Comparison of Adhesive Tape Impression Cytology, Hair Plucks, and Fungal Culture for the Diagnosis of Dermatophytosis in Dogs and Cats
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Diagnostic Tests
2.2. Statistical Analysis
3. Results
3.1. Dogs
3.2. Cats
3.3. Statistical Analysis Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chermette, R.; Ferreiro, L.; Guillot, J. Dermatophytoses in animals. Mycopathologia 2008, 166, 385–405. [Google Scholar] [CrossRef]
- Bond, R. Superficial veterinary mycoses. Clin. Dermatol. 2010, 28, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Moriello, K.A.; Coyner, K.; Paterson, S.; Mignon, B. Diagnosis and treatment of dermatophytosis in dogs and cats. Clinical Consensus Guidelines of the World Association for Veterinary Dermatology. Vet. Dermatol. 2017, 28, 266-e68. [Google Scholar] [CrossRef]
- Boehm, T.M.; Mueller, R.S. Dermatophytosis in dogs and cats- an update. Tierarztl. Prax. Ausg. K Kleintiere Heimtiere 2019, 47, 257–268. [Google Scholar] [PubMed]
- Moskaluk, A.E.; VandeWoude, S. Current Topics in Dermatophyte Classification and Clinical Diagnosis. Pathogens 2022, 11, 957. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, J. Feline dermatophytosis: Clinical features and diagnostic testing. Can. Vet. J. 2020, 61, 1217–1220. [Google Scholar]
- Cornegliani, L.; Persico, P.; Colombo, S. Canine nodular dermatophytosis (kerion): 23 cases. Vet. Dermatol. 2009, 20, 185–190. [Google Scholar] [CrossRef]
- Colombo, S.; Cornegliani, L.; Beccati, M.; Albanese, F. Comparison of two sampling methods for microscopic examination of hair shafts in feline and canine dermatophytosis. Veterinaria 2010, 24, 27–33. [Google Scholar]
- Carlotti, D.N.; Bensignor, E. Dermatophytosis due to Microsporum persicolor (13 cases) or Microsporum gypseum (20 cases) in dogs. Vet. Dermatol. 1999, 10, 17–27. [Google Scholar] [CrossRef]
- Kaufmann, R.; Blum, S.E.; Elad, D.; Zur, G. Comparison between point-of-care dermatophyte test medium and mycology laboratory culture for diagnosis of dermatophytosis in dogs and cats. Vet. Dermatol. 2016, 27, 284-e68. [Google Scholar] [CrossRef]
- Piri, F.; Zarei Mahmoudabadi, A.; Ronagh, A.; Ahmadi, B.; Makimura, K.; Rezaei-Matehkolaei, A. Assessment of a pan-dermatophyte nested-PCR compared with conventional methods for direct detection and identification of dermatophytosis agents in animals. Mycoses 2018, 61, 837–844. [Google Scholar] [CrossRef]
- Barillas, O.F.; Bajwa, J.; Guillot, J.; Arcique, A.J.M. Comparison of acetate tape impression, deep skin scraping, and microscopic examination of hair for therapeutic monitoring of dogs with juvenile generalized demodicosis: A pilot study. Can. Vet. J. 2019, 60, 596–600. [Google Scholar] [PubMed]
- Vogelnest, L.J.; Ludwig, C.B.; Ravens, P.A. Adhesive tape impression cytology in dermatophytosis and pemphigus foliaceus. In Special Issue: Abstracts from the 9th World Congress of Veterinary Dermatology, October 2020–April 2021, Sydney, Australia. Vet. Dermatol. 2020, 31 (Suppl. S1), 45 (abstract). [Google Scholar]
- Miller, W.H.; Griffin, C.E.; Campbell, K.L. Muller & Kirk’s Small Animal Dermatology, 7th ed.; Elsevier: St. Louis, MO, USA, 2013. [Google Scholar]
- Newbold, G.M.; Outerbridge, C.A.; Kass, P.H.; Maggs, D.J. Malassezia spp. on the periocular skin of dogs and their association with blepharitis, ocular discharge, and the application of ophthalmic medications. J. Am. Vet. Med. Assoc. 2014, 244, 1304–1308. [Google Scholar] [CrossRef]
- Lo, K.L.; Rosenkrantz, W.S. Evaluation of cytology collection techniques and prevalence of Malassezia yeast and bacteria in claw folds of normal and allergic dogs. Vet. Dermatol. 2016, 27, 279-e67. [Google Scholar] [CrossRef] [PubMed]
- Layne, E.A.; Zabel, S. Impression smear agreement with acetate tape preparation for cytologic sampling. J. Am. Anim. Hosp. Assoc. 2017, 53, 193–197. [Google Scholar] [CrossRef]
- Albanese, F. Canine and Feline Skin Cytology: A Comprehensive and Illustrated Guide to the Interpretation of Skin Lesions via Cytological Examination, 1st ed.; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Pereira, A.V.; Pereira, S.A.; Gremião, I.D.F.; Campos, M.P.; Ferreira, A.M.R. Comparison of acetate tape impression with squeezing versus skin scraping for the diagnosis of canine demodicosis. Aust. Vet. J. 2012, 90, 448–450. [Google Scholar] [CrossRef]
- Sampaio, K.O.; de Oliveira, L.M.; Burmann, P.M.; Sousa Filho, R.P.; Evangelista, J.S.; Cunha, M.G. Acetate tape impression test for diagnosis of notoedric mange in cats. J. Feline Med. Surg. 2017, 19, 702–705. [Google Scholar] [CrossRef] [PubMed]
- Mueller, R.S.; Rosenkrantz, W.; Bensignor, E.; Karaś-Tęcza, J.; Paterson, T.; Shipstone, M.A. Diagnosis and treatment of demodicosis in dogs and cats: Clinical consensus guidelines of the World Association for Veterinary Dermatology. Vet. Dermatol. 2020, 31, 4-e2. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria 2020. Available online: https://www.R-project.org/ (accessed on 1 December 2022).
- Lewis, D.T.; Foil, C.S.; Hosgood, G. Epidemiology and clinical features of dermatophytosis in dogs and cats at Louisiana State University: 1981–1990. Vet. Dermatol. 1991, 2, 53–58. [Google Scholar] [CrossRef]
- Mancianti, F.; Nardoni, S.; Cecchi, S.; Corazza, M.; Taccini, F. Dermatophytes isolated from symptomatic dogs and cats in Tuscany, Italy during a 15-year-period. Mycopathologia 2002, 156, 13–18. [Google Scholar] [CrossRef]
- Seker, E.; Dogan, N. Isolation of dermatophytes from dogs and cats with suspected dermatophytosis in Western Turkey. Prev. Vet. Med. 2011, 98, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Anzawa, K.; Mochizuki, T. An epidemiological study of feline and canine dermatophytoses in Japan. Med. Mycol. J. 2019, 60, 39–44. [Google Scholar] [CrossRef]
- Long, S.; Carveth, H.; Chang, Y.M.; O’Neill, D.; Bond, R. Isolation of dermatophytes from dogs and cats in the South of England between 1991 and 2017. Vet. Rec. 2020, 187, e87. [Google Scholar] [CrossRef] [PubMed]
- Cafarchia, C.; Romito, D.; Sasanelli, M.; Lia, R.; Capelli, G.; Otranto, D. The epidemiology of canine and feline dermatophytoses in southern Italy. Mycoses 2004, 47, 508–513. [Google Scholar] [CrossRef] [PubMed]
- da Silva Machado, R.D.C.; da Cruz, F.A.C.S.; Lima, S.R.; Torres, M.M.; Dutra, V.; Sousa, V.R.F. A retrospective of dermatophytosis in dogs and cats Veterinary Hospital at the Universidade Federal de Mato Grosso, in the years 2006 to 2008. Cienc. Rural 2011, 41, 1405–1410. [Google Scholar] [CrossRef]
- Zwierzyńska, E.; Dworecka-Kaszak, B. Mixed dermatophyte infection in a cat. Wiad. Parazytol. 2001, 47, 639–646. [Google Scholar] [PubMed]
- Koutinas, A.F.; Saridomichelakis, M.; Lekkas, S.; Koutinas, C.K. Clinical and histopathological aspects of dermatophyte kerion in the dog: A retrospective study of 20 spontaneous cases. In ESVD and ECVD 2002 Abstracts (Nice, France). Vet. Dermatol. 2003, 14, 243 (abstract). [Google Scholar]
- Albanese, F.; Caruso, C. Il kerio dermatofico: Aspetti eziologici, clinici, diagnostici e terapeutici in 39 cani. Veterinaria 2007, 21, 9–18. [Google Scholar]
- DeTar, L.G.; Dubrovsky, V.; Scarlett, J.M. Descriptive epidemiology and test characteristics of cats diagnosed with Microsporum canis dermatophytosis in a Northwestern US animal shelter. J. Feline Med. Surg. 2019, 21, 1198–1205. [Google Scholar] [CrossRef]
- Scarampella, F.; Zanna, G.; Peano, A.; Fabbri, E.; Tosti, A. Dermoscopic features in 12 cats with dermatophytosis and in 12 cats with self-induced alopecia due to other causes: An observational descriptive study. Vet. Dermatol. 2015, 26, 282-e63. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Angus, J.; Scarampella, F.; Neradilek, M. Evaluation of dermoscopy in the diagnosis of naturally occurring dermatophytosis in cats. Vet. Dermatol. 2016, 27, 275-e65. [Google Scholar] [CrossRef] [PubMed]
- Gross, T.L.; Ihrke, P.J.; Walder, E.J.; Affolter, V.K. Pustular and nodular diseases with adnexal destruction. In Skin Diseases of the Dog and the Cat: Clinical and Histopathologic Diagnosis, 2nd ed.; Blackwell Science Ltd.: Oxford, UK, 2005; pp. 420–459. [Google Scholar]
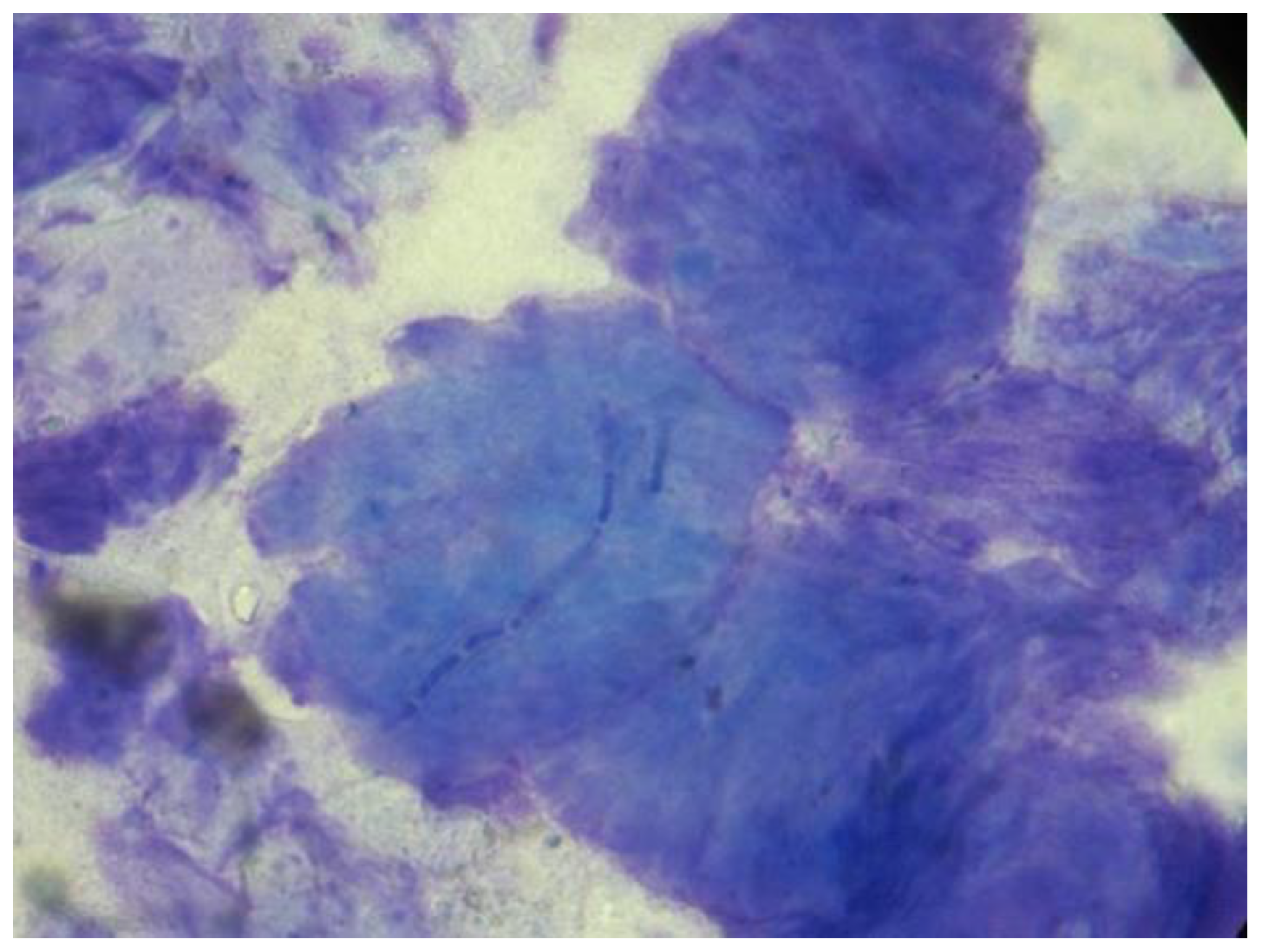
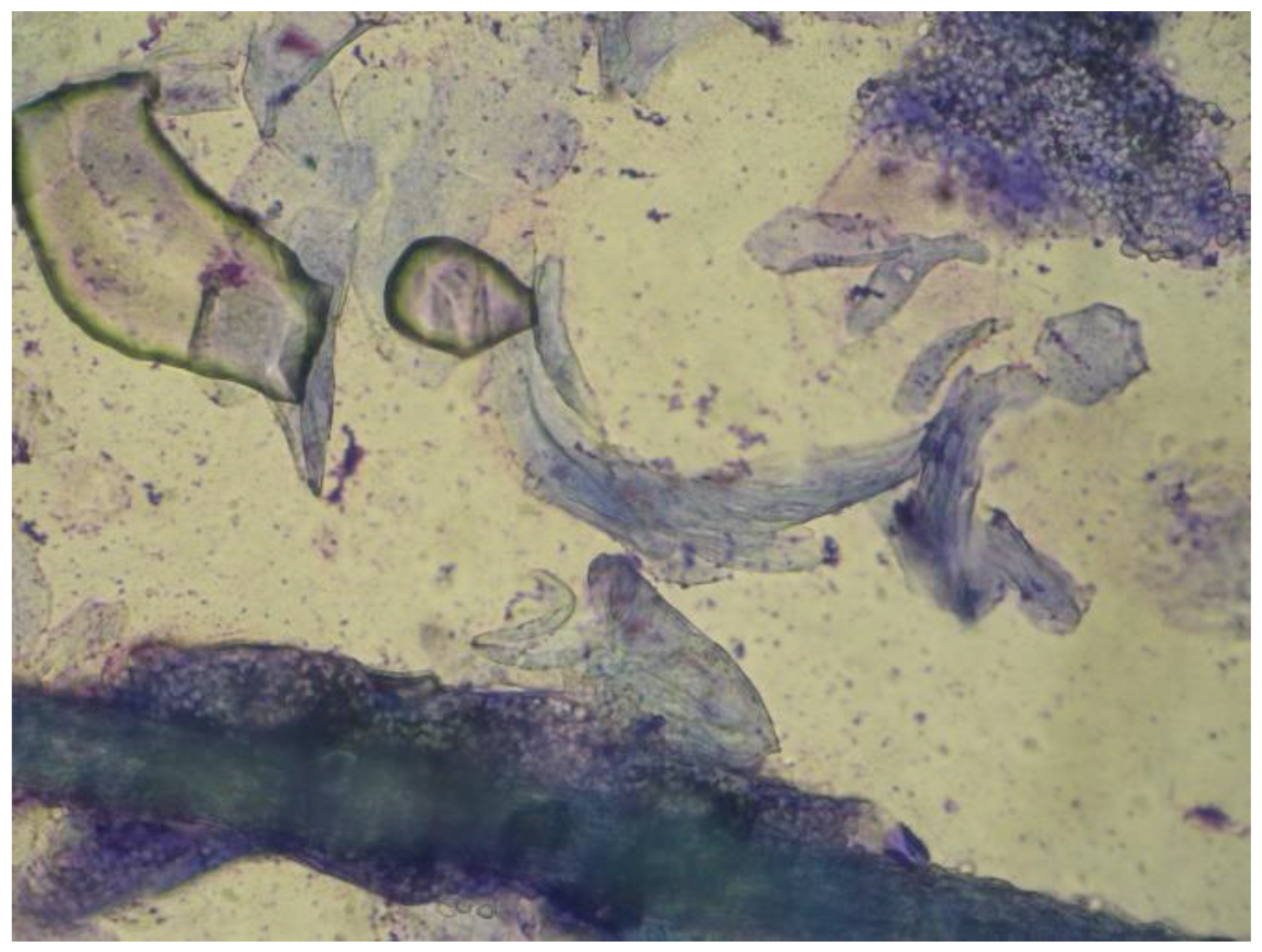
| Data | Percentage |
|---|---|
| Breed | |
| Mixed breed | 12/30 (40%) |
| Boxer | 3/30 (10%) |
| Pitbull | 3/30 (10%) |
| Yorkshire terrier | 2/30 (6.7%) |
| Others | 10/30 (33.3%) |
| Gender | |
| Male | 15/30 (50%) |
| Female | 15/30 (50%) |
| Age (median:2, range:1.5–14 y) | |
| ≤1 year | 13/30 (43.3%) |
| 1–2 Years | 3/30 (10%) |
| >2 years | 14/30 (46.7%) |
| Case | Clinical Form | Hair Plucks | Adhesive Tape Impression Cytology | Fungal Culture (DTM) |
|---|---|---|---|---|
| 1 | A | Positive | Positive | M. canis |
| 2 | A | Positive | Negative | Negative |
| 3 | A | Positive | Positive | Negative |
| 4 | A | Positive | Positive | M. canis |
| 5 | A | Negative | Positive | Negative |
| 6 | A | Positive | Negative | M. canis |
| 7 | A | Positive | Positive | M. canis |
| 8 | A | Negative | Positive | M. canis |
| 9 | A | Negative | Positive | M. canis T. mentagrophytes |
| 10 | A | Positive | Negative | M. canis |
| 11 | A | Positive | Negative | Negative |
| 12 | A | Positive | Negative | M. canis |
| 13 | A | Positive | Positive | M. canis |
| 14 | A | Positive | Positive | Negative |
| 15 | A | Positive | Positive | Negative |
| 16 | A | Positive | Positive | M. canis |
| 17 | A | Negative | Positive | M. canis |
| 18 | A | Negative | Negative | M. canis |
| 19 | A | Positive | Positive | M. canis |
| 20 | K | Positive | Negative | M. canis |
| 21 | K | Positive | Positive | M. canis |
| 22 | K | Negative | Positive | M. canis |
| 23 | K | Negative | Positive | M. canis |
| 24 | K | Negative | Positive | M. canis |
| 25 | K | Negative | Positive | M. canis |
| 26 | K | Negative | Positive | M. canis |
| 27 | K | Positive | Positive | M. canis |
| 28 | K | Negative | Positive | Negative |
| 29 | K | Negative | Positive | M. canis |
| 30 | K | Positive | Positive | M. canis |
| Diagnostic Test | Dogs (n = 30) | Dogs with Alopecia (n = 19) | Dogs with Kerion (n = 11) | Cats (n = 15) | Dogs and Cats (n = 45) | Dogs and Cats with Alopecia (n = 34) |
|---|---|---|---|---|---|---|
| Hair plucks | 18/30, 60.0% (40.6, 77.3) | 14/19, 73.7% (48.8, 90.9) | 4/11, 36.4% (10.9, 69.2) | 12/15, 80.0% (51.9, 95.7) | 30/45, 66.7% (51.1, 80.0) | 26/34, 76.5% (58.8, 89.3) |
| Adhesive tape impression cytology | 23/30, 76.7% (57.7, 90.1) | 13/19, 68.4% (43.5, 87.4) | 10/11, 90.9% (58.7, 99.8) | 14/15, 93.3% (68.1, 99.8) | 37/45, 82.2% (67.9, 92.0) | 27/34, 79.4% (62.1, 91.3) |
| Fungal culture (DTM) | 23/30, 76.7% (57.7, 90.1) | 13/19, 68.4% (43.5, 87.4) | 10/11, 90.9% (58.7, 99.8) | 13/15, 86.7% (59.5, 98.3) | 36/45, 80.0% (65.4, 90.4) | 26/34, 76.5% (58.8, 89.3) |
| Data | Percentage |
|---|---|
| Breed | |
| DSH | 14/15 (93.3%) |
| Persian | 1/15 (6.7%) |
| Gender | |
| Male | 9/15 (60%) |
| Female | 6/15 (40%) |
| Age (median: 2.5 months, range: 1.5 month–11 y) | |
| ≤6 months | 13/15 (86.7%) |
| >6 months | 2/15 (13.3%) |
| Case | Hair Plucks | Adhesive Tape Impression Cytology | Fungal Culture (DTM) |
|---|---|---|---|
| 1 | Negative | Positive | M. canis |
| 2 | Positive | Positive | M. canis |
| 3 | Positive | Positive | Negative |
| 4 | Positive | Positive | Negative |
| 5 | Positive | Positive | M. canis |
| 6 | Negative | Negative | M. canis |
| 7 | Positive | Positive | M. canis |
| 8 | Positive | Positive | M. canis |
| 9 | Positive | Positive | T. mentagrophytes |
| 10 | Positive | Positive | M. canis |
| 11 | Positive | Positive | M. canis |
| 12 | Positive | Positive | M. canis |
| 13 | Positive | Positive | M. canis |
| 14 | Negative | Positive | M. canis |
| 15 | Positive | Positive | M. canis |
| Comparison of Diagnostic Tests | Measures of Agreement | Cats (n = 15) | Dogs (n = 30) | Cats and Dogs (n = 45) | Cats and Dogs with Alopecia (n = 34) | Dogs with Alopecia (n = 19) | Dogs with Kerion (n = 11) |
|---|---|---|---|---|---|---|---|
| ATI cytology vs. Hair plucks | % Agreement | 86.67 | 43.33 | 57.78 | 67.65 | 52.63 | 23.08 |
| Cohen’s k | 0.444 | −0.269 | −0.075 | 0.06 | −0.148 | NA | |
| P-McNemar Test | 0.475 | 0.332 | 0.169 | 1 | 1 | 0.078 | |
| ATI cytology vs. fungal culture (DTM) | % Agreement | 80 | 66.67 | 71.11 | 67.65 | 52.38 | 81.82 |
| Cohen’s k | NA | 0.068 | 0.058 | 0.06 | −0.061 | NA | |
| P-McNemar Test | 1 | 0.752 | 1 | 1 | 0.724 | 0.48 | |
| Hair plucks vs. Fungal culture (DTM) | % Agreement | 66.67 | 50 | 55.56 | 58.82 | 52.63 | 45.45 |
| Cohen’s k | NA | −0.119 | −0.111 | −0.144 | −0.148 | 0.108 | |
| P-McNemar Test | 1 | 0.302 | 0.359 | 0.789 | 1 | 0.041 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouza-Rapti, P.; Karafylia, A.; Tamvakis, A.; Farmaki, R. Comparison of Adhesive Tape Impression Cytology, Hair Plucks, and Fungal Culture for the Diagnosis of Dermatophytosis in Dogs and Cats. Vet. Sci. 2023, 10, 183. https://doi.org/10.3390/vetsci10030183
Bouza-Rapti P, Karafylia A, Tamvakis A, Farmaki R. Comparison of Adhesive Tape Impression Cytology, Hair Plucks, and Fungal Culture for the Diagnosis of Dermatophytosis in Dogs and Cats. Veterinary Sciences. 2023; 10(3):183. https://doi.org/10.3390/vetsci10030183
Chicago/Turabian StyleBouza-Rapti, Pavlina, Anastasia Karafylia, Androniki Tamvakis, and Rania Farmaki. 2023. "Comparison of Adhesive Tape Impression Cytology, Hair Plucks, and Fungal Culture for the Diagnosis of Dermatophytosis in Dogs and Cats" Veterinary Sciences 10, no. 3: 183. https://doi.org/10.3390/vetsci10030183
APA StyleBouza-Rapti, P., Karafylia, A., Tamvakis, A., & Farmaki, R. (2023). Comparison of Adhesive Tape Impression Cytology, Hair Plucks, and Fungal Culture for the Diagnosis of Dermatophytosis in Dogs and Cats. Veterinary Sciences, 10(3), 183. https://doi.org/10.3390/vetsci10030183






