Histological Findings and T2 Relaxation Time in Canine Menisci of Elderly Dogs—An Ex Vivo Study in Stifle Joints
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Sample Preparation
2.3. X-ray Grading
2.4. MR Imaging
2.5. Histology
2.6. Statistical Analysis
3. Results
3.1. Study Population
3.2. Radiographic Osteoarthritis Score
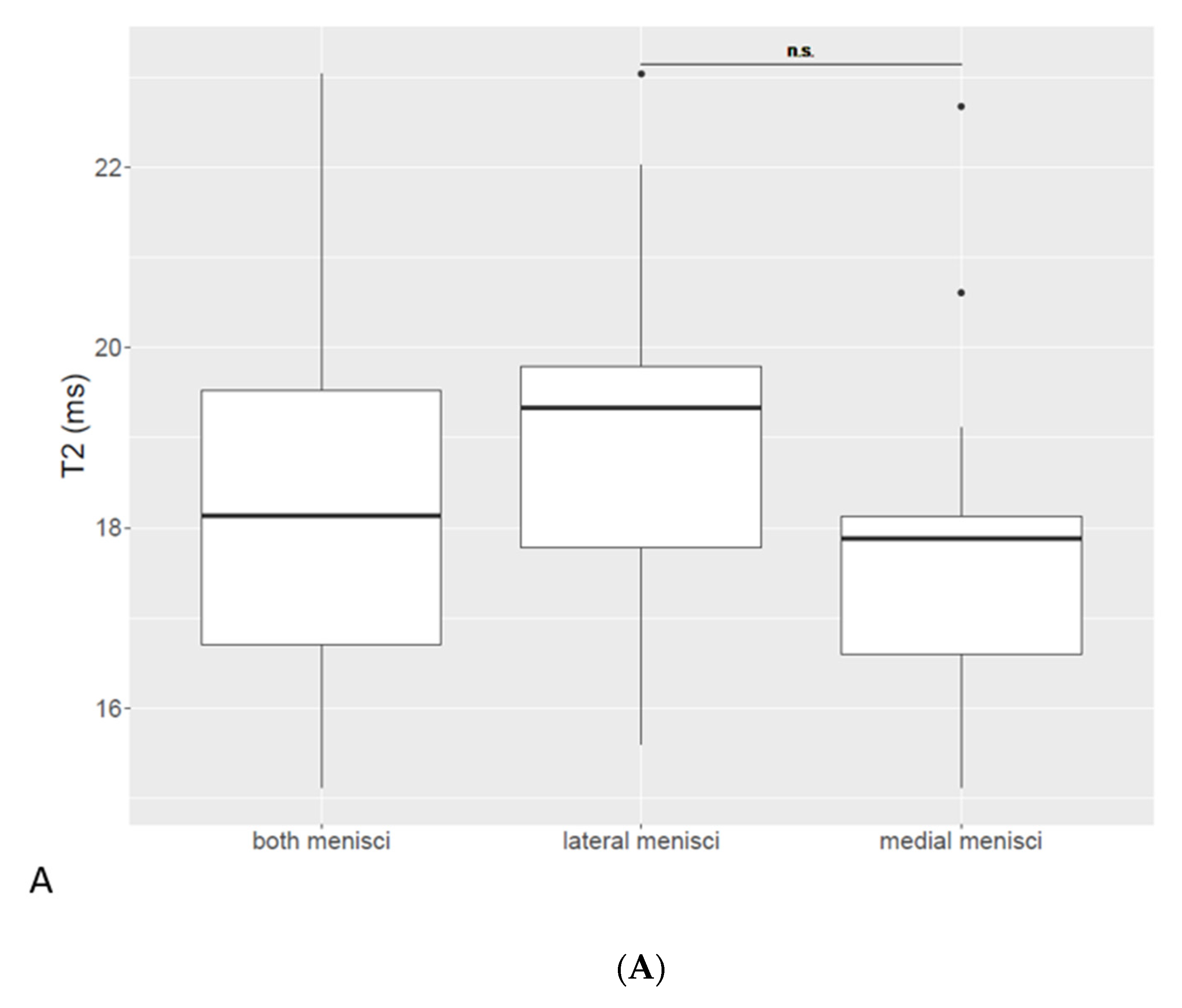
3.3. T2 Relaxation Time in Meniscal Tissue
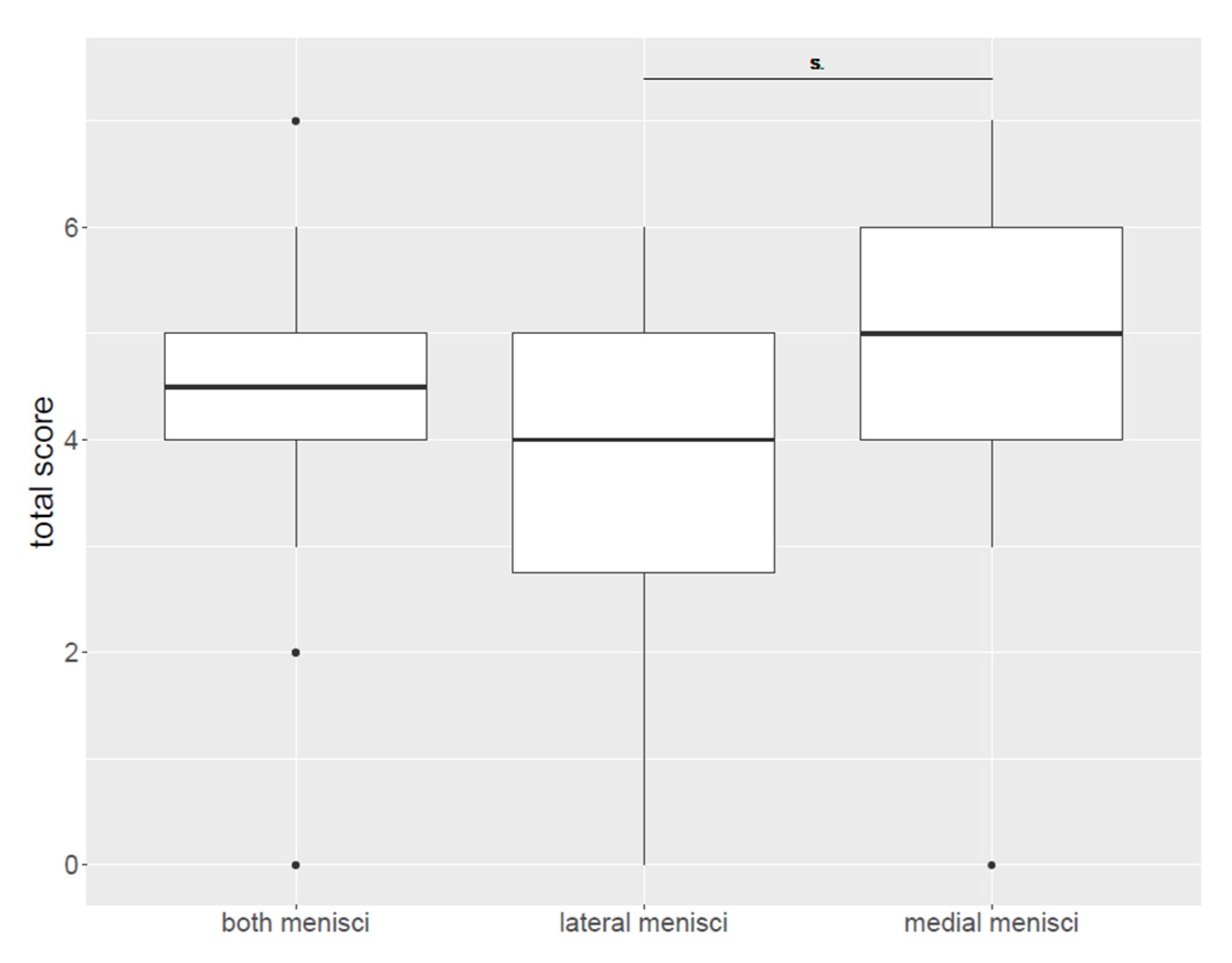
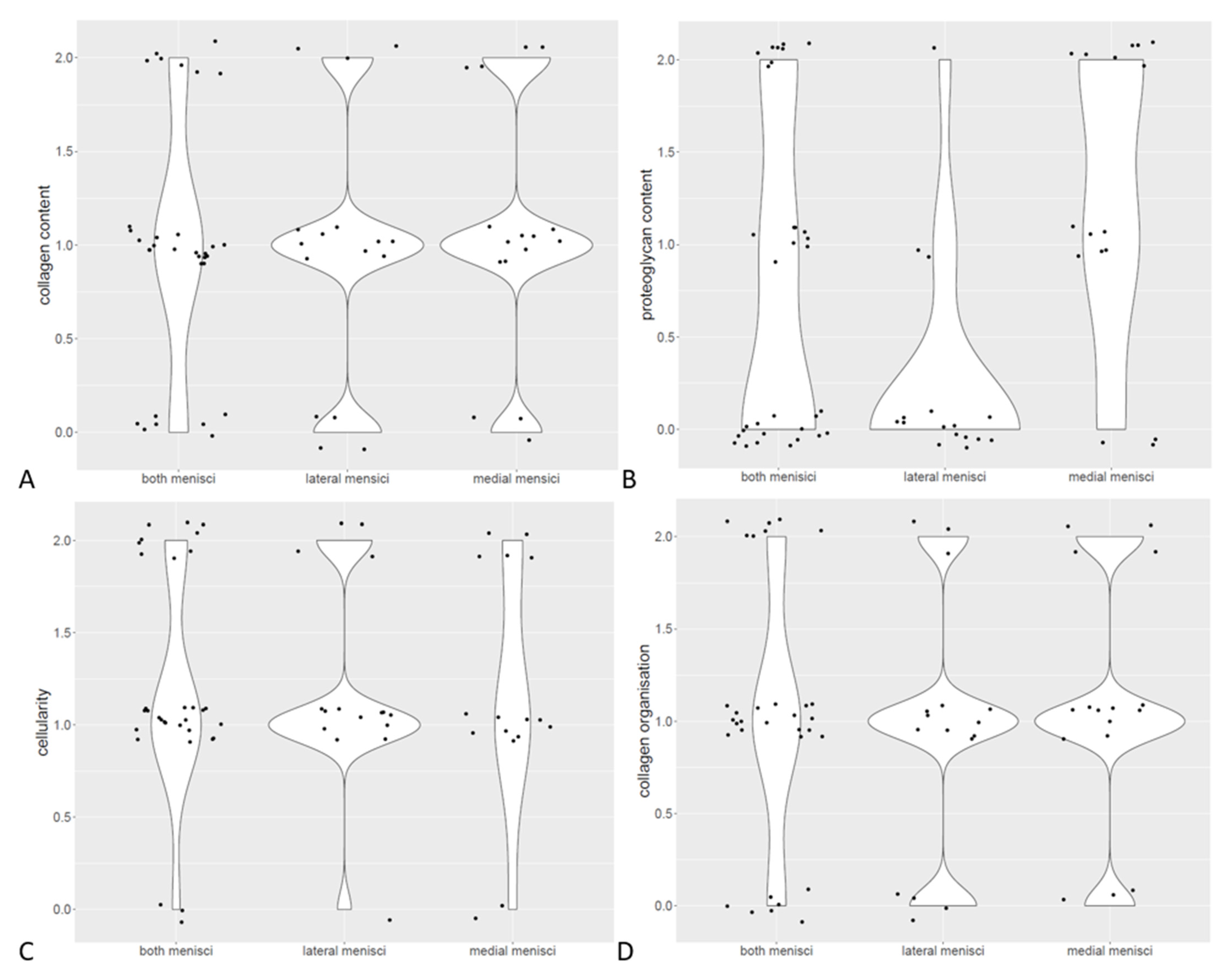
3.4. Histological Findings
3.5. T2 Relaxation Time and Histological Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pownder, S.L.; Hayashi, K.; Caserto, B.G.; Norman, M.L.; Potter, H.G.; Koff, M.F. Magnetic Resonance Imaging T2 Values of Stifle Articular Cartilage in Normal Beagles. Vet. Comp. Orthop. Traumatol. 2018, 31, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Cicuttini, F.; Jones, G. How important is MRI for detecting early osteoarthritis? Nat. Clin. Pract. Rheumatol. 2008, 4, 4–5. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, L.; Yang, H.; Collins, J.; Guermazi, A.; Jones, M.; Teeple, E.; Xu, L.; Losina, E.; Katz, J. Associations among meniscal damage, meniscal symptoms and knee pain severity. Osteoarthr. Cartil. 2016, 25, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Antony, B.; Driban, J.; Price, L.; Lo, G.; Ward, R.; Nevitt, M.; Lynch, J.; Eaton, C.; Ding, C.; McAlindon, T. The relationship between meniscal pathology and osteoarthritis depends on the type of meniscal damage visible on magnetic resonance images: Data from the Osteoarthritis Initiative. Osteoarthr. Cartil. 2016, 25, 76–84. [Google Scholar] [CrossRef]
- Hu, J.; Xin, H.; Chen, Z.; Zhang, Q.; Peng, Y.; Jin, Z. The role of menisci in knee contact mechanics and secondary kinematics during human walking. Clin. Biomech. 2019, 61, 58–63. [Google Scholar] [CrossRef]
- Fox, A.J.; Wanivenhaus, F.; Burge, A.J.; Warren, R.F.; Rodeo, S.A. The human meniscus: A review of anatomy, function, injury, and advances in treatment. Clin. Anat. 2014, 28, 269–287. [Google Scholar] [CrossRef]
- McDermott, I. Meniscal tears, repairs and replacement: Their relevance to osteoarthritis of the knee. Br. J. Sports Med. 2011, 45, 292–297. [Google Scholar] [CrossRef]
- Mosher, T.J.; Dardzinski, B.J. Cartilage MRI T2 relaxation time mapping: Overview and applications. Semin. Musculoskelet. Radiol. 2004, 8, 355–368. [Google Scholar] [CrossRef]
- Oei, E.H.G.; van Tiel, J.; Robinson, W.H.; Gold, G.E. Quantitative Radiologic Imaging Techniques for Articular Cartilage Composition: Toward Early Diagnosis and Development of Disease-Modifying Therapeutics for Osteoarthritis. Arthritis Care Res. 2014, 66, 1129–1141. [Google Scholar] [CrossRef]
- Arno, S.; Bell, C.P.; Xia, D.; Regatte, R.R.; Krasnokutsky, S.; Samuels, J.; Oh, C.; Abramson, S.; Walker, P.S. Relationship between meniscal integrity and risk factors for cartilage degeneration. Knee 2016, 23, 686–691. [Google Scholar] [CrossRef]
- Hofmann, F.C.; Neumann, J.; Heilmeier, U.; Joseph, G.B.; Nevitt, M.C.; McCulloch, C.E.; Link, T.M. Conservatively treated knee injury is associated with knee cartilage matrix degeneration measured with MRI-based T2 relaxation times: Data from the osteoarthritis initiative. Skelet. Radiol. 2017, 47, 93–106. [Google Scholar] [CrossRef]
- Banjar, M.; Horiuchi, S.; Gedeon, D.N.; Yoshioka, H. Review of Quantitative Knee Articular Cartilage MR Imaging. Magn. Reson. Med. Sci. 2022, 21, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Baum, T.; Joseph, G.; Karampinos, D.; Jungmann, P.; Link, T.; Bauer, J. Cartilage and meniscal T2 relaxation time as non-invasive biomarker for knee osteoarthritis and cartilage repair procedures. Osteoarthr. Cartil. 2013, 21, 1474–1484. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Pedoia, V.; Su, F.; Abramson, E.; Kretzschmar, M.; Nardo, L.; Link, T.M.; McCulloch, C.E.; Jin, C.; Ma, C.B.; et al. MR T1ρ and T2 of meniscus after acute anterior cruciate ligament injuries. Osteoarthr. Cartil. 2015, 24, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Rauscher, I.; Stahl, R.; Cheng, J.; Li, X.; Huber, M.B.; Luke, A.; Majumdar, S.; Link, T.M. Meniscal Measurements of T1ρ and T2 at MR Imaging in Healthy Subjects and Patients with Osteoarthritis. Radiology 2008, 249, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Nebelung, S.; Tingart, M.; Pufe, T.; Kuhl, C.; Jahr, H.; Truhn, D. Ex vivo quantitative multiparametric MRI mapping of human meniscus degeneration. Skelet. Radiol. 2016, 45, 1649–1660. [Google Scholar] [CrossRef] [PubMed]
- Diekmann, H.U. Analysis of the pathogenesis and progression of osteoarthritis in canine stifle joints considering three bone healing markers. 13 September 2022. Available online: https://elib.tiho-hannover.de/receive/etd_mods_00000009 (accessed on 9 January 2023).
- Kellgren, J.H.; Lawrence, J.S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef]
- Puchtler, H.; Waldrop, F.S.; Valentine, L.S. Polarization microscopic studies of connective tissue stained with picro-sirius red FBA. Beitr Pathol. 1973, 150, 174–187. [Google Scholar] [CrossRef]
- Junqueira, L.C.; Bignolas, G.; Brentani, R.R. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem. J. 1979, 11, 447–455. [Google Scholar] [CrossRef]
- Whittaker, P. Polarlzed light microscopy in biomedical research. Microsc. Anal. 1995, 15–17. [Google Scholar]
- Romeis, B. Mikroskopische Technik. 17. Aufl; Spektrum Akademischer Verlag: Heidelberg, Germany, 1989. [Google Scholar]
- Sun, Y.; Mauerhan, D.R.; Kneisl, J.S.; Norton, H.J.; Zinchenko, N.; Ingram, J.; Hanley, E.N.; Gruber, H.E. Histological Exam-ination of Collagen and Proteoglycan Changes in Osteoarthritic Menisci. Open Rheumatol. J. 2012, 6, 24–32. [Google Scholar] [CrossRef]
- Pauli, C.; Grogan, S.; Patil, S.; Otsuki, S.; Hasegawa, A.; Koziol, J.; Lotz, M.; D’Lima, D. Macroscopic and histopathologic analysis of human knee menisci in aging and osteoarthritis. Osteoarthr. Cartil. 2011, 19, 1132–1141. [Google Scholar] [CrossRef]
- Harper, T.A.M.; Jones, J.C.; Saunders, G.K.; Daniel, G.B.; Leroith, T.; Rossmeissl, E. Sensitivity of low-field T2 images for detecting the presence and severity of histopathologic meniscal lesions in dogs. Veter. Radiol. Ultrasound 2011, 52, 428–435. [Google Scholar] [CrossRef]
- Hayashi, K.; Caserto, B.G.; Breighner, R.E.; Norman, M.L.; Potter, H.G.; Koff, M.F.; Pownder, S.L. Quantitative Magnetic Resonance Imaging and Histological Comparison of Normal Canine Menisci. Vet. Comp. Orthop. Traumatol. 2018, 31, 452–457. [Google Scholar] [CrossRef]
- Jackson, J.; Vasseur, P.B.; Griffey, S.; Walls, C.M.; Kass, P.H. Pathologic changes in grossly normal menisci in dogs with rupture of the cranial cruciate ligament. J. Am. Veter. Med. Assoc. 2001, 218, 1281–1284. [Google Scholar] [CrossRef]
- Roughley, P.J.; Lee, E.R. Cartilage proteoglycans: Structure and potential functions. Microsc Res Tech. 1994, 28, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Dijkgraaf, L.C.; de Bont, L.G.; Boering, G.; Liem, R.S. Normal cartilage structure, biochemistry, and metabolism. J. Oral Maxillofac. Surg. 1995, 53, 924–929. [Google Scholar] [CrossRef] [PubMed]
- Eijgenraam, S.M.; Bovendeert, F.A.T.; Verschueren, J.; van Tiel, J.; Bastiaansen-Jenniskens, Y.M.; Wesdorp, M.A.; Nasserinejad, K.; Meuffels, D.E.; Guenoun, J.; Klein, S.; et al. T2 mapping of the meniscus is a biomarker for early osteoarthritis. Eur. Radiol. 2019, 29, 5664–5672. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, C.B.; Link, T.; Castillo, D.-D.; Blumenkrantz, G.; Lozano, J.; Carballido-Gamio, J.; Ries, M.; Majumdar, S. In vivo T1ρ and T2 mapping of articular cartilage in osteoarthritis of the knee using 3T MRI. Osteoarthr. Cartil. 2007, 15, 789–797. [Google Scholar] [CrossRef]
- Li, H.; Chen, S.; Tao, H.; Chen, S. Quantitative MRI T2 Relaxation Time Evaluation of Knee Cartilage. Am. J. Sports Med. 2015, 43, 865–872. [Google Scholar] [CrossRef]
- Williams, A.; Qian, Y.; Golla, S.; Chu, C. UTE-T2∗ mapping detects sub-clinical meniscus injury after anterior cruciate ligament tear. Osteoarthr. Cartil. 2012, 20, 486–494. [Google Scholar] [CrossRef]
- Zarins, Z.; Bolbos, R.; Pialat, J.; Link, T.; Li, X.; Souza, R.; Majumdar, S. Cartilage and meniscus assessment using T1rho and T2 measurements in healthy subjects and patients with osteoarthritis. Osteoarthr. Cartil. 2010, 18, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Pradhan, G.; Singh, S.; Batra, R. T1 and T2 mapping of articular cartilage and menisci in early osteoarthritis of the knee using 3-Tesla magnetic resonance imaging. Pol. J. Radiol. 2019, 84, 549–564. [Google Scholar] [CrossRef]
- Van Tiel, J.; Kotek, G.; Reijman, M.; Bos, P.K.; Bron, E.E.; Klein, S.; Nasserinejad, K.; Van Osch, G.J.V.M.; Verhaar, J.; Krestin, G.P.; et al. Is T1ρ Mapping an Alternative to Delayed Gadolinium-enhanced MR Imaging of Cartilage in the Assessment of Sulphated Glycosaminoglycan Content in Human Osteoarthritic Knees? An in Vivo Validation Study. Radiology 2016, 279, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Son, M.; Goodman, S.; Chen, W.; Hargreaves, B.; Gold, G.; Levenston, M. Regional variation in T1ρ and T2 times in osteoarthritic human menisci: Correlation with mechanical properties and matrix composition. Osteoarthr. Cartil. 2013, 21, 796–805. [Google Scholar] [CrossRef]
- Ghadially, F.N.; Lalonde, J.M.; Wedge, J.H. Ultrastructure of normal and torn menisci of the human knee joint. J. Anat. 1983, 136, 773–791. [Google Scholar] [PubMed]
- Tsai, P.-H.; Chou, M.-C.; Lee, H.-S.; Lee, C.-H.; Chung, H.-W.; Chang, Y.-C.; Huang, G.-S. MR T2 values of the knee menisci in the healthy young population: Zonal and sex differences. Osteoarthr. Cartil. 2009, 17, 988–994. [Google Scholar] [CrossRef] [PubMed]
- McWalter, E.J.; Gold, G.E. UTE T2∗ mapping detects sub-clinical meniscus degeneration. Osteoarthr. Cartil. 2012, 20, 471–472. [Google Scholar] [CrossRef]
- Seneag, D.B.; Shah, P.; Koff, M.F.; Lim, W.Y.; Rodeo, S.A.; Potter, H.G. Quantitative Ultrashort Echo Time Magnetic Resonance Imaging Evaluation of Postoperative Menisci: A Pilot Study. HSS J. 2014, 11, 123–129. [Google Scholar] [CrossRef]



| Number of menisci | 32 |
| Number of patients | 8 |
| Median age Mean age Range (age) | 13 12.94 10–17 |
| Median body weight Mean body weight Range (body weight) | 27.5 25.5 20–30 |
| Median BCS Mean BCS Range (BCS) | 6 6 4–7 |
| Female (neutered) | 4 |
| Male | 3 |
| Female | 1 |
| Total score (all menisci) | |
| Median Mean Range | 4.5 4.25 0–7 |
| Total score (medial menisci) | 4.25 |
| Median Mean Range | 5 4.75 0–7 |
| Total score (lateral menisci) | |
| Median Mean Range | 4 3.75 0–6 |
| Total Score | Medial Menisci | Lateral Menisci |
|---|---|---|
| Score 0 | 6.25% | 6.25% |
| Score 1 | - | - |
| Score 2 | - | 18.75% |
| Score 3 | 6.25% | 6.25% |
| Score 4 | 25% | 31.25% |
| Score 5 | 31.25% | 31.25% |
| Score 6 | 18.75% | 6.25% |
| Score 7 | 12.5% | - |
| Score 8 | - | - |
| Score 9 | - | - |
| Score 10 | - | - |
| Scoring of collagen content | Medial menisci | Lateral menisci |
| Score 0 | 18.75% | 25% |
| Score 1 | 56.25% | 56.25% |
| Score 2 | 25% | 18.75% |
| Scoring of proteoglycan content | Medial menisci | Lateral menisci |
| Score 0 | 18.75% | 81.25% |
| Score 1 | 37.5% | 12.5% |
| Score 2 | 43.75% | 6.25% |
| Scoring of cellularity | Medial menisci | Lateral menisci |
| Score 0 | 12.5% | 6.25% |
| Score 1 | 56.25% | 68,75% |
| Score 2 | 31.25 | 25% |
| Score 3 | - | - |
| Scoring of collagen organization | Medial menisci | Lateral menisci |
| Score 0 | 6.25% | 6.25% |
| Score 1 | 75% | 56.25% |
| Score 2 | 18.75% | 31.25 |
| Score 3 | - | 6.25% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bunzendahl, L.; Moussavi, A.; Bleyer, M.; Dehnert, J.; Boretius, S.; Neumann, S. Histological Findings and T2 Relaxation Time in Canine Menisci of Elderly Dogs—An Ex Vivo Study in Stifle Joints. Vet. Sci. 2023, 10, 182. https://doi.org/10.3390/vetsci10030182
Bunzendahl L, Moussavi A, Bleyer M, Dehnert J, Boretius S, Neumann S. Histological Findings and T2 Relaxation Time in Canine Menisci of Elderly Dogs—An Ex Vivo Study in Stifle Joints. Veterinary Sciences. 2023; 10(3):182. https://doi.org/10.3390/vetsci10030182
Chicago/Turabian StyleBunzendahl, Lena, Amir Moussavi, Martina Bleyer, Jana Dehnert, Susann Boretius, and Stephan Neumann. 2023. "Histological Findings and T2 Relaxation Time in Canine Menisci of Elderly Dogs—An Ex Vivo Study in Stifle Joints" Veterinary Sciences 10, no. 3: 182. https://doi.org/10.3390/vetsci10030182
APA StyleBunzendahl, L., Moussavi, A., Bleyer, M., Dehnert, J., Boretius, S., & Neumann, S. (2023). Histological Findings and T2 Relaxation Time in Canine Menisci of Elderly Dogs—An Ex Vivo Study in Stifle Joints. Veterinary Sciences, 10(3), 182. https://doi.org/10.3390/vetsci10030182








