Prymak, C.; McKee, L.J.; Goldschmidt, M.H.; Glickman, L.T. Epidemiologic, Clinical, Pathologic, and Prognostic Characteristics of Splenic Hemangiosarcoma and Splenic Hematoma in Dogs: 217 Cases (1985). J. Am. Vet. Med. Assoc. 1988, 193, 706–712. [Google Scholar]
- Johnson, K.A.; Powers, B.E.; Withrow, S.J.; Sheetz, M.J.; Curtis, C.R.; Wrigley, R.H. Splenomegaly in Dogs: Predictors of Neoplasia and Survival After Splenectomy. J. Vet. Intern. Med. 1989, 3, 160–166. [Google Scholar] [CrossRef]
- Spangler, W.L.; Culbertson, M.R. Prevalence, Type, and Importance of Splenic Diseases in Dogs: 1480 Cases (1985–1989). J. Am. Vet. Med. Assoc. 1992, 200, 829–834. [Google Scholar] [PubMed]
- Pintar, J.; Breitschwerdt, E.B.; Hardie, E.M.; Spaulding, K.A. Acute Nontraumatic Hemoabdomen in the Dog: A Retrospective Analysis of 39 Cases (1987–2001). J. Am. Anim. Hosp. Assoc. 2003, 39, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Story, A.L.; Wavreille, V.; Abrams, B.; Egan, A.; Cray, M.; Selmic, L.E. Outcomes of 43 Small Breed Dogs Treated for Splenic Hemangiosarcoma. Vet. Surg. 2020, 49, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Davies, O.; Taylor, A.J. Refining the “Double Two-Thirds” Rule: Genotype-Based Breed Grouping and Clinical Presentation Help Predict the Diagnosis of Canine Splenic Mass Lesions in 288 Dogs. Vet. Comp. Oncol. 2020, 18, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Meuten, D.J. Tumors in Domestic Animals, 5th ed.; Wiley/Blackwell: Ames, IA, USA, 2017; pp. 203–321. [Google Scholar]
- Bettini, G.; Mandrioli, L.; Brunetti, B.; Marcato, P. Canine Splenic Pathology: A Retrospective Study of 109 Surgical Samples, with Special Emphasis on Fibrohistiocytic Nodules. Eur. J. Vet. Pathol. 2001, 7, 101–109. [Google Scholar]
- Fernandez, S.; Lang, J.M.; Maritato, K.C. Evaluation of Nodular Splenic Lesions in 370 Small-Breed Dogs (<15 Kg). J. Am. Anim. Hosp. Assoc. 2019, 55, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Corbin, E.E.; Cavanaugh, R.P.; Schwartz, P.; Zawadzki, K.I.; Donovan, T. Splenomegaly in Small-breed Dogs: 45 Cases (2005–2011). J. Am. Vet. Med. Assoc. 2017, 250, 1148–1154. [Google Scholar] [CrossRef]
- Stewart, S.D.; Ehrhart, E.J.; Davies, R.; Khanna, C. Prospective Observational Study of Dogs with Splenic Mass Rupture Suggests Potentially Lower Risk of Malignancy and More Favourable Perioperative Outcomes. Vet. Comp. Oncol. 2020, 18, 811–817. [Google Scholar] [CrossRef]
- O’Byrne, K.; Hosgood, G. Splenic Mass Diagnosis in Dogs Undergoing Splenectomy According to Breed Size. Vet. Rec. 2019, 184. [Google Scholar] [CrossRef]
- Kim, E.; Choe, C.; Yoo, J.G.; Oh, S.I.; Jung, Y.; Cho, A.; Kim, S.; Do, Y.J. Major Medical Causes by Breed and Life Stage for Dogs Presented at Veterinary Clinics in the Republic of Korea: A Survey of Electronic Medical Records. PeerJ 2018, 2018, e5161. [Google Scholar] [CrossRef]
- Patten, S.G.; Boston, S.E.; Monteith, G.J. Outcome and Prognostic Factors for Dogs with a Histological Diagnosis of Splenic Hematoma Following Splenectomy: 35 Cases (2001–2013). Can. Vet. J. 2016, 57, 842. [Google Scholar]
- Maharani, A.; Aoshima, K.; Onishi, S.; Christian, K.; Gulay, M.; Kobayashi, A.; Kimura, T. Cellular Atypia Is Negatively Correlated with Immunohistochemical Reactivity of CD31 and VWF Expression Levels in Canine Hemangiosarcoma. J. Vet. Med. Sci. 2018, 80, 213–218. [Google Scholar] [CrossRef]
- Göritz, M.; Müller, K.; Krastel, D.; Staudacher, G.; Schmidt, P.; Kühn, M.; Nickel, R.; Schoon, H.A. Canine Splenic Haemangiosarcoma: Influence of Metastases, Chemotherapy and Growth Pattern on Post-Splenectomy Survival and Expression of Angiogenic Factors. J. Comp. Pathol. 2013, 149, 30–39. [Google Scholar] [CrossRef]
- Tabaran, F. Immunohistochemical Characterization of Hemangiosarcoma in Dogs. Vet. Med. 2010, 67, 372. [Google Scholar]
- Giuffrida, M.A.; Bacon, N.J.; Kamstock, D.A. Use of Routine Histopathology and Factor VIII-Related Antigen/von Willebrand Factor Immunohistochemistry to Differentiate Primary Hemangiosarcoma of Bone from Telangiectatic Osteosarcoma in 54 Dogs. Vet. Comp. Oncol. 2017, 15, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Bryant, R.J.; Banks, P.M.; O’Malley, D.P. Ki67 Staining Pattern as a Diagnostic Tool in the Evaluation of Lymphoproliferative Disorders. Histopathology 2006, 48, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Thalheim, L.; Williams, L.E.; Borst, L.B.; Fogle, J.E.; Suter, S.E. Lymphoma Immunophenotype of Dogs Determined by Immunohistochemistry, Flow Cytometry, and Polymerase Chain Reaction for Antigen Receptor Rearrangements. J. Vet. Intern. Med. 2013, 27, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Valli, V.E.; Myint, M.; Barthel, A.; Bienzle, D.; Caswell, J.; Colbatzky, F.; Durham, A.; Ehrhart, E.J.; Johnson, Y.; Jones, C.; et al. Classification of Canine Malignant Lymphomas According to the World Health Organization Criteria. Vet. Pathol. 2011, 48, 198–211. [Google Scholar] [CrossRef]
- Valli, V.E.; Vernau, W.; de Lorimier, L.P.; Graham, P.S.; Moore, P.F. Canine Indolent Nodular Lymphoma. Vet. Pathol. 2006, 43, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Valli, V.E.; Kass, P.H.; Myint, M.S.; Scott, F. Canine Lymphomas: Association of Classification Type, Disease Stage, Tumor Subtype, Mitotic Rate, and Treatment With Survival. Vet. Pathol. 2013, 50, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, M.J.; Carpenter, J.L.; Schunk, C.J. Nonangiogenic and Nonlymphomatous Sarcomas of the Canine Spleen: 57 Cases (1975–1987). J. Am. Vet. Med. Assoc. 1989, 195, 784–788. [Google Scholar] [PubMed]
- Spangler, W.L.; Culbertson, M.R.; Kass, P.H. Primary Mesenchymal (Nonangiomatous/Nonlymphomatous) Neoplasms Occurring in the Canine Spleen: Anatomic Classification, Immunohistochemistry, and Mitotic Activity Correlated with Patient Survival. Vet. Pathol. 1994, 31, 37–47. [Google Scholar] [CrossRef] [PubMed]


| Antibody | Supplier | Clone | Isotype | Dilution | Antigen Retrieval |
|---|---|---|---|---|---|
| CD31 | Dako | JC70A | Mouse IgG1 | 1:100 | Tris-EDTA (15 min) |
| CD3 | Dako | Polyclonal | Rabbit polyclonal | 1:200 | Citric acid (15 min) |
| PAX5 | BD Biosciences | 24/Pax-5 | Mouse IgG1 | 1:100 | Citric acid (15 min) |
| Iba1 | Fujifilm | Polyclonal | Rabbit polyclonal | 1:2000 | Citric acid (15 min) |
| C-kit | Dako | Polyclonal | Rabbit polyclonal | 1:400 | Citric acid (15 min) |
| Diagnosis | Number | Percentage | Total | |
|---|---|---|---|---|
| Non-neoplastic | Nodular hyperplasia | 66 | 48.2% | 99 (72.3%) |
| Hematoma | 33 | 24.1% | ||
| Neoplastic | Hemangiosarcoma | 14 | 10.2% | 38 (27.7%) |
| Lymphoma | 11 | 8.0% | ||
| Stromal sarcomas | 10 | 7.3% | ||
| Myelolipoma | 2 | 1.5% | ||
| Mast cell tumor | 1 | 0.7% | ||
| Total | 137 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, Y.-U.; Bae, M.-K.; Sur, J.-H.; Choe, N.-H. Analysis of the Prevalence of Canine Splenic Mass Lesions in Republic of Korea via Histopathological Diagnosis with Immunohistochemistry. Vet. Sci. 2023, 10, 247. https://doi.org/10.3390/vetsci10040247
Ko Y-U, Bae M-K, Sur J-H, Choe N-H. Analysis of the Prevalence of Canine Splenic Mass Lesions in Republic of Korea via Histopathological Diagnosis with Immunohistochemistry. Veterinary Sciences. 2023; 10(4):247. https://doi.org/10.3390/vetsci10040247
Chicago/Turabian StyleKo, Yeong-Ung, Min-Kyung Bae, Jung-Hyang Sur, and Nong-Hoon Choe. 2023. "Analysis of the Prevalence of Canine Splenic Mass Lesions in Republic of Korea via Histopathological Diagnosis with Immunohistochemistry" Veterinary Sciences 10, no. 4: 247. https://doi.org/10.3390/vetsci10040247
APA StyleKo, Y.-U., Bae, M.-K., Sur, J.-H., & Choe, N.-H. (2023). Analysis of the Prevalence of Canine Splenic Mass Lesions in Republic of Korea via Histopathological Diagnosis with Immunohistochemistry. Veterinary Sciences, 10(4), 247. https://doi.org/10.3390/vetsci10040247




