Effect of Feeding Pomegranate (Punica granatum) Peel and Garlic (Allium sativum) on Antioxidant Status and Reproductive Efficiency of Female Rabbits
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Diet
2.3. Chemical Analysis of PP and GP
2.4. Experimental Design and Ethic
2.5. Bodyweight
2.6. Blood Collection
2.7. Hematological Estimation
2.8. Determination of Liver and Kidney Functions
2.9. Estimation of Immunoglobulin and Hormones
2.10. Oxidative Status and Antioxidants
2.11. Statistical Analysis
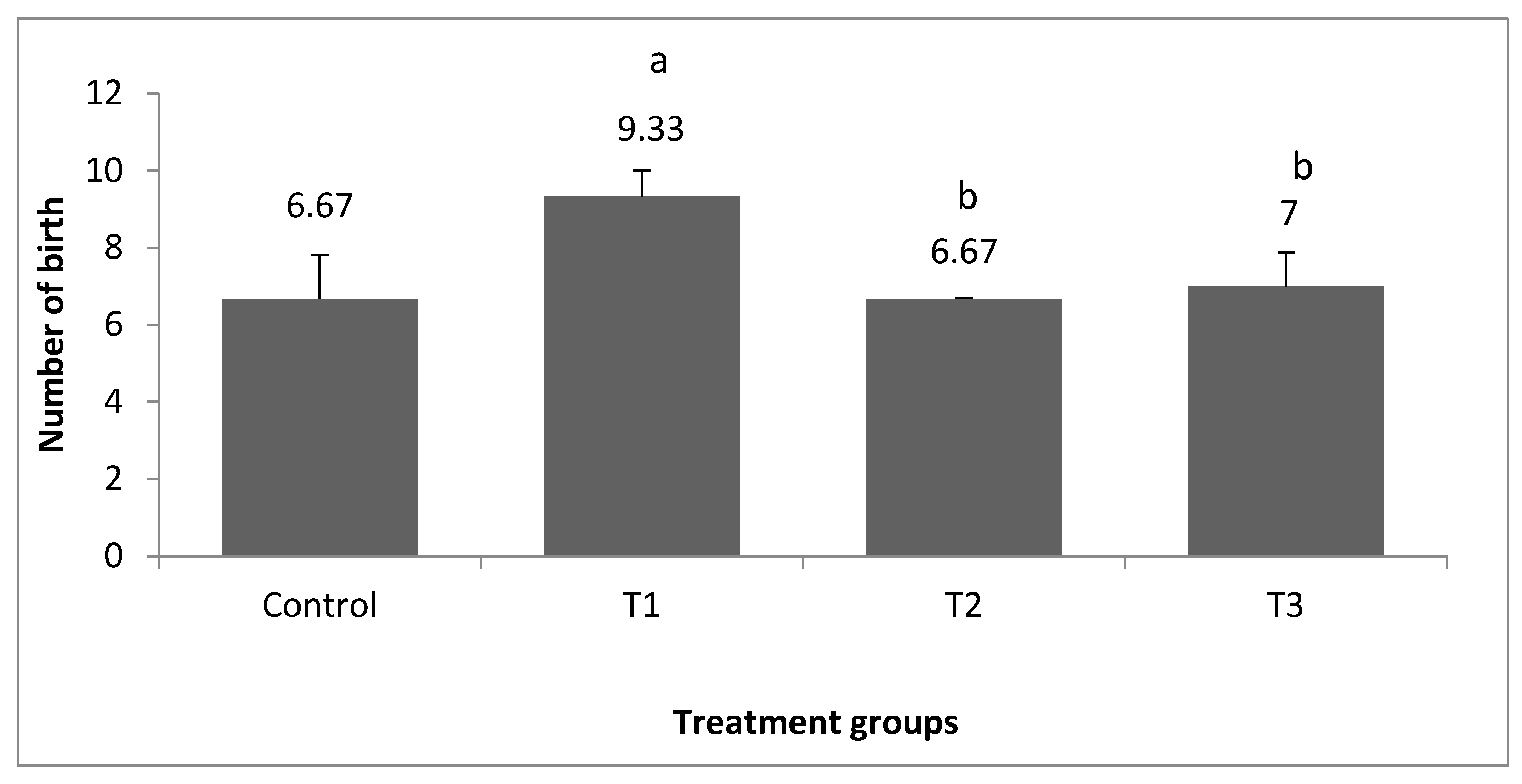
3. Results
3.1. Bodyweight
3.2. Hematological Parameters
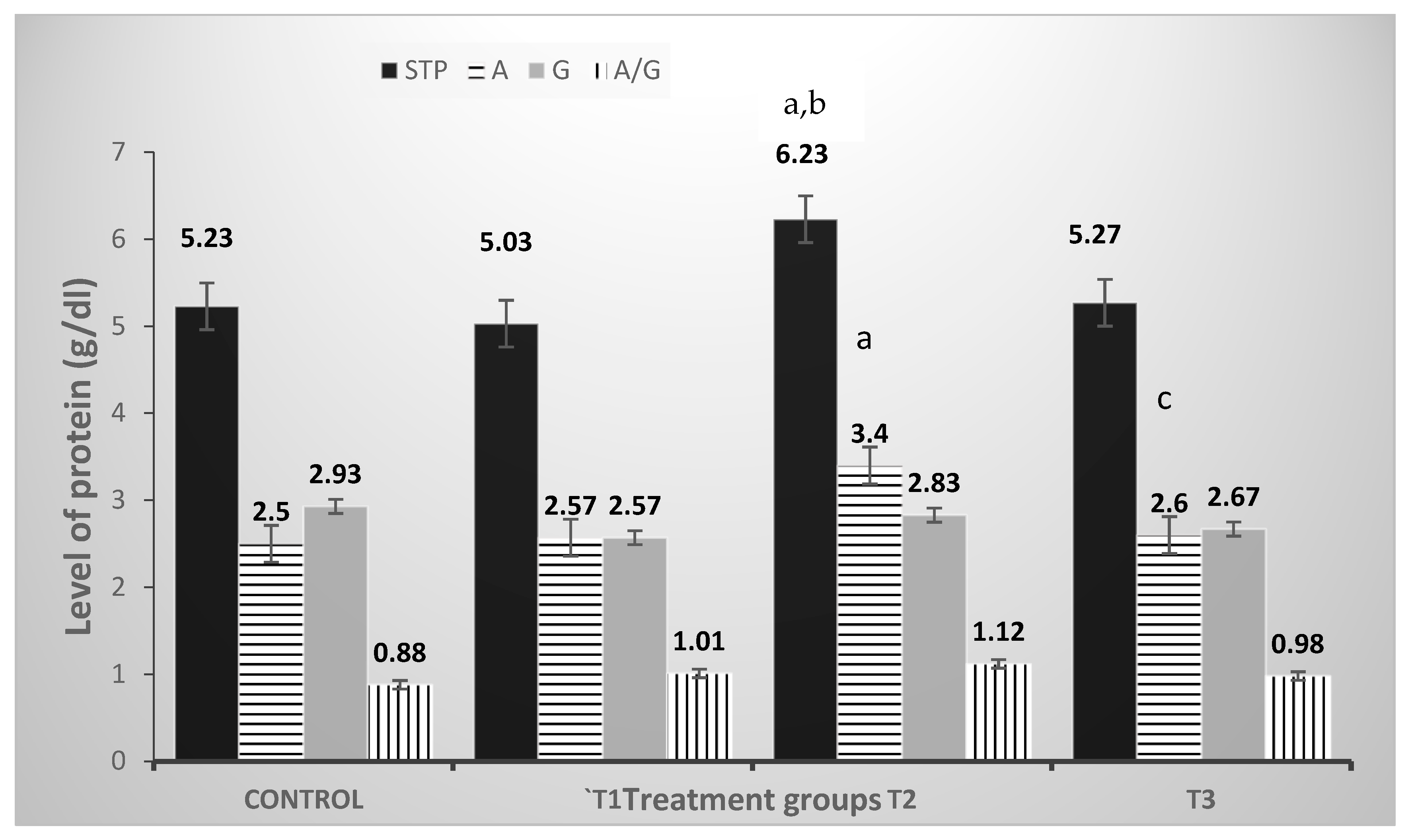
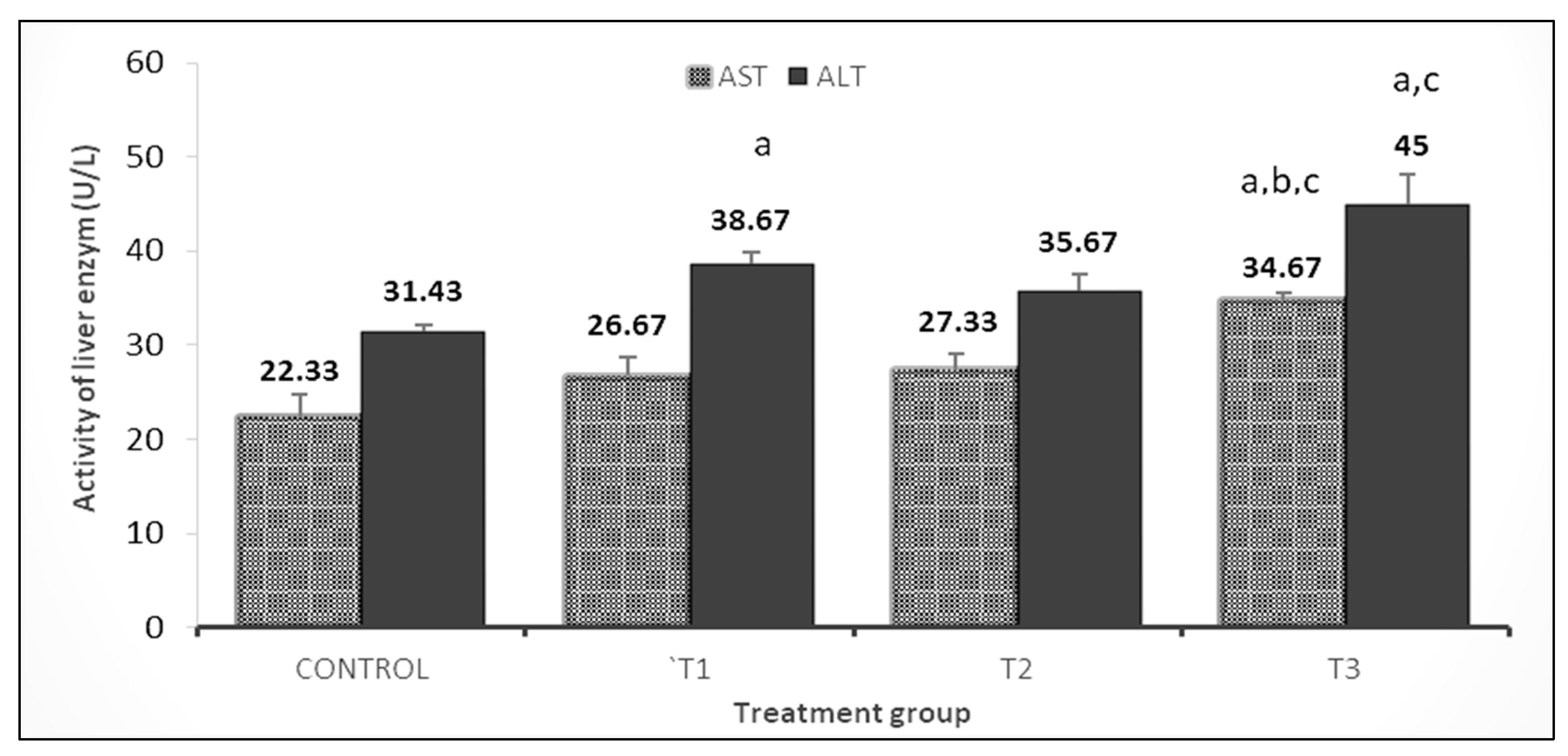
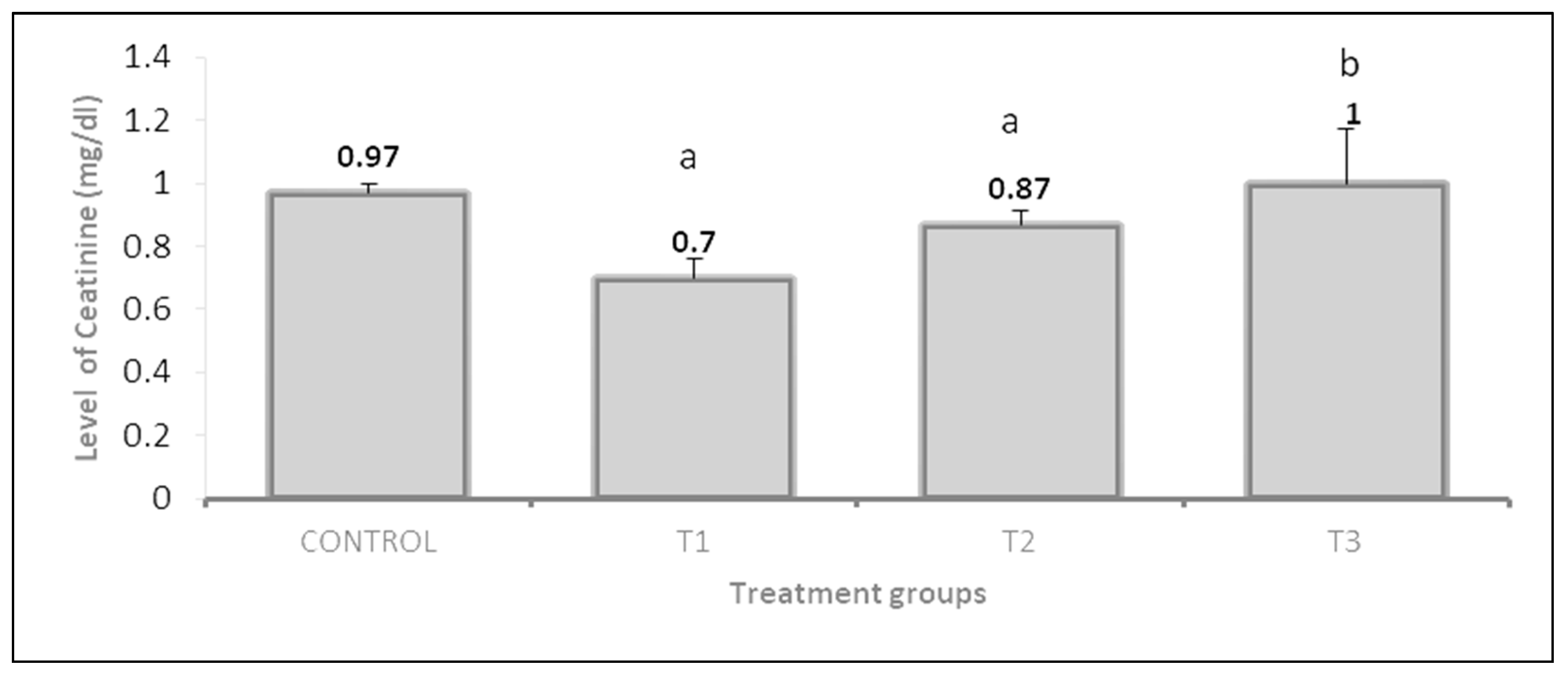
3.3. Liver and Kidney Functions
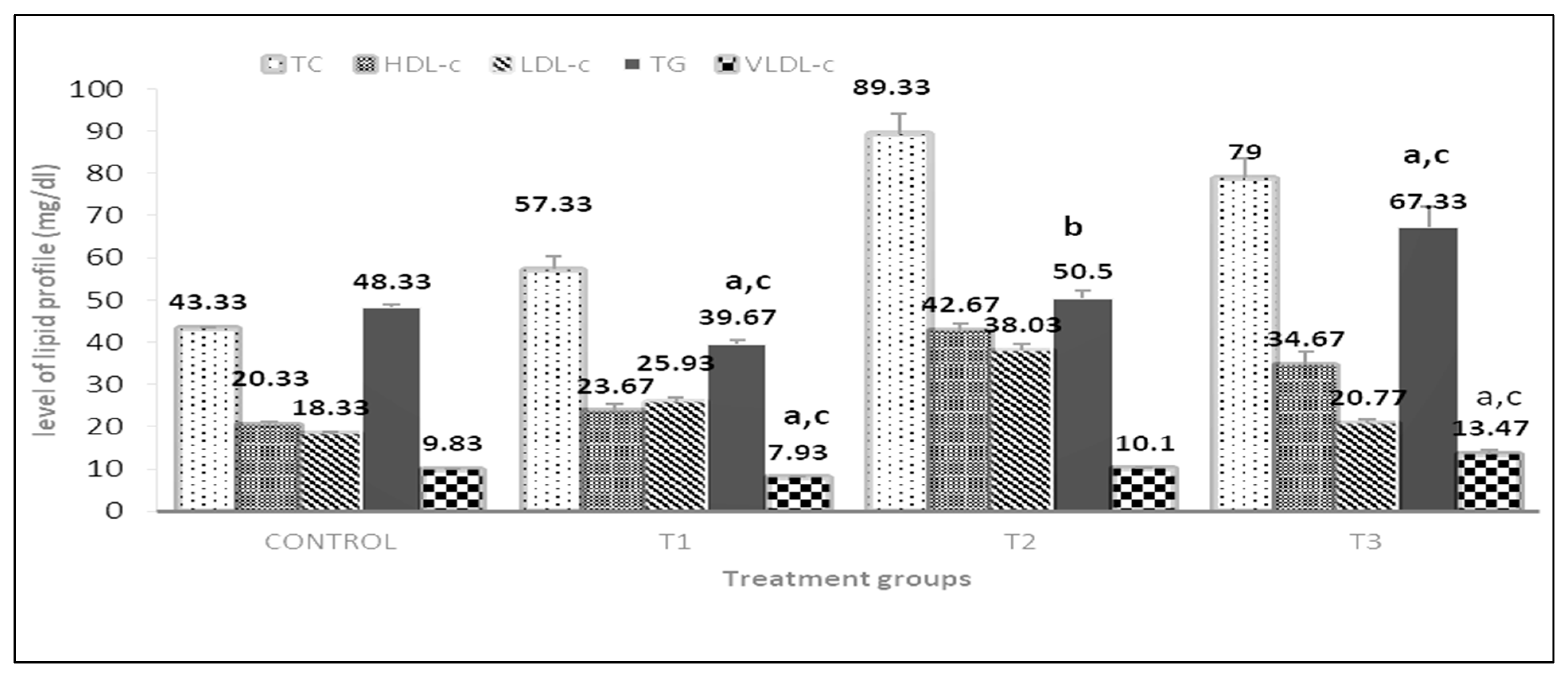
3.4. Effect of Supplementation on Lipid Profile
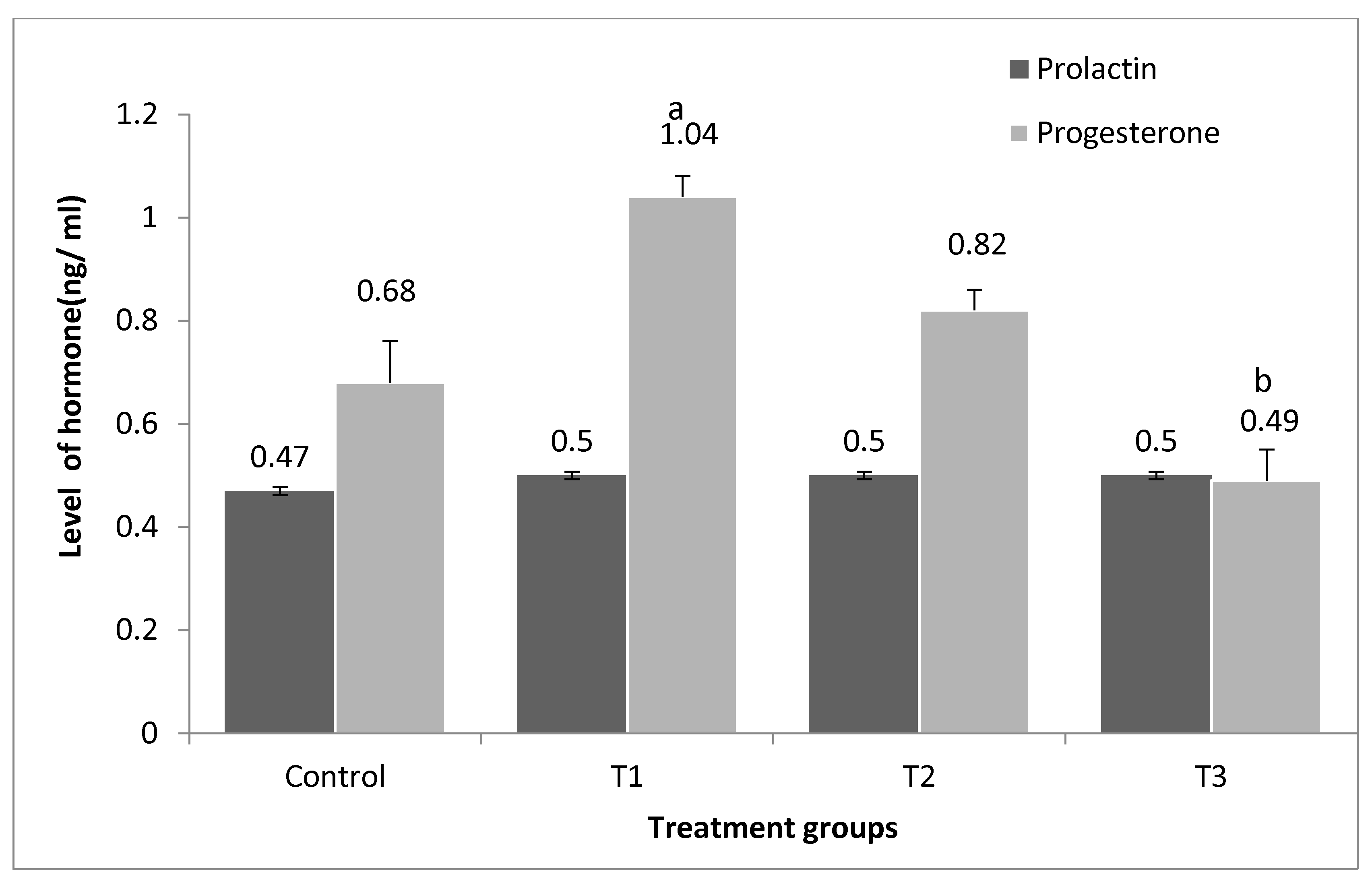
3.5. Effect of Supplementation on Progesterone and Prolactin Hormones
3.6. Effect of Supplementation on Immunoglobulin IgG and IgM
3.7. Oxidative Stress/Antioxidant Status
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Omer, H.A.A.; Abdel-Magid, S.S.; Awadalla, I.M. Pomegranate (Punica granatum L.) peels and studying the impact of level of inclusion in ration formulation on productive performance of growing Ossimi lambs. Bull. Natl. Res. Cent. 2019, 43, 182–192. [Google Scholar] [CrossRef]
- Hossian, M.K.; Ali, M.Y.; Kamruzzaman, M.; Khan, M.J. Efficacy study of garlic leaf on feed utilization and growth performances of rabbits. Acta Sci. Nutrit. Health 2020, 4, 8–11. [Google Scholar]
- Ashour, G.; Abdel-Rahman, S.M.; Morsy, W.A.; Abdel-Azeem, N.M.; Barakat, S.A. Physiological, reproductive, and productive performance of rabbit does as influence by N-acetylcysteine administration. Egypt. J. Rabbit Sci. 2018, 28, 63–92. [Google Scholar] [CrossRef]
- Cullere, M.; Zotte, A.D. Rabbit meat production and consumption: State of knowledge and future perspectives. Meat Sci. 2018, 143, 137–146. [Google Scholar] [CrossRef]
- Sikiru, A.B.; Alemede, I.C.; Egena, S.S.A.; Ijaiya, A.T. Oxidative stress, and reproductive inefficiencies: The science, evidence, and solutions. Agric. Ext. J. 2018, 2, 17–26. [Google Scholar]
- Kumar, N.; Neeraj. Study on physico-chemical and antioxidant properties of pomegranate peel. Pharmacog. Phytochem. 2018, 7, 2141–2147. [Google Scholar]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef]
- Zeweil, H.; Elgindy, Y. Pomegranate peel as a natural antioxidant enhanced reproductive performance and milk yield of female rabbits. World Rabbit Sci. 2016, 24, 207–212. [Google Scholar] [CrossRef]
- Pathak, P.D.; Mandavgane, S.A.; Kulkarni, B.D. Valorization of pomegranate peels: A biorefinery approach. Waste Biomass Valorization 2017, 8, 1127–1137. [Google Scholar] [CrossRef]
- Mphahlele, R.R.; Fawole, O.A.; Makunga, N.P.; Umezuruike, L.; Opara, U.L. Effect of drying on the bioactive compounds, antioxidant, antibacterial, and antityrosinase activities of pomegranate peel. BMC Complem. Altern. Med. 2016, 16, 143–155. [Google Scholar] [CrossRef]
- Shadab, K.; Anjum, P.; Bhise, K.S. Antioxidant activity of pomegranate peel powder. J. Drug Delive. Therap. 2017, 7, 81–84. [Google Scholar]
- El-Gogary, M.R.; Mansour, A.M.; El-Said, E.A. Blood biochemical and immunological responses to garlic oil administration in growing rabbits’ diet. J. Agric. Sci. 2018, 10, 217–224. [Google Scholar] [CrossRef]
- Kandylis, P.; Kokkinomagoulos, E. Food Applications and Potential Health Benefits of Pomegranate and its Derivatives. Foods 2020, 9, 122. [Google Scholar] [CrossRef]
- Mancini, S.; Mattioli, S.; Nuvoloni, R.; Pedonese, F.; Bosco, A.D.; Paci, G. Effects of garlic powder and salt on meat quality and microbial loads of rabbit burgers. Foods 2020, 9, 1022. [Google Scholar] [CrossRef]
- Al-Shuwaili, M.A.; Ibrahim, I.E.; Al-Bayati, M.T.N. Effect of dietary herbal plants supplement in turkey diet on performance and some blood biochemical parameters. Global, J. Biosci. Biotechnol. 2015, 4, 1–5. [Google Scholar]
- El-Nomeary, Y.A.; Abedo, A.A.; Salman, F.M.; Sedera, S.A.; Nasr, S.M.; Nassar, S.A.; Ibraheim, S.A. Effect of Adding Cinnamon, Garlic and Juniper Essential Oils on Productive Performance of New-Zealand White Rabbits. Egypt. J. Nutr. Feed. 2020, 23, 409–424. [Google Scholar] [CrossRef]
- AOAC International Official. Methods of Analysis of AOAC International. 2nd Revision, 17th ed.; Association of Analytical Communities: Gaithersburg, MD, USA, 2003. [Google Scholar]
- Doumas, B.T.; Bayse, D.D.; Carter, R.J.; Peters, T.J.; Schaffer, R.A. Candidate reference method for determination of total protein in serum. I. Development and validation. Clin. Chem. 1981, 27, 1642–1650. [Google Scholar] [CrossRef]
- Reitman, S.; Frankel, S. A colorimetric method for determination of serum glutamate oxaloacetate and glutamic pyruvate transaminase. Am. J. Clin. Pathol. 1957, 28, 56–58. [Google Scholar] [CrossRef]
- Patton, C.J.; Crouch, S.R. Spectrophotometric and kinetics investigation of the Berthelot reaction for the determination of ammonia. Anal. Chem. 1977, 49, 464–469. [Google Scholar] [CrossRef]
- Bartles, H.; Bohmer, M.; Heierli, C. Serum creatinine determination without protein precipitation. Clin. Chem. Acta 1972, 37, 193–197. [Google Scholar]
- Otchoumou, A.K.; Yao, F.K.; Wognin, M.F.; Niamké, S. Effect of dietary restriction on the growth and survival of young rabbits Oryctolagus cuniculus (Linnaeus, 1758). J. Anim. Plant Sci. 2021, 47, 8426–8432. [Google Scholar]
- Bahakaim, A.S.A.; Hekal, A.M.; Osman, A.O. Effect of using pomegranate (Punica Granatum, L.) peels and its extract on productive performance and milk yield of does rabbit. Egypt. Poul. Sci. J. 2020, 40, 769–781. [Google Scholar] [CrossRef]
- Bello, K.O.; Akanji, A.O.; Irekhore, O.T.; Lala, A.O. Anti-Endo parasitic effects of garlic (Allium sativum) as supplement in the diets of rabbits reared under deep litter system. Appl. Trop. Agric. 2016, 21, 1–7. [Google Scholar]
- Onu, P.N.; Aja, P.M. Growth performance and hematological indices of weaned rabbits fed garlic (Alium sativum) and ginger (Zingiber officinale) supplemented diets. Int. J. Food 2011, 1, 51–59. [Google Scholar]
- Alagawany, M.; Ashour, E.A.; Reda, F.M. Effect of dietary supplementation of garlic (Allium sativum) and turmeric (Curcuma longa) on growth performance, carcass traits, blood profile, and oxidative status in growing rabbits. Ann. Anim. Sci. 2016, 16, 489–505. [Google Scholar] [CrossRef]
- Azoz, A.A.; Basyony, M. Influence of supplementation of pomegranate dried waste as of natural antioxidative potential source in feeding does rabbits on some productive and reproductive performance under hot climate condition. Egypt. J. Rabbit Sci. 2012, 22, 23–39. [Google Scholar] [CrossRef]
- El-Ratel, I.T.; Abdel-Khalek, E.A.; El-Harairy, M.A.; Fouda, S.F.; El-Bnawy, L.Y. Impact of green tea extract on reproductive performance, hematology, lipid metabolism and histogenesis of liver and kidney of rabbit does. Asian J. Ani. Vet. Adv. 2017, 12, 51–60. [Google Scholar] [CrossRef]
- Manthou, E.; Georgakouli, K.; Deli, C.K.; Sotiropoulos, A.; Fatouros, I.G.; Kouretas, D.; Haroutounian, S.; Matthaiou, C.; Koutedakis, Y.; Jamurtas, A.Z. Effect of pomegranate juice consumption on biochemical parameters and complete blood count. Exp. Ther. Med. 2017, 14, 1756–1762. [Google Scholar] [CrossRef]
- El-Gindy, Y.M. Hematological and antioxidant status of pregnant rabbits at third trimester as affected by pomegranate peels under heat stress condition. Egypt. Poult. Sci. J. 2018, 38, 1–10. [Google Scholar]
- Riaz, A.; Khan, R.A. Anticoagulant, antiplatelet and antianemic effects of Punica granatum (pomegranate) juice in rabbits. Blood Coagul. Fibrinolysis 2016, 27, 287–293. [Google Scholar] [CrossRef]
- Onyimonyi, A.E.; Chukwuma, P.C.; Igbokwe, C. Growth and hypocholesterolemic properties of dry garlic powder (Allium sativum) on broilers. Afr. J. Biotechnol. 2012, 11, 2666–2671. [Google Scholar] [CrossRef]
- Fazlolahzadeh, F.; Keramati, K.; Nazifi, S.; Shirian, S.; Seifi, S. Effect of Garlic (Allium sativum) on Hematological parameters and plasma activities of ALT and AST of rainbow trout in temperature stress. Aust. J. Basic Appl. Sci. 2011, 5, 84–90. [Google Scholar]
- Al-Jowari, S.A. Effect of garlic powder (Allium sativum) on blood constituents in male rabbits. J. Al-Nahrain Univ. 2014, 17, 132–137. [Google Scholar] [CrossRef]
- Moskaug, J.Ø.; Carlsen, H.; Myhrstad, M.C.; Blomhoff, R. Polyphenols, and glutathione synthesis regulation. Am. J. Clin. Nutr. 2005, 81, 277S–283S. [Google Scholar] [CrossRef]
- Mohany, M.; Badr, G.; Refaat, I.; El-Feki, M. Immunological and histological effects of exposure to imidacloprid insecticide in male albino rats. Afr. J. Pharm. Pharmacol. 2011, 5, 2106–2114. [Google Scholar] [CrossRef]
- Nassrallah, M.M.; Saba, F.E.; Abo-Wardah, M.A. Effect of feeding pomegranate (Punica Granatum, L.) peels and its extract on growth performance and carcass characteristics of growing V-line male rabbits. Egypt. J. Nutr. Feeds 2016, 19, 511–520. [Google Scholar] [CrossRef]
- Ibrahim, M.R.M.; Arafa, S.A.; Hassan, F.A.; Zaki, E.E. Utilization of pomegranate (Punica Granatum, L.) byproduct powder as a natural growth promoter in growing rabbit diets. Egypt. J. Rabbit Sci. 2017, 27, 197–217. [Google Scholar]
- El-Ratel, I.T.; Abdel-Khalek, A.E.; Gabr, S.A.; Hammad, M.E.; El-Morsy, H.I. Influence of allicin administration on reproductive efficiency, immunity, and lipid peroxidation of rabbit does under high ambient temperature. Anim. Physiol. Ani. Nutr. 2020, 104, 539–548. [Google Scholar] [CrossRef]
- Ajayi, G.O.; Adeniyi, T.T.; Babayemi, D.O. Hepatoprotective and some hematological effects of Allium sativum and vitamin C in lead-exposed Wistar rats. Int. J. Med. Med. Sci. 2009, 1, 64–67. [Google Scholar]
- El-Katcha, M.I.; Soltan, M.A.; Sharaf, M.M.; Hasen, A. Growth performance, immune response, blood serum parameters, nutrient digestibility, and carcass traits of broiler chicken as affected by dietary supplementation of garlic extract (Allicin). Alex. J. Vet. Sci. 2016, 49, 50–64. [Google Scholar]
- Lan, J.; Lei, F.; Hua, L.; Wang, Y.; Xing, D.; Du, L. Transport behavior of ellagic acid of pomegranate leaf tannins and its correlation with total cholesterol alteration in HepG2 cells. Biomed. Chromatog. 2009, 23, 531–536. [Google Scholar] [CrossRef]
- Abdel-Maksoud, H.A.; Abdelhamid, M.O.; Ismaeel, T.E.A.; Elkharadly, W.A. Biochemical effects of pomegranate grinded peels on high sucrose stressed rabbits. Benha Vet. Med. J. 2018, 35, 152–163. [Google Scholar]
- Subashini, R. Pretreatment with Nelumbo Nucifera leaf extract ameliorates on lipids, lipoproteins, marker enzymes of lipid metabolism, and ECG pattern against isoproterenol-induced cardiotoxicity. Int. J. Pharm. Pharm. Sci. 2014, 6, 459–464. [Google Scholar]
- Codoñer-Franch, P.; Valls-Bellés, V.; Arilla-Codoñer, A.; Alonso-Iglesias, E. Oxidant mechanisms in childhood obesity: The link between inflammation and oxidative stress. Transl. Res. 2011, 158, 369–384. [Google Scholar] [CrossRef]
- Imbabi, T.A.; Ahmed-Farid, O.; Selim, D.A.; Sabeq, I.I. Antioxidant and anti-apoptotic potential of whole-pomegranate extract promoted growth performance, physiological homeostasis, and meat quality of V-line rabbits under hot summer conditions. Anim. Feed. Sci. Technol. 2021, 276, 114911. [Google Scholar] [CrossRef]
- Orgil, O.; Schwartz, E.; Baruch, L.; Matityahu, I.; Mahajna, J.; Amir, R. The antioxidative and anti-proliferative potential of non-edible organs of the pomegranate fruit and tree. LWT-Food Sci. Technol. 2014, 58, 571–577. [Google Scholar] [CrossRef]
- Abdel-Wareth, A.; Ahmed, A.; Hassan, H.A.; El-Sadek, M.S.; Ghazalah, A.; Lohakare, J. Nutritional impact of nano-selenium, garlic oil, and their combination on growth and reproductive performance of male Californian rabbits. Anim. Feed. Sci. Technol. 2019, 249, 37–45. [Google Scholar] [CrossRef]
- DeMayo, F.J.; Zhao, B.; Takamoto, N.; Tsai, S.Y. Mechanisms of action of estrogen and progesterone. Ann. N. Y. Acad. Sci. 2002, 955, 48–59. [Google Scholar] [CrossRef]
- Liebler, D. Peroxyl radical trapping reactions of α-tocopherol in biomimetic systems. In Vitamin E in Health and Disease; Packer, L., Fuchs, J., Eds.; Marcel Dekker: New York, NY, USA, 1992; pp. 85–97. [Google Scholar]
- Behnaz, H. Effects of garlic (Allium sativum L.) hydroalcoholic extract on estrogen, progesterone, and testosterone levels in rats exposed to cell phone radiation. Zahedan J. Res. Med. Sci. 2014, 16, 19–24. [Google Scholar]
- El-Sissi, A.F.; Bahakim, A.S.A.; Saba, F.S.; Osman, A.O. Assessment of dietary supplementation with pomegranate peel powder or its extract on productive performance and immune status of rabbits. Anim. Health Res. J. 2018, 6, 51–62. [Google Scholar]
- Gracious, R.R.; Selvasubramanian, S.; Jayasundar, S. Immunomodulatory activity of Punica granatum in rabbits a preliminary study. J. Ethnopharmacol. 2001, 78, 85–87. [Google Scholar] [CrossRef]
- Li, P.; Yin, Y.L.; Li, D.; Kim, S.W.; Guoyao, W.U. Review article amino acids and immune function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef]
- Hermes-Lima, M.; Storey, J.M.; Storey, K.B. Antioxidant defenses and metabolic depression. The hypothesis of preparation for oxidative stress in land snails. Comp. Biochem. Physiol. 1998, 120, 437–448. [Google Scholar] [CrossRef]
- El-Hafidi, M.; Ruiz-Ramírez, A.; Chávez-Salgado, M.; Peñeda-Flores, J.A.; Zapata, E.; Masso, F. High-sucrose diet increases ROS generation, FFA accumulation, UCP2 level, and proton leak in liver mitochondria. Am. J. Physiol. Endocrinol. Metab. 2011, 301, 1198–1207. [Google Scholar]
- Rouhi, S.Z.T.; Sarker, M.M.R.; Rahmat, A.; Alkahtani, S.A.; Othman, F. The effect of pomegranate fresh juice versus pomegranate seed powder on metabolic indices, lipid profile, inflammatory biomarkers, and the histopathology of pancreatic islets of Langerhans in streptozocin-nicotinamide induced type 2 diabetic Sprague-Dawley rats. MBC Complement. Altern. Med. 2017, 17, 156–169. [Google Scholar]
- Nabil, M.I.; Esam, A.E.; El-Beltagi, H.; Yasmin, E.A.M. Effect of lead acetate toxicity on experimental male albino rate. Asian Pac. J. Trop. Biomed. 2012, 2, 41–46. [Google Scholar]
- Erisir, M.; Benzer, E.M.; Kandemir, F.M. Changes in the rate of lipid peroxidation in plasma and selected blood antioxidants before and during pregnancy in Ewes. Acta Vet. Brno. 2009, 78, 237–242. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Ali, S.E. Protective effect of pomegranate peel ethanol extract against ferric nitrilotriacetate induced renal oxidative damage in rats. J. Cell. Mol. Biol. 2010, 8, 35–43. [Google Scholar]




| Chemical Analysis (%) | PP | GP |
|---|---|---|
| CP | 17.51 ± 0.1 | 18.6 ± 0.2 |
| Moisture | 13.80 ± 0.3 | 9.44 ± 0.2 a |
| Ash | 4.40 ± 0.1 | 3.7 ± 0.1 |
| EE | 3.65 ± 0.1 | 2.52 ± 0.1 |
| Ingredients % | Basal Diet (BD) | Pomegranate (PP-3%) | Garlic Powder (GP-3%) | Both Materials (PP1.5% + GP-1.5%) |
|---|---|---|---|---|
| Yellow corn | 5 | 5 | 5 | 5 |
| Wheat bran | 22 | 22 | 22 | 22 |
| Barley | 12 | 12 | 12 | 12 |
| Clover hay | 30 | 28.5 | 28.5 | 28.5 |
| Hay | 4 | 2.5 | 2.5 | 2.5 |
| Soybean meal (20%CP) | 23 | 23 | 23 | 23 |
| Pomegranate peel | 0 | 3 | 0 | 1.5 |
| Garlic powder (GP) | 0 | 0 | 3 | 1.5 |
| Limestone | 1.15 | 1.15 | 1.15 | 1.15 |
| Di-calcium phosphate | 0.5 | 0.5 | 0.5 | 0.5 |
| DI-Methionine | 0.2 | 0.2 | 0.2 | 0.2 |
| Anti-aflatoxin + anticoccidial1 | 0.5 | 0.5 | 0.5 | 0.5 |
| Vitamin and minerals premix * | 0.3 | 0.3 | 0.3 | 0.3 |
| Di-sodium | 0.5 | 0.5 | 0.5 | 0.5 |
| NaCl | 0.35 | 0.35 | 0.35 | 0.35 |
| Groups | Parameters of Blood Profile | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RBC (106/μL) | HB (g/dl) | HCT (%) | MCV (FL) | MCH (Pgs.) | MCHC (g/dl) | RDW-CV % | RDW-SD (FL) | WBC (103/μL) | Lymph (103/μL) | Mid (103/μL) | Grand (103/μL) | PLT (103/UL) | MPV (FL) | |
| Control | 5.61 ± 0.37 | 9.37 ± 0.58 | 32.13 ± 2.34 | 57.23 ± 0.58 | 16.37 ± 0.25 | 29.17 ± 0.48 | 14.9 ± 0.10 | 29.47 ± 0.33 | 13.9 ± 1.09 | 3.67 ± 0.45 | 0.63 ± 0.07 | 9.0 ± 1.82 | 322 ± 8.69 | 8.13 ± 0.32 |
| T1 | 4.83 ± 0.21 | 8.70 ± 0.90 | 25.27 ± 0.03 a | 58.03 ± 0.64 | 16.30 ± 1.7 | 29 ± 0.96 | 14.7 ± 0.60 | 29.53 ± 1.27 | 11.27 ± 1.12 | 3.23 ± 1.16 | 0.70 ± 0.12 | 6.6 ± 0.41 | 236 ± 18.61 a | 8.23 ± 0.27 |
| T2 | 6.39 ± 0.71 b | 9.63 ± 0.41 | 37.30 ± 4.63 | 62.33 ± 2.28 b | 16.80 ± 0.72 | 26.17 ± 1.99 | 14.0 ± 0.83 | 31.07 ± 1.39 | 8.57 ± 0.43 a | 3.97 ± 0.03 | 0.40 ± 0.06 | 4.2 ± 0.38 a,b | 288.7 ± 14.19 | 8.10 ± 0.30 |
| T3 | 5.79 ± 0.29 | 9.80 ± 0.12 | 31.53 ± 1.21b | 54.63 ± 1.25 c | 16.67 ± 0.13 | 29.97 ± 0.27 | 14.6 ± 0.41 | 27.33 ± 0.67 a | 16.73 ± 1.05 b,c | 4.87 ± 0.32c | 1.30 ± 0.06 a–c | 11.0 ± 0.58 b,c | 224.3 ± 7.06 a,c | 7.67 ± 0.15 |
| Groups | Parameters of Blood Profile | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RBC (106/μL) | HB (g/dl) | HCT (%) | MCV (FL) | MCH (Pgs.) | MCHC (g/dl) | RDW-CV % | RDW-SD (FL) | WBC (103/μL) | Lymph (103/μL) | Mid (103/μL) | Grand (103/μL) | PLT (103/UL) | MPV (FL) | |
| Control | 5.11 ± 0.16 | 8.2 ± 0.15 | 30.4 ± 0.87 | 62.5 ± 2.16 | 17.3 ± 0.85 | 27.8 ± 0.6 | 17 ± 1.3 | 33.9 ± 1.68 | 7.7 ± 0.32 | 1.83 ± 0.32 | 0.4 ± 0.03 | 5.5 ± 0.35 | 287 ± 32.13 | 8.3 ± 0.09 |
| T1 | 5.7 ± 0.56 | 9.1 ± 0.45 | 35.6 ± 4.23 | 61.6 ± 1.3 | 16.8 ± 0.2 | 27.53 ± 0.28 | 16.6 ± 0.28 | 34.5 ± 1.4 | 5.6 ± 0.6a | 2.4 ± 0.12 | 0.37 ± 0.09 | 2.7 ± 0.38 a | 348 ± 77.7 | 8.3 ± 0.27 |
| T2 | 5.36 ± 0.16 | 9.20 ± 0.0.6 a | 32.97 ± 0.39 | 60.3 ± 1.03 | 17.1 ± 0.55 | 27.9 ± 0.47 | 17.5 ± 0.48 | 38.6 ± 0.85 | 7.6 ± 1.15 | 4.0 ± 0.49 b | 0.43 ± 0.09 a | 3.1 ± 0.58 a | 430.3 ± 72.80 | 7.9 ± 0.26 |
| T3 | 5.55 ± 0.12 | 9.67 ± 0.50 a | 33.43 ± 1.66 | 58 ± 1.81 | 17.1 ± 0.6 | 29.1 ± 0.45b | 20.6 ± 0.84 bc | 37.9 ± 0.81 | 3.9 ± 0.21 ac | 1.8 ± 0.03 cb | 0.17 ± 0.03 ac | 1.9 ± 0.17 a | 334.6 ± 60.3 | 8.1 ± 0.20 |
| Groups | MDA (nmol/mL) | SOD (U/mL) | CAT (ng/mL) | GSH (ng/mL) | TAC (ng/mL) |
|---|---|---|---|---|---|
| Control | 0.50 ± 0.057 | 248.00 ± 15.51 | 16.84 ± 1.77 | 195.50 ± 6.12 | 17.23 ± 0.89 |
| T1 | 3.51 ± 0.61 a | 155.50 ± 6.12 a | 3.42 ± 0.84 a | 126.00 ± 7.35 a | 6.49 ± 0.82 a |
| T2 | 10.82 ± 1.98 a | 54.50 ± 10.21 a,b | 0.63 ± 0.12 a | 28.00 ± 2.45 a,b | 0.86 ± 0.04 a,b |
| T3 | 0.93 ± 0.05 a–c | 221.50 ± 8.57 b,c | 9.10 ± 029 a–c | 157.00 ± 6.53 a–c | 12.00 ± 1.63 a–c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hagag, O.Y.A.-E.; Younis, F.E.-E.; Al-Eisa, R.A.; Fayad, E.; El-Shenawy, N.S. Effect of Feeding Pomegranate (Punica granatum) Peel and Garlic (Allium sativum) on Antioxidant Status and Reproductive Efficiency of Female Rabbits. Vet. Sci. 2023, 10, 179. https://doi.org/10.3390/vetsci10030179
Hagag OYA-E, Younis FE-E, Al-Eisa RA, Fayad E, El-Shenawy NS. Effect of Feeding Pomegranate (Punica granatum) Peel and Garlic (Allium sativum) on Antioxidant Status and Reproductive Efficiency of Female Rabbits. Veterinary Sciences. 2023; 10(3):179. https://doi.org/10.3390/vetsci10030179
Chicago/Turabian StyleHagag, Omnia Y. Abd-Elfadiel, Fawzy El-Essawy Younis, Rasha A. Al-Eisa, Eman Fayad, and Nahla S. El-Shenawy. 2023. "Effect of Feeding Pomegranate (Punica granatum) Peel and Garlic (Allium sativum) on Antioxidant Status and Reproductive Efficiency of Female Rabbits" Veterinary Sciences 10, no. 3: 179. https://doi.org/10.3390/vetsci10030179
APA StyleHagag, O. Y. A.-E., Younis, F. E.-E., Al-Eisa, R. A., Fayad, E., & El-Shenawy, N. S. (2023). Effect of Feeding Pomegranate (Punica granatum) Peel and Garlic (Allium sativum) on Antioxidant Status and Reproductive Efficiency of Female Rabbits. Veterinary Sciences, 10(3), 179. https://doi.org/10.3390/vetsci10030179






