Molecular Tracking of the Origin of Vesicular Stomatitis Outbreaks in 2004 and 2018, Ecuador
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus Isolation in Cell Culture
2.2. RNA Extraction and Reverse Transcriptase-PCR (RT-PCR)
2.3. Phylogenetic Analysis
2.3.1. Construction of Phylogenetic Trees
2.3.2. Haplotype Network of the VSNJV Sequences
3. Results
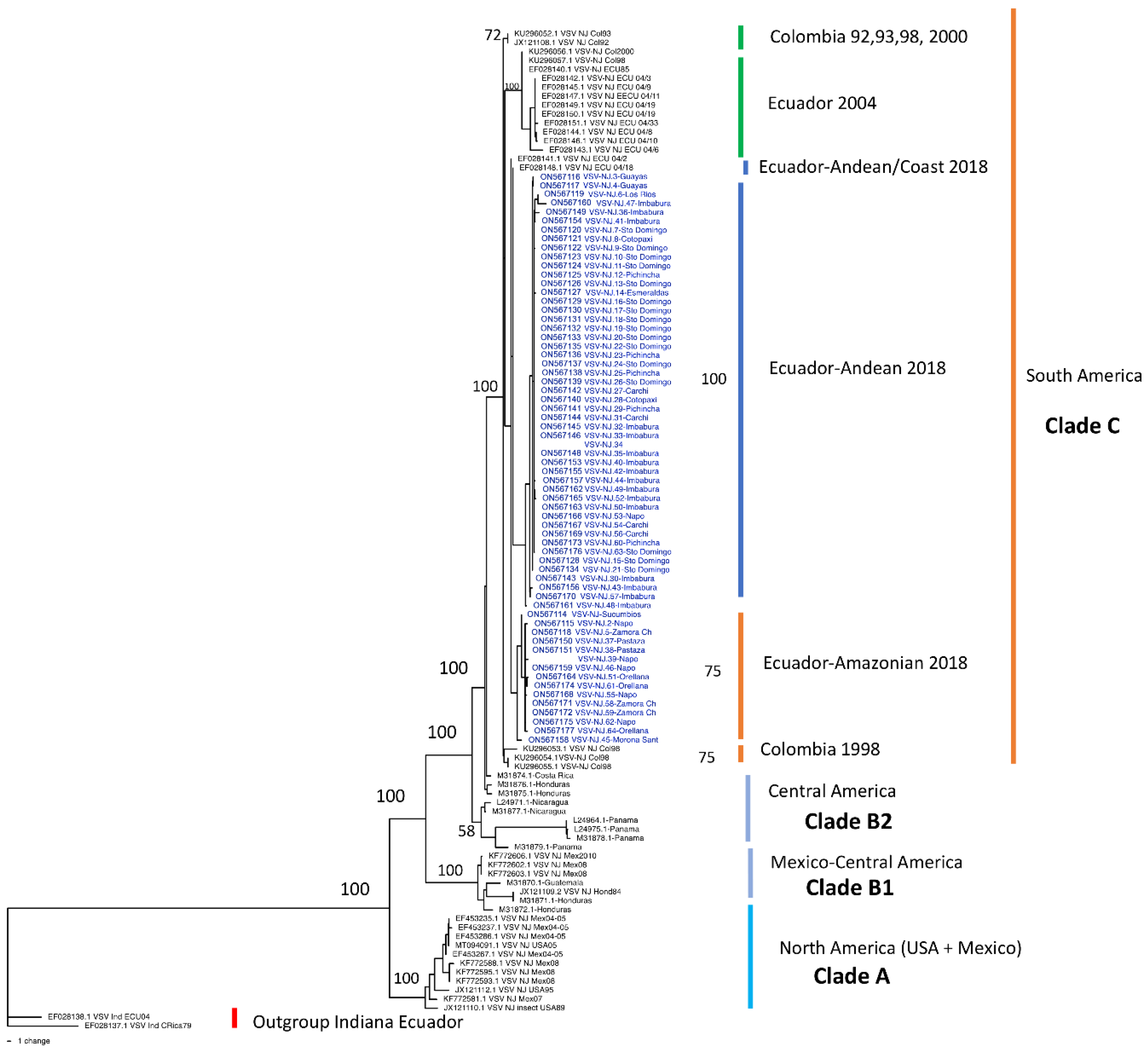
3.1. New-Jersey Phylogenetic Tree
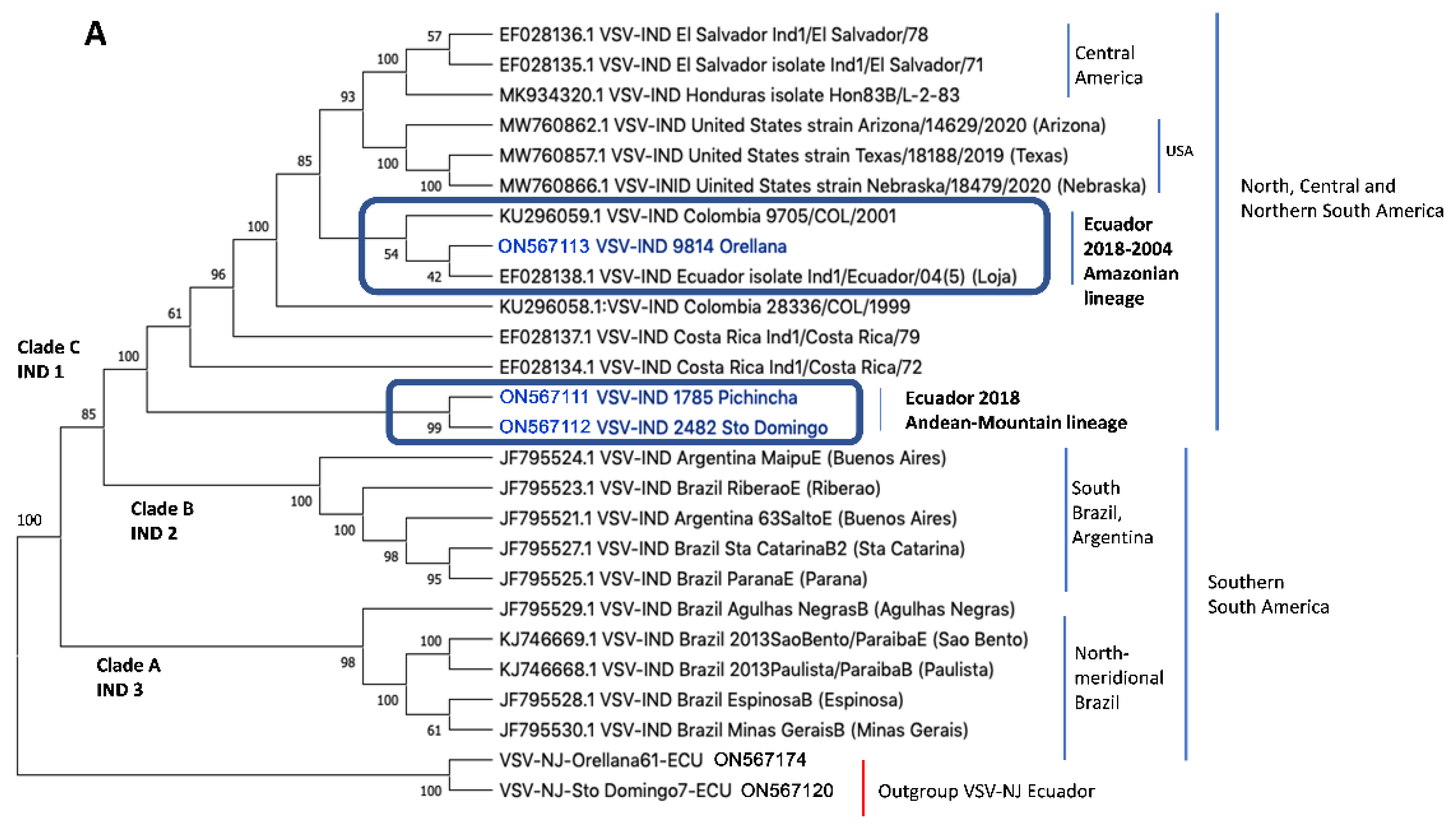
3.2. Indiana Phylogenetic Tree
3.3. Haplotype Network Topology of VSV-NJ
4. Discussion
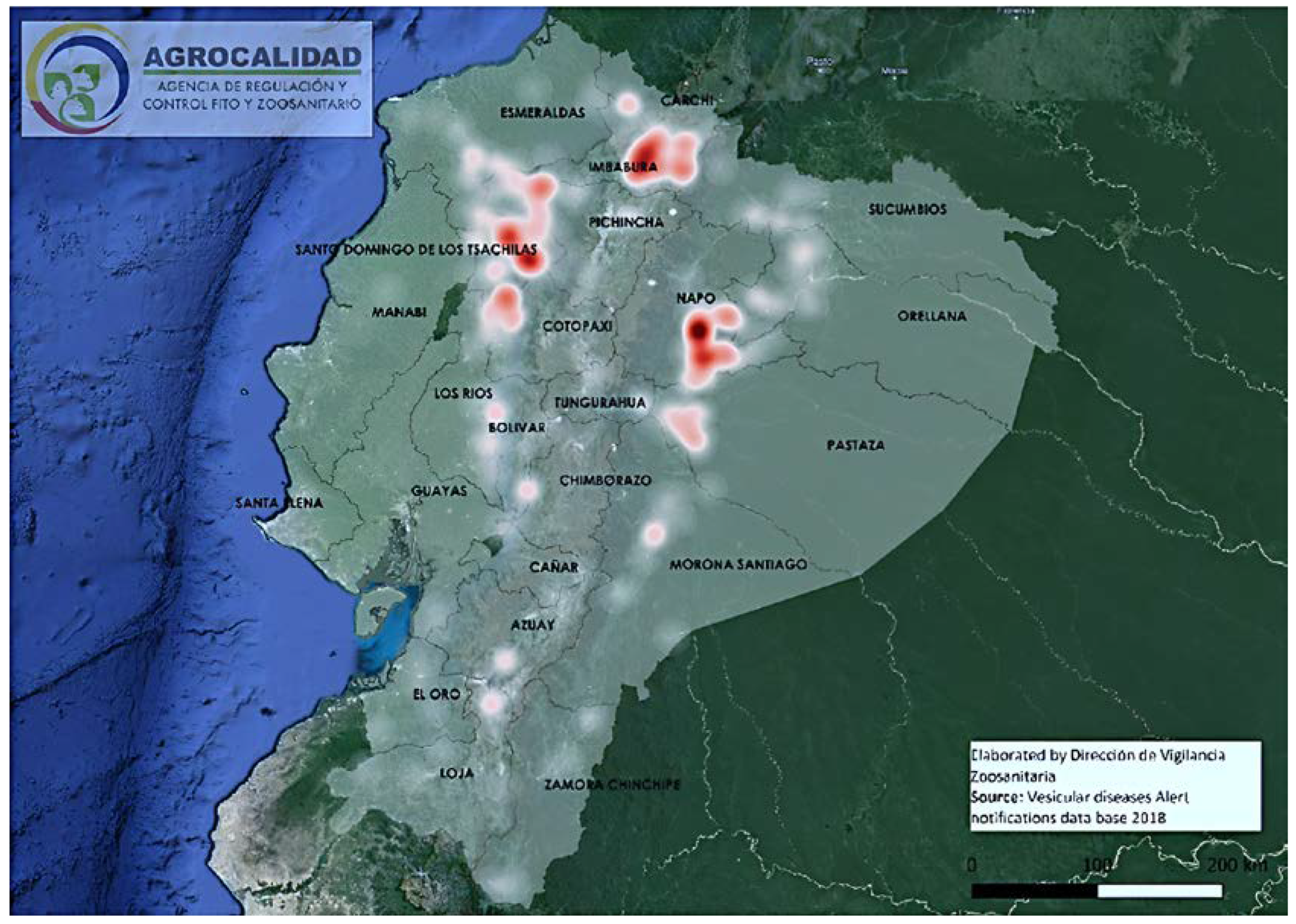
4.1. VSNJV Epidemiology
4.2. VSIV Epidemiology
4.3. Transmission by Vectors
5. Conclusions
6. Recommendations
7. Limitations of the Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Velazquez-Salinas, L.; Zarate, S.; Eschbaumer, M.; Lobo, F.P.; Gladue, D.P.; Arzt, J.; Novella, I.S.; Rodriguez, L.L. Selective Factors Associated with the Evolution of Codon Usage in Natural Populations of Arboviruses. PLoS ONE 2016, 11, e0159943. [Google Scholar] [CrossRef]
- Velazquez-Salinas, L.; Pauszek, S.J.; Holinka, L.G.; Gladue, D.P.; Rekant, S.I.; Bishop, E.A.; Stenfeldt, C.; Verdugo-Rodriguez, A.; Borca, M.V.; Arzt, J.; et al. A Single Amino Acid Substitution in the Matrix Protein (M51R) of Vesicular Stomatitis New Jersey Virus Impairs Replication in Cultured Porcine Macrophages and Results in Significant Attenuation in Pigs. Front. Microbiol. 2020, 11, 1123. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, L.L. Emergence and re-emergence of vesicular stomatitis in the United States. Virus Res. 2002, 85, 211–219. [Google Scholar] [CrossRef] [PubMed]
- CFSPH. Estomatitis Vesicular; Center for Food Security & Public Health: Arnes, IA, USA, 2008; Available online: https://www.cfsph.iastate.edu/Factsheets/es/!replaced/!estomatitis_vesicular.pdf (accessed on 24 April 2021).
- Rodriguez, L.L.; Bunch, T.A.; Fraire, M.; Llewellyn, Z.N. Re-emergence of vesicular stomatitis in the western United States is associated with distinct viral genetic lineages. Virology 2000, 271, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Rozo-Lopez, P.; Drolet, B.S.; Londoño-Renteria, B. Vesicular Stomatitis Virus Transmission: A Comparison of Incriminated Vectors. Insects 2018, 9, 190. [Google Scholar] [CrossRef] [PubMed]
- Hastie, E.; Cataldi, M.; Marriott, I.; Grdzelishvili, V.Z. Understanding and altering cell tropism of vesicular stomatitis virus. Virus Res. 2013, 176, 16–32. [Google Scholar] [CrossRef]
- Drolet, B.S.; Campbell, C.L.; Stuart, M.A.; Wilson, W.C. Vector competence of Culicoides sonorensis (Diptera: Ceratopogonidae) for vesicular stomatitis virus. J. Med. Entomol. 2005, 42, 409–418. [Google Scholar] [CrossRef]
- McGregor, B.L.; Rozo-Lopez, P.; Davis, T.M.; Drolet, B.S. Detection of Vesicular Stomatitis Virus Indiana from Insects Collected during the 2020 Outbreak in Kansas, USA. Pathogens 2021, 10, 1126. [Google Scholar] [CrossRef]
- Arboleda, J.J.; Valbuena, R.M.; Nancy, N.; Velásquez, J.I.; Rodas, J.D.; Londoño, A.F.; García, G. A Respuesta inmune humoral de una vacuna comercial contra la estomatitis vesicular en cerdos. Rev. Colomb. Ciencias Pecu 2005, 18, 115–121. [Google Scholar]
- Mead, D.G.; Ramberg, F.B.; Besselsen, D.G.; Maré, C.J. Transmission of vesicular stomatitis virus from infected to noninfected black flies co-feeding on nonviremic deer mice. Science 2000, 287, 485–487. [Google Scholar] [CrossRef]
- Timoney, P. Vesicular stomatitis. Vet. Rec. 2016, 179, 119–120. [Google Scholar] [CrossRef]
- Letchworth, G.J.; Rodriguez, L.L.; Del Cbarrera, J. Vesicular stomatitis. Vet J. 1999, 157, 239–260. [Google Scholar] [CrossRef]
- World Organization for Animal Health (OIE). Vesicular Stomatitis: Aetiology, Epidemiology, Diagnosis, Prevention and Control References. OIE Scientific and Technical Department, Editor. 2013. Available online: https://www.oie.int/fileadmin/Home/eng/Animal_Health_in_the_World/docs/pdf/Disease_cards/VESICULAR_STOMATITIS.pdf (accessed on 15 January 2022).
- Salinas, M.T.; De La Torre, E.J.; Moreno, P.K.; Vaca, A.A.; Maldonado, R.A. A Spatio-Temporal Distribution Analysis of Vesicular Stomatitis Outbreak in Ecuador, 2018. Bionatura. 2021. Available online: https://www.revistabionatura.com/2021.06.02.19.html (accessed on 15 January 2022).
- Sepúlveda, L.M.; Malirat, V.; Bergmann, I.E.; Mantilla, A.; Nascimento, E.R.D. Rapid diagnosis of vesicular stomatitis virus in Ecuador by the use of polymerase chain reaction. Braz. J. Microbiol. 2007, 38, 3. [Google Scholar] [CrossRef]
- Organización Mundial de Sanidad Animal (OIE). Estomatitis Vesicular. 2021. Report No.: 3.1.23. Available online: https://www.ecuadorencifras.gob.ec/encuesta-de-superficie-y-produccion-agropecuaria-continua-2018/ (accessed on 15 January 2022).
- MacVEctor Inc. Sequence Analysis Tools for Molecular Biologist; Mac Vector, Inc.: Apex, NC, USA, 2020. [Google Scholar]
- Pauszek, S.J.; Rodriguez, L.L. Full-length genome analysis of vesicular stomatitis New Jersey virus strains representing the phylogenetic and geographic diversity of the virus. Arch. Virol. 2012, 157, 2247–2251. [Google Scholar] [CrossRef]
- Cargnelutti, J.F.; Olinda, R.G.; Maia, L.A.; de Aguiar, G.M.N.; Neto, E.G.M.; Simões, S.V.D. Outbreaks of Vesicular stomatitis Alagoas virus in horses and cattle in northeastern Brazil. J. Vet. Diagn. Investig. 2014, 26, 788–794. [Google Scholar] [CrossRef]
- Nichol, S.T.; Rowe, J.E.; Fitch, W.M. Punctuated equilibrium and positive Darwinian evolution in vesicular stomatitis virus. Proc. Natl. Acad. Sci. USA 1993, 90, 10424–10428. [Google Scholar] [CrossRef] [PubMed]
- Fowler, V.L.; King, D.J.; Howson, E.L.A.; Madi, M.; Pauszek, S.J.; Rodriguez, L.L.; Knowles, N.J.; Mioulet, V.; King, D.P. Genome Sequences of Nine Vesicular Stomatitis Virus Isolates from South America. Genome Announc. 2016, 4, e00249-16. [Google Scholar] [CrossRef] [PubMed]
- Palinski, R.; Pauszek, S.; Burruss, N.D.; Savoy, H.; Pelzel-McCluskey, A.; Arzt, J.; Peters, D.P.; Rodriguez, L. Full Genomic Sequencing of Vesicular Stomatitis Virus Isolates from the 2004–2006 US Outbreaks Reveals Associations of Viral Genetics to Environmental Variables. Proceedings 2020, 50, 76. [Google Scholar]
- Koster, L.; Killian, M. Direct Submission Diagnostic Virology Laboratory, National Veterinary Services Laboratories, Ames Iowa; Diagnostic Virology Laboratory, United States Department of Agriculture: Washington, DC, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/nuccore/MW760857.1 (accessed on 15 January 2022).
- Rainwater-Lovett, K.; Pauszek, S.J.; Kelley, W.N.; Rodriguez, L.L. Molecular epidemiology of vesicular stomatitis New Jersey virus from the 2004-2005 US outbreak indicates a common origin with Mexican strains. J. Gen. Virol. 2007, 88, 2042–2051. [Google Scholar] [CrossRef] [PubMed]
- Velazquez-Salinas, L.; Pauszek, S.J.; Zarate, S.; Basurto-Alcantara, F.J.; Verdugo-Rodriguez, A.; Perez, A.M.; Rodriguez, L.L. Phylogeographic characteristics of vesicular stomatitis New Jersey viruses circulating in Mexico from 2005 to 2011 and their relationship to epidemics in the United States. Virology 2014, 449, 17–24. [Google Scholar] [CrossRef]
- Bilsel, P.; Rowe, J.; Fitch, W.M.; Nichol, S.T. Phosphoprotein and nucleocapsid protein evolution of vesicular stomatitis virus New Jersey. J. Virol. 1990, 64, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Morozov, I.; Davis, A.S.; Ellsworth, S.; Trujillo, J.D.; McDowell, C.; Shivanna, V.; Dasen, E.J.; Nichols, R.; Martin, B.K.; Monath, T.P.; et al. Comparative evaluation of pathogenicity of three isolates of vesicular stomatitis virus (Indiana serotype) in pigs. J. Gen. Virol. 2019, 100, 1478–1490. [Google Scholar] [CrossRef] [PubMed]
- Pauszek, S.J.; Barrera, J.D.C.; Goldberg, T.; Allende, R.; Rodriguez, L.L. Genetic and antigenic relationships of vesicular stomatitis viruses from South America. Arch. Virol. 2011, 156, 1961–1968. [Google Scholar] [CrossRef]
- Swofford, D. Phylogenetic Analysis Using Parsimony (*and Other Methods); Sinauer Associates: Sunderland, MA, USA. Available online: https://paup.phylosolutions.com/ (accessed on 15 January 2022).
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G+C-content biases. Mol. Biol. Evol. 1992, 9, 678–687. [Google Scholar]
- Rambaut, A. FigTree 1.4.4. Edinburgh: Institute of Evolutionary Biology, University of Edinburg; 2018. p. 1. Rambaut A. FigTree 1.4.4 [Internet]. Edinburgh. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 15 January 2022).
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Leight, J.; Bryant, D. PopART: Full-Feature Software for Haplotype Network Construction. Methods [Internet]. Methods in Ecology and Evolution. 2015. Available online: http://popart.otago.ac.nz/ (accessed on 15 January 2022).
- Ar, T.; Crandall, K.; Sing, C. A cladistic analysis of phenotypic associations with haplotypes inferred from restriction endonuclease mapping and DNA sequence data. III. Cladogram estimation. Genetics 1992, 132, 619–633. [Google Scholar]
- Clement, M.; Posada, D.; Crandall, K.A. TCS: A computer program to estimate gene genealogies. Mol. Ecol. 2000, 9, 1657–1659. [Google Scholar] [CrossRef]
- Elias, E.; McVey, D.S.; Peters, D.; Derner, J.D.; Pelzel-McCluskey, A.; Schrader, T.S.; Rodriguez, L. Contributions of Hydrology to Vesicular Stomatitis Virus Emergence in the Western USA. Ecosystems 2019, 22, 416–433. [Google Scholar] [CrossRef]
- Jimenez, A.E.; Herrera, F.V.; Salman, M.; Herrero, M.V. Survey of small rodents and hematophagous flies in three sentinel farms in a Costa Rican vesicular stomatitis endemic region. Ann. NY Acad. Sci. 2000, 916, 453–463. [Google Scholar] [CrossRef]
- Migné, C.; Moutailler, S.; Attoui, H. Strategies for Assessing Arbovirus Genetic Variability in Vectors and/or Mammals. Pathogens 2020, 9, 915. [Google Scholar] [CrossRef]
- Agrocalidad. Dirección de Vigilancia Zoosanitaria. 2021. Available online: https://www.agrocalidad.gob.ec/direccion-de-vigilancia-zoosanitaria-3/ (accessed on 25 January 2022).
- Aguirre, A.A.; McLean, R.G.; Cook, R.S.; Quan, T.J. Serologic survey for selected arboviruses and other potential pathogens in wildlife from Mexico. J. Wildl. Dis. 1992, 28, 435–442. [Google Scholar] [CrossRef]
- Jimenez, A.E.; Jiménez, C.; Castro, L.; Rodriguez, L. Serological survey of small mammals in a vesicular stomatitis virus enzootic area. J. Wildl. Dis. 1996, 32, 274–279. [Google Scholar] [CrossRef]
- INEC. Encuesta de Superficie y Producción Agropecuaria Continua (ESPAC) 2018. 2018. Available online: https://www.ecuadorencifras.gob.ec/documentos/web-inec/Estadisticas_agropecuarias/espac/espac-2018/Presentaciondeprincipalesresultados.pdf (accessed on 15 January 2022).
- Comer, J.A.; Tesh, R.B.; Modi, G.B.; Corn, J.L.; Nettles, V.F. Vesicular stomatitis virus, New Jersey serotype: Replication in and transmission by Lutzomyia shannoni (Diptera: Psychodidae). Am. J. Trop. Med. Hyg. 1990, 42, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Drolet, B.S.; Reeves, W.K.; Bennett, K.E.; Pauszek, S.J.; Bertram, M.R.; Rodriguez, L.L. Identical Viral Genetic Sequence Found in Black Flies (Simulium bivittatum) and the Equine Index Case of the 2006 U.S. Vesicular Stomatitis Outbreak. Pathogens 2021, 10, 929. [Google Scholar] [CrossRef] [PubMed]
- Mead, D.G.; Gray, E.W.; Noblet, R.; Murphy, M.D.; Howerth, E.W.; Stallknecht, D.E. Biological Transmission of Vesicular Stomatitis Virus (New Jersey Serotype) by Simulium vittatum (Diptera: Simuliidae) to Domestic Swine (Sus scrofa). J. Med. Entomol. 2004, 41, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Rozo-Lopez, P.; Londono-Renteria, B.; Drolet, B.S. Venereal Transmission of Vesicular Stomatitis Virus by Culicoides sonorensis Midges. Pathogens 2020, 9, 316. [Google Scholar] [CrossRef]
- Tesh, R.B.; Chaniotis, B.N.; Johnson, K.M. Vesicular stomatitis virus (Indiana serotype): Transovarial transmission by phlebotomine sandflies. Science 1972, 175, 1477–1479. [Google Scholar] [CrossRef]
- Webb, P.A.; McLean, R.G.; Smith, G.C.; Ellenberger, J.H.; Francy, D.B.; Walton, T.E.; Monath, T.P. Epizootic vesicular stomatitis in Colorado, 1982: Some observations on the possible role of wildlife populations in an enzootic maintenance cycle. J. Wildl. Dis. 1987, 23, 192–198. [Google Scholar] [CrossRef]
- Mesquita, L.P.; Diaz, M.H.; Howerth, E.W.; Stallknecht, D.E.; Noblet, R.; Gray, E.W.; Mead, D.G. Pathogenesis of Vesicular Stomatitis New Jersey Virus Infection in Deer Mice (Peromyscus maniculatus) Transmitted by Black Flies (Simulium vittatum). Vet. Pathol. 2017, 54, 74–81. [Google Scholar] [CrossRef]
- Nichol, S.T. Genetic diversity of enzootic isolates of vesicular stomatitis virus New Jersey. J. Virol. 1988, 62, 572–579. [Google Scholar] [CrossRef]
- Calisher, C.H.; Gutierrez, V.E.; Francy, D.B.; Alava, A.A.; Muth, D.J.; Lazuick, J.S. Identification of Hitherto Unrecognized Arboviruses from Ecuador: Members of Serogroups B, C, Bunyamwera, Patois, and Minatitlan. Am. J. Trop. Med. Hyg. 1983, 32, 877–885. [Google Scholar] [CrossRef]
- Alfonzo, D.; Grillet, M.E.; Liria, J.; Navarro, J.-C.; Weaver, S.C.; Barrera, R. Ecological characterization of the aquatic habitats of mosquitoes (Diptera: Culicidae) in enzootic foci of Venezuelan equine encephalitis virus in western Venezuela. J. Med. Entomol. 2005, 42, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Gong, Y.; Zhang, C.; Sun, J.; Wong, G.; Shi, W. Co-existence and co-infection of influenza A viruses and coronaviruses: Public health challenges. Innov 2022, 3, 100306. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.C.; Ferro, C.; Barrera, R.; Boshell, J.; Navarro, J.-C. Venezuelan equine encephalitis. Annu. Rev. Entomol. 2004, 49, 141–174. [Google Scholar] [CrossRef] [PubMed]
- Méndez, W.; Liria, J.; Navarro, J.-C.; García, C.Z.; Freier, J.E.; Salas, R.; Weaver, S.C.; Barrera, R. Spatial Dispersion of Adult Mosquitoes (Diptera: Culicidae) in a Sylvatic Focus of Venezuelan Equine Encephalitis Virus. J. Med. Entomol. 2001, 38, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Peters, D.P.C.; Mcvey, D.S.; Elias, E.H.; Pelzel-Mccluskey, A.M.; Derner, J.D.; Dylan Burruss, N. Big data–model integration and AI for vector-borne disease prediction. Ecosphere 2020, 11, e03157. [Google Scholar] [CrossRef]
- Peck, D.E.; Reeves, W.K.; Pelzel-McCluskey, A.M.; Derner, J.D.; Drolet, B.; Cohnstaedt, L.W.; Swanson, D.; McVey, D.S.; Rodriguez, L.L.; Peters, D.P. Management Strategies for Reducing the Risk of Equines Contracting Vesicular Stomatitis Virus (VSV) in the Western United States. J. Equine Vet. Sci. 2020, 90, 103026. [Google Scholar] [CrossRef]
- Combe, M.; Sanjuán, R. Variation in RNA virus mutation rates across host cells. PLoS Pathog. 2014, 10, e1003855. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasco-Julio, D.; Aguilar, D.; Maldonado, A.; de la Torre, E.; Cisneros-Montufar, M.S.; Bastidas-Caldes, C.; Navarro, J.-C.; de Waard, J.H. Molecular Tracking of the Origin of Vesicular Stomatitis Outbreaks in 2004 and 2018, Ecuador. Vet. Sci. 2023, 10, 181. https://doi.org/10.3390/vetsci10030181
Vasco-Julio D, Aguilar D, Maldonado A, de la Torre E, Cisneros-Montufar MS, Bastidas-Caldes C, Navarro J-C, de Waard JH. Molecular Tracking of the Origin of Vesicular Stomatitis Outbreaks in 2004 and 2018, Ecuador. Veterinary Sciences. 2023; 10(3):181. https://doi.org/10.3390/vetsci10030181
Chicago/Turabian StyleVasco-Julio, David, Dayana Aguilar, Alexander Maldonado, Euclides de la Torre, Maria Soledad Cisneros-Montufar, Carlos Bastidas-Caldes, Juan-Carlos Navarro, and Jacobus H. de Waard. 2023. "Molecular Tracking of the Origin of Vesicular Stomatitis Outbreaks in 2004 and 2018, Ecuador" Veterinary Sciences 10, no. 3: 181. https://doi.org/10.3390/vetsci10030181
APA StyleVasco-Julio, D., Aguilar, D., Maldonado, A., de la Torre, E., Cisneros-Montufar, M. S., Bastidas-Caldes, C., Navarro, J.-C., & de Waard, J. H. (2023). Molecular Tracking of the Origin of Vesicular Stomatitis Outbreaks in 2004 and 2018, Ecuador. Veterinary Sciences, 10(3), 181. https://doi.org/10.3390/vetsci10030181




