Chemical Profile of Essential Oils of Selected Lamiaceae Plants and In Vitro Activity for Varroosis Control in Honeybees (Apis mellifera)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants and EOs’ Extraction
2.2. Gas Chromatography–Mass Spectrometry (GC-MS) Analyses
2.3. Mite Harvesting
2.4. Toxicity towards V. destructor
2.5. Toxicity towards Honeybees
2.6. Statistical Analysis
3. Results
3.1. Chemical Composition
3.2. V. destructor Toxicity
3.3. Toxicity towards Honeybees
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khalifa, S.A.M.; Elshafiey, E.H.; Shetaia, A.A.; El-Wahed, A.A.A.; Algethami, A.F.; Musharraf, S.G.; AlAjmi, M.F.; Zhao, C.; Masry, S.H.D.; Abdel-Daim, M.M. Overview of bee pollination and its economic value for crop production. Insects 2021, 12, 688. [Google Scholar] [CrossRef] [PubMed]
- Kolayli, S.; Keskin, M. Natural bee products and their apitherapeutic applications. Stud. Nat. Prod. Chem. 2020, 66, 175–196. [Google Scholar]
- Brown, R. Hive products: Pollen, propolis and royal jelly. Bee World 1989, 70, 109–117. [Google Scholar] [CrossRef]
- Breeze, T.D.; Bailey, A.P.; Balcombe, K.G.; Potts, S.G. Pollination services in the UK: How important are honeybees? Agric. Ecosyst. Environ. 2011, 142, 137–143. [Google Scholar] [CrossRef]
- Mutinelli, F.; Costa, C.; Lodesani, M.; Baggio, A.; Medrzycki, P.; Formato, G.; Porrini, C. Honey bee colony losses in Italy. J. Apic. Res. 2010, 49, 119–120. [Google Scholar] [CrossRef]
- Neumann, P.; Carreck, N.L. Honey bee colony losses. J. Apic. Res. 2010, 49, 1–6. [Google Scholar] [CrossRef]
- Guzmán-Novoa, E.; Eccles, L.; Calvete, Y.; McGowan, J.; Kelly, P.G.; Correa-Benítez, A. Varroa destructor is the main culprit for the death and reduced populations of overwintered honey bee (Apis mellifera) colonies in Ontario, Canada. Apidologie 2010, 41, 443–450. [Google Scholar] [CrossRef]
- Boecking, O.; Genersch, E. Varroosis—The ongoing crisis in bee keeping. J. Für Verbraucherschutz Leb. 2008, 3, 221–228. [Google Scholar] [CrossRef]
- Francis, R.M.; Nielsen, S.L.; Kryger, P. Varroa-Virus Interaction in Collapsing Honey Bee Colonies. PLoS ONE 2013, 8, e57540. [Google Scholar] [CrossRef]
- Flores, J.M.; Gámiz, V.; Jiménez-Marín, Á.; Flores-Cortés, A.; Gil-Lebrero, S.; Garrido, J.J.; Hernando, M.D. Impact of Varroa destructor and associated pathologies on the colony collapse disorder affecting honey bees. Res. Vet. Sci. 2021, 135, 85–95. [Google Scholar] [CrossRef]
- Highfield, A.C.; El Nagar, A.; Mackinder, L.C.M.; Noël, L.M.-L.; Hall, M.J.; Martin, S.J.; Schroeder, D.C. Deformed wing virus implicated in overwintering honeybee colony losses. Appl. Environ. Microbiol. 2009, 75, 7212–7220. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, D.C.; Martin, S.J. Deformed wing virus: The main suspect in unexplained honeybee deaths worldwide. Virulence 2012, 3, 589–591. [Google Scholar] [CrossRef] [PubMed]
- Steiner, J.; Dittmann, F.; Rosenkranz, P.; Engels, W. The first gonocycle of the parasitic mite (Varroa jacobsoni) in relation to preimaginal development of its host, the honey bee (Apis mellifra carnica). Invertebr. Reprod. Dev. 1994, 25, 175–183. [Google Scholar] [CrossRef]
- Nazzi, F.; Le Conte, Y. Ecology of Varroa destructor, the Major Ectoparasite of the Western Honey Bee, Apis mellifera. Annu. Rev. Entomol. 2016, 61, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Noël, A.; Le Conte, Y.; Mondet, F. Varroa destructor: How does it harm Apis mellifera honey bees and what can be done about it? Emerg. Top. Life Sci. 2020, 4, 45–57. [Google Scholar]
- Harris, J.W.; Harbo, J.R.; Villa, J.D.; Danka, R.G. Variable population growth of Varroa destructor (Mesostigmata: Varroidae) in colonies of honey bees (Hymenoptera: Apidae) during a 10-year period. Environ. Entomol. 2003, 32, 1305–1312. [Google Scholar] [CrossRef]
- Bava, R.; Castagna, F.; Carresi, C.; Cardamone, A.; Federico, G.; Roncada, P.; Palma, E.; Musella, V.; Britti, D. Comparison of Two Diagnostic Techniques for the Apis mellifera Varroatosis: Strengths, Weaknesses and Impact on the Honeybee Health. Vet. Sci. 2022, 9, 354. [Google Scholar] [CrossRef]
- Descamps, C.; Quinet, M.; Jacquemart, A.-L. Climate change–induced stress reduce quantity and alter composition of nectar and pollen from a bee-pollinated species (Borago officinalis, Boraginaceae). Front. Plant Sci. 2021, 12, 755843. [Google Scholar] [CrossRef]
- Borghi, M.; Perez de Souza, L.; Yoshida, T.; Fernie, A.R. Flowers and climate change: A metabolic perspective. New Phytol. 2019, 224, 1425–1441. [Google Scholar] [CrossRef]
- Di Pasquale, G.; Salignon, M.; Le Conte, Y.; Belzunces, L.P.; Decourtye, A.; Kretzschmar, A.; Suchail, S.; Brunet, J.-L.; Alaux, C. Influence of pollen nutrition on honey bee health: Do pollen quality and diversity matter? PLoS ONE 2013, 8, e72016. [Google Scholar] [CrossRef]
- Van Dooremalen, C.; Stam, E.; Gerritsen, L.; Cornelissen, B.; Van der Steen, J.; Van Langevelde, F.; Blacquière, T. Interactive effect of reduced pollen availability and Varroa destructor infestation limits growth and protein content of young honey bees. J. Insect Physiol. 2013, 59, 487–493. [Google Scholar] [CrossRef]
- Bava, R.; Castagna, F.; Palma, E.; Ceniti, C.; Millea, M.; Lupia, C.; Britti, D.; Musella, V. Prevalence of Varroa destructor in Honeybee (Apis mellifera) Farms and Varroosis Control Practices in Southern Italy. Microorganisms 2023, 11, 1228. [Google Scholar] [CrossRef] [PubMed]
- Medici, S.K.; Maggi, M.D.; Sarlo, E.G.; Ruffinengo, S.; Marioli, J.M.; Eguaras, M.J. The presence of synthetic acaricides in beeswax and its influence on the development of resistance in Varroa destructor. J. Apic. Res. 2015, 54, 267–274. [Google Scholar] [CrossRef]
- Higes, M.; Martín-Hernández, R.; Sara Hernández-Rodríguez, C.; González-Cabrera, J. Assessing the resistance to acaricides in Varroa destructor from several Spanish locations. Parasitol. Res. 2020, 119, 3595–3601. [Google Scholar] [CrossRef]
- Lodesani, M.; Costa, C.; Serra, G.; Colombo, R.; Sabatini, A.G. Acaricide residues in beeswax after conversion to organic beekeeping methods. Apidologie 2008, 39, 324–333. [Google Scholar] [CrossRef]
- Milani, N. The resistance of Varroa jacobsoni Oud. to acaricides. Apidologie 1999, 30, 229–234. [Google Scholar] [CrossRef]
- Bade, R.; Chan, H.-F.; Reynisson, J. Characteristics of known drug space. Natural products, their derivatives and synthetic drugs. Eur. J. Med. Chem. 2010, 45, 5646–5652. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Castagna, F.; Bava, R.; Musolino, V.; Piras, C.; Cardamone, A.; Carresi, C.; Lupia, C.; Bosco, A.; Rinaldi, L.; Cringoli, G. Potential new therapeutic approaches based on Punica granatum fruits compared to synthetic anthelmintics for the sustainable control of gastrointestinal nematodes in sheep. Animals 2022, 12, 2883. [Google Scholar] [CrossRef]
- Štrbac, F.; Bosco, A.; Maurelli, M.P.; Ratajac, R.; Stojanović, D.; Simin, N.; Orčić, D.; Pušić, I.; Krnjajić, S.; Sotiraki, S.; et al. Anthelmintic Properties of Essential Oils to Control Gastrointestinal Nematodes in Sheep—In Vitro and In Vivo Studies. Vet. Sci. 2022, 9, 93. [Google Scholar] [CrossRef]
- Štrbac, F.; Krnjajić, S.; Maurelli, M.P.; Stojanović, D.; Simin, N.; Orčić, D.; Ratajac, R.; Petrović, K.; Knežević, G.; Cringoli, G. A potential anthelmintic phytopharmacological source of Origanum vulgare (L.) essential oil against gastrointestinal nematodes of sheep. Animals 2022, 13, 45. [Google Scholar] [CrossRef]
- Maiuolo, J.; Bulotta, R.M.; Oppedisano, F.; Bosco, F.; Scarano, F.; Nucera, S.; Guarnieri, L.; Ruga, S.; Macri, R.; Caminiti, R. Potential Properties of Natural Nutraceuticals and Antioxidants in Age-Related Eye Disorders. Life 2022, 13, 77. [Google Scholar] [CrossRef] [PubMed]
- Castagna, F.; Piras, C.; Palma, E.; Musolino, V.; Lupia, C.; Bosco, A.; Rinaldi, L.; Cringoli, G.; Musella, V.; Britti, D. Green veterinary pharmacology applied to parasite control: Evaluation of Punica granatum, Artemisia campestris, Salix caprea aqueous macerates against gastrointestinal nematodes of sheep. Vet. Sci. 2021, 8, 237. [Google Scholar] [CrossRef] [PubMed]
- Bosco, A.; Kießler, J.; Amadesi, A.; Varady, M.; Hinney, B.; Ianniello, D.; Maurelli, M.P.; Cringoli, G.; Rinaldi, L. The threat of reduced efficacy of anthelmintics against gastrointestinal nematodes in sheep from an area considered anthelmintic resistance-free. Parasites Vectors 2020, 13, 1–12. [Google Scholar] [CrossRef]
- Mollace, V.; Scicchitano, M.; Paone, S.; Casale, F.; Calandruccio, C.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Nucera, S. Hypoglycemic and hypolipemic effects of a new lecithin formulation of bergamot polyphenolic fraction: A double blind, randomized, placebo-controlled study. Endocr. Metab. Immune Disord.-Drug Targets (Former. Curr. Drug Targets-Immune Endocr. Metab. Disord.) 2019, 19, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Kiruthika, S.; Vishali, S. Industrial Application of Essential Oils. Essent. Oils Extr. Methods Appl. 2023, 49–67. [Google Scholar] [CrossRef]
- Bava, R.; Castagna, F.; Piras, C.; Palma, E.; Cringoli, G.; Musolino, V.; Lupia, C.; Perri, M.R.; Statti, G.; Britti, D.; et al. In vitro evaluation of acute toxicity of five Citrus spp. Essential oils towards the parasitic mite Varroa destructor. Pathogens 2021, 10, 1182. [Google Scholar] [CrossRef] [PubMed]
- Maggi, M.; Tourn, E.; Negri, P.; Szawarski, N.; Marconi, A.; Gallez, L.; Medici, S.; Ruffinengo, S.; Brasesco, C.; De Feudis, L. A new formulation of oxalic acid for Varroa destructor control applied in Apis mellifera colonies in the presence of brood. Apidologie 2016, 47, 596–605. [Google Scholar] [CrossRef]
- Hýbl, M.; Bohatá, A.; Rádsetoulalová, I.; Kopecký, M.; Hoštičková, I.; Vaníčková, A.; Mráz, P. Evaluating the Efficacy of 30 Different Essential Oils against Varroa destructor and Honey Bee Workers (Apis mellifera). Insects 2021, 12, 1045. [Google Scholar] [CrossRef]
- Schneider, S.; Eisenhardt, D.; Rademacher, E. Sublethal effects of oxalic acid on Apis mellifera (Hymenoptera: Apidae): Changes ins behaviour and longevity. Apidologie 2012, 43, 218–225. [Google Scholar] [CrossRef]
- Ostermann, D.J.; Currie, R.W. Effect of formic acid formulations on honey bee (Hymenoptera: Apidae) colonies and influence of colony and ambient conditions on formic acid concentration in the hive. J. Econ. Entomol. 2004, 97, 1500–1508. [Google Scholar] [CrossRef] [PubMed]
- Siegfried, B.D. The Effect of Oxalic Acid Treatments on Queen Survival and Drone Semen Viability. Available online: https://rd.almondboard.com (accessed on 20 October 2023).
- Papežíková, I.; Palíková, M.; Kremserová, S.; Zachová, A.; Peterová, H.; Babák, V.; Navrátil, S. Effect of oxalic acid on the mite Varroa destructor and its host the honey bee Apis mellifera. J. Apic. Res. 2017, 56, 400–408. [Google Scholar] [CrossRef]
- Strachecka, A.; Paleolog, J.; Olszewski, K.; Borsuk, G. Influence of amitraz and oxalic acid on the cuticle proteolytic system of Apis mellifera L. workers. Insects 2012, 3, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Bava, R.; Castagna, F.; Palma, E.; Marrelli, M.; Conforti, F.; Musolino, V.; Carresi, C.; Lupia, C.; Ceniti, C.; Tilocca, B. Essential Oils for a Sustainable Control of Honeybee Varroosis. Vet. Sci. 2023, 10, 308. [Google Scholar] [CrossRef]
- Asbahani, A.E.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.H.A.; Casabianca, H.; Mousadik, A.E.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef]
- Abdelgaleil, S.A.M.; Mohamed, M.I.E.; Badawy, M.E.I.; El-Arami, S.A.A. Fumigant and contact toxicities of monoterpenes to Sitophilus oryzae (L.) and Tribolium castaneum (Herbst) and their inhibitory effects on acetylcholinesterase activity. J. Chem. Ecol. 2009, 35, 518–525. [Google Scholar] [CrossRef]
- Safavi, S.A.; Mobki, M. Susceptibility of Tribolium castaneum (Herbst, 1797) larvae to essential oils of Citrus reticulata blanco fruit peels and the synergist, diethyl maleate. Biharean Biol. 2016, 10, 82–85. [Google Scholar]
- Aboelhadid, S.M.; Arafa, W.M.; Abdel-Baki, A.A.S.; Sokmen, A.; Al-Quraishy, S.; Hassan, A.O.; Kamel, A.A. Acaricidal activity of Foeniculum vulgare against Rhipicephalus annulatus is mainly dependent on its constituent from trans-anethone. PLoS ONE 2021, 16, e0260172. [Google Scholar] [CrossRef]
- Chou, J.T.; Rossignol, P.A.; Ayres, J.W. Evaluation of Commercial Insect Repellents on Human Skin Against Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 1997, 34, 624–630. [Google Scholar] [CrossRef]
- Romo-Chacón, A.; Martínez-Contreras, L.J.; Molina-Corral, F.J.; Acosta-Muñiz, C.H.; Ríos-Velasco, C.; De León-Door, A.P.; Rivera, R. Evaluation of oregano (Lippia berlandieri) essential oil and Entomopathogenic Fungi for Varroa destructor control in colonies of honey bee, Apis mellifera. Southwest. Entomol. 2016, 41, 971–982. [Google Scholar] [CrossRef]
- Aglagane, A.; Laghzaoui, E.-M.; Soulaimani, B.; Er-Rguibi, O.; Abbad, A.; El Mouden, E.H.; Aourir, M. Acaricidal activity of Mentha suaveolens subsp. timija, Chenopodium ambrosioides, and Laurus nobilis essential oils, and their synergistic combinations against the ectoparasitic bee mite, Varroa destructor (Acari: Varroidae). J. Apic. Res. 2022, 61, 9–18. [Google Scholar] [CrossRef]
- Rajendran, S.; Sriranjini, V. Plant products as fumigants for stored-product insect control. J. Stored Prod. Res. 2008, 44, 126–135. [Google Scholar] [CrossRef]
- Lee, B.H.; Annis, P.C.; Tumaalii, F.; Choi, W.S. Fumigant toxicity of essential oils from the Myrtaceae family and 1,8-cineole against 3 major stored-grain insects. J. Stored Prod. Res. 2004, 40, 553–564. [Google Scholar] [CrossRef]
- Conti, B.; Bocchino, R.; Cosci, F.; Ascrizzi, R.; Flamini, G.; Bedini, S. Essential oils against Varroa destructor: A soft way to fight the parasitic mite of Apis mellifera. J. Apic. Res. 2020, 59, 774–782. [Google Scholar] [CrossRef]
- Bava, R.; Castagna, F.; Palma, E.; Musolino, V.; Carresi, C.; Cardamone, A.; Lupia, C.; Marrelli, M.; Conforti, F.; Roncada, P. Phytochemical Profile of Foeniculum vulgare subsp. piperitum Essential Oils and Evaluation of Acaricidal Efficacy against Varroa destructor in Apis mellifera by In Vitro and Semi-Field Fumigation Tests. Vet. Sci. 2022, 9, 684. [Google Scholar] [CrossRef]
- Štrbac, F.; Krnjajić, S.; Stojanović, D.; Ratajac, R.; Simin, N.; Orčić, D.; Rinaldi, L.; Ciccone, E.; Maurelli, M.P.; Cringoli, G. Invitro and in vivo anthelmintic efficacy of peppermint (Mentha x piperita L.) essential oil against gastrointestinal nematodes of sheep. Front. Vet. Sci. 2023, 10, 1232570. [Google Scholar] [CrossRef] [PubMed]
- Eguaras, M.J.; Fuselli, S.; Gende, L.; Fritz, R.; Ruffinengo, S.R.; Clemente, G.; Gonzalez, A.; Bailac, P.N.; Ponzi, M.I. An in vitro evaluation of Tagetes minuta essential oil for the control of the honeybee pathogens Paenibacillus larvae and Ascosphaera apis, and the parasitic mite Varroa destructor. J. Essent. Oil Res. 2005, 17, 336–340. [Google Scholar] [CrossRef]
- Knobloch, K.; Pauli, A.; Iberl, B.; Weigand, H.; Weis, N. Antibacterial and antifungal properties of essential oil components. J. Essent. Oil Res. 1989, 1, 119–128. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 5th ed.; Texensis Publication: Gruver, TX, USA, 2017. [Google Scholar]
- Gashout, H.A.; Guzmán-Novoa, E. Acute toxicity of Essential oils and other natural compounds to the parasitic mite, Varroa destructor, and to larval and adult worker honey bees (Apis mellifera L.). J. Apic. Res. 2009, 48, 263–269. [Google Scholar] [CrossRef]
- Tseng, S.-H.; Chang, P.-C.; Chou, S.-S. Determination of amitraz residue in fruits by high performance liquid chromatography. J. Food Drug Anal. 1999, 7, 6. [Google Scholar] [CrossRef]
- Mutete, F.B.; Bweupe, N.; Chiyenu, K.O.R.; Mutengo, K.H. Amitraz poisoning—A case report of a common but highly misconstrued cause of poisoning in Zambia. Med. J. Zamb. 2019, 46, 254–258. [Google Scholar]
- Natrajan, D.; Srinivasan, S.; Sundar, K.; Ravindran, A. Formulation of essential oil-loaded chitosan–alginate nanocapsules. J. Food Drug Anal. 2015, 23, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Samfira, I.; Rodino, S.; Petrache, P.; Cristina, R.T.; Butu, M.; Butnariu, M. Characterization and identity confirmation of essential oils by mid infrared absorption spectrophotometry. Dig. J. Nanomater. Biostruct. 2015, 10, 557–566. [Google Scholar]
- Lide, D.R. (Ed.) CRC Handbook of Chemistry and Physics, 88th ed.; National Institute of Standards and Technology; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2007; 2640p, ISBN 0-8493-0488-1. [Google Scholar] [CrossRef]
- Le Conte, Y.; Arnold, G.; Desenfant, P. Influence of brood temperature and hygrometry variations on the development of the honey bee ectoparasite Varroa jacobsoni (Mesostigmata: Varroidae). Environ. Entomol. 1990, 19, 1780–1785. [Google Scholar] [CrossRef]
- Williams, G.R.; Alaux, C.; Costa, C.; Csaki, T.; Doublet, V.; Eisenhardt, D.; Fries, I.; Kuhn, R.; McMahon, D.P.; Medrzycki, P. Standard methods for maintaining adult Apis mellifera in cages under in vitro laboratory conditions. J. Apic. Res. 2013, 52, 1–36. [Google Scholar] [CrossRef]
- Sharpe, R.M.; Irvine, D.S. How strong is the evidence of a link between environmental chemicals and adverse effects on human reproductive health? BMJ 2004, 328, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; Chatterjee, C.; Mandal, F.B. Synthetic chemical pesticides and their effects on birds. Res. J. Env. Toxicol. 2011, 5, 81–96. [Google Scholar] [CrossRef]
- Jagne, J.; White, D.; Jefferson, F. Endocrine-disrupting chemicals: Adverse effects of bisphenol A and parabens to women’s health. Water Air Soil Pollut. 2016, 227, 1–10. [Google Scholar] [CrossRef]
- Hamilton, D.; Crossley, S. Pesticide Residues in Food and Drinking Water: Human Exposure and Risks; John Wiley & Sons: Hoboken, NJ, USA, 2004; ISBN 0470091606. [Google Scholar]
- Batish, D.R.; Singh, H.P.; Kohli, R.K.; Kaur, S. Eucalyptus essential oil as a natural pesticide. For. Ecol. Manag. 2008, 256, 2166–2174. [Google Scholar] [CrossRef]
- Regnault-Roger, C.; Vincent, C.; Arnason, J.T. Essential oils in insect control: Low-risk products in a high-stakes world. Annu. Rev. Entomol. 2012, 57, 405–424. [Google Scholar] [CrossRef]
- Venkateshappa, S.M.; Sreenath, K.P. Potential medicinal plants of Lamiaceae. Am. Int. J. Res. Form. Appl. Nat. Sci. 2013, 1, 82–87. [Google Scholar]
- El-Bolok, D.M.R.; Mahfouz, H.M. Efficacy Of Some Plant Extracts Against Varroa destructor and Their Sideeffect On Honeybee Colonies. Zagazig J. Agric. Res. 2021, 48, 1023–1032. [Google Scholar] [CrossRef]
- Damiani, N.; Gende, L.B.; Bailac, P.; Marcangeli, J.A.; Eguaras, M.J. Acaricidal and insecticidal activity of essential oils on Varroa destructor (Acari: Varroidae) and Apis mellifera (Hymenoptera: Apidae). Parasitol. Res. 2009, 106, 145–152. [Google Scholar] [CrossRef]
- Ghasemi, V.; Moharramipour, S.; Tahmasbi, G.H. Laboratory cage studies on the efficacy of some medicinal plant essential oils for controlling varroosis in Apis mellifera (Hym.: Apidae). Syst. Appl. Acarol. 2016, 21, 1681–1692. [Google Scholar] [CrossRef]
- Milani, N. Activity of oxalic and citric acids on the mite Varroa destructor in laboratory assays. Apidologie 2001, 32, 127–138. [Google Scholar] [CrossRef]
- Koc, S.; Oz, E.; Cinbilgel, I.; Aydin, L.; Cetin, H. Acaricidal activity of Origanum bilgeri PH Davis (Lamiaceae) essential oil and its major component, carvacrol against adults Rhipicephalus turanicus (Acari: Ixodidae). Vet. Parasitol. 2013, 193, 316–319. [Google Scholar] [CrossRef]
- Kintzios, S.E. Oregano: The Genera Origanum and Lippia; CRC Press: Boca Raton, FL, USA, 2002; ISBN 0203222091. [Google Scholar]
- Çetin, H.; Cilek, J.E.; Oz, E.; Aydin, L.; Deveci, O.; Yanikoglu, A. Acaricidal activity of Satureja thymbra L. essential oil and its major components, carvacrol and γ-terpinene against adult Hyalomma marginatum (Acari: Ixodidae). Vet. Parasitol. 2010, 170, 287–290. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Cruz, E.M.; Costa-Junior, L.M.; Pinto, J.A.O.; de Alexandria Santos, D.; de Araujo, S.A.; de Fátima Arrigoni-Blank, M.; Bacci, L.; Alves, P.B.; de Holanda Cavalcanti, S.C.; Blank, A.F. Acaricidal activity of Lippia gracilis essential oil and its major constituents on the tick Rhipicephalus (Boophilus) microplus. Vet. Parasitol. 2013, 195, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, G.; Kumar, S.; Chhabra, L.; Mahant, S.; Rao, R. Essential oil–cyclodextrin complexes: An updated review. J. Incl. Phenom. Macrocycl. Chem. 2017, 89, 39–58. [Google Scholar] [CrossRef]
- Das, S.; Singh, V.K.; Chaudhari, A.K.; Dwivedy, A.K.; Dubey, N.K. Fabrication, physico-chemical characterization, and bioactivity evaluation of chitosan-linalool composite nano-matrix as innovative controlled release delivery system for food preservation. Int. J. Biol. Macromol. 2021, 188, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Martín, Á.; Varona, S.; Navarrete, A.; Cocero, M.J. Encapsulation and co-precipitation processes with supercritical fluids: Applications with essential oils. Open Chem. Eng. J. 2010, 4, 31–41. [Google Scholar] [CrossRef]
- Vishwakarma, G.S.; Gautam, N.; Babu, J.N.; Mittal, S.; Jaitak, V. Polymeric encapsulates of essential oils and their constituents: A review of preparation techniques, characterization, and sustainable release mechanisms. Polym. Rev. 2016, 56, 668–701. [Google Scholar] [CrossRef]
- Agency, E.M. Guideline on Veterinary Medicinal Products Controlling Varroa destructor Parasitosis in Bees. 2007. Available online: https://www.ema.europa.eu/en/homepage (accessed on 20 October 2023).
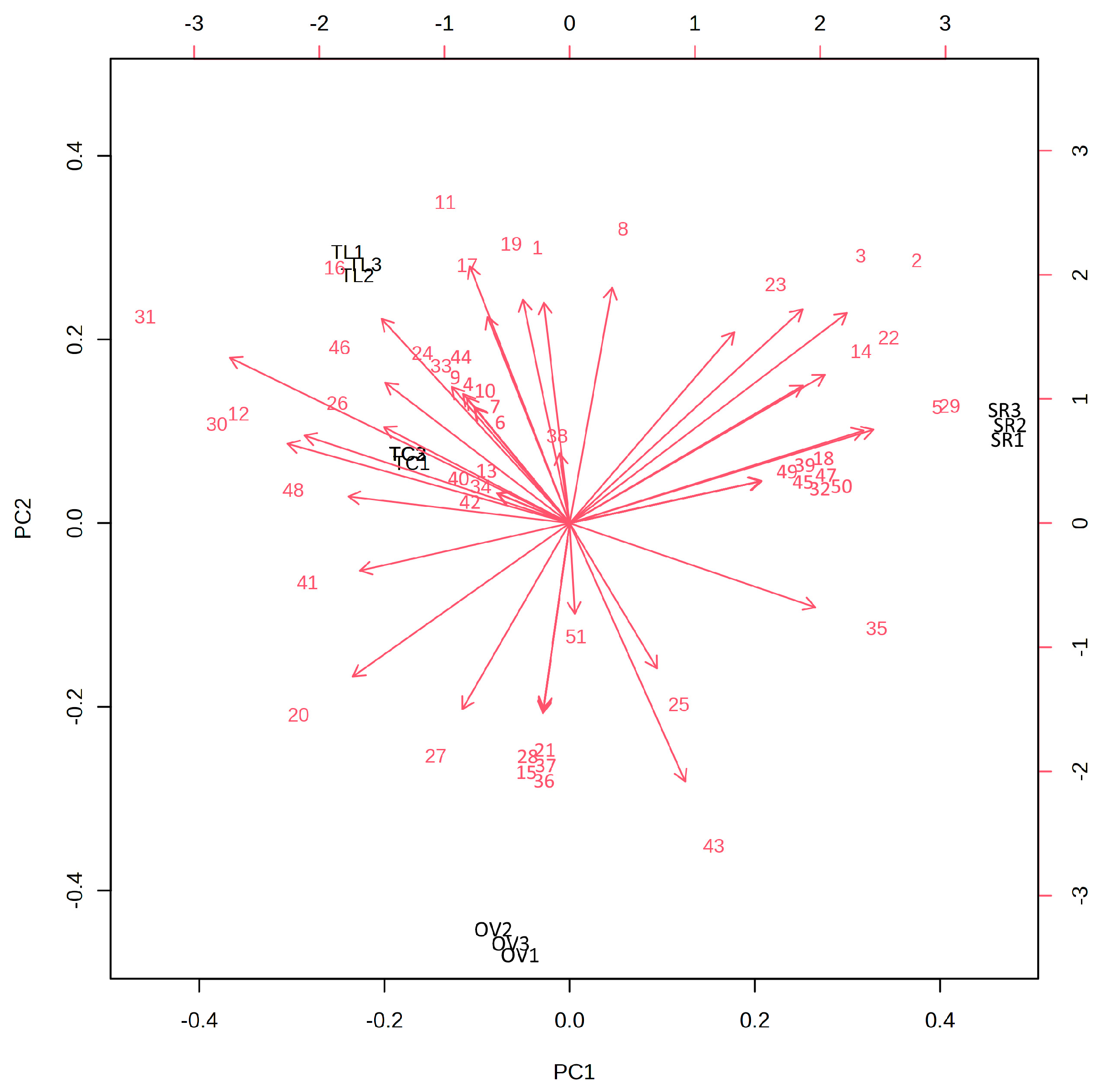
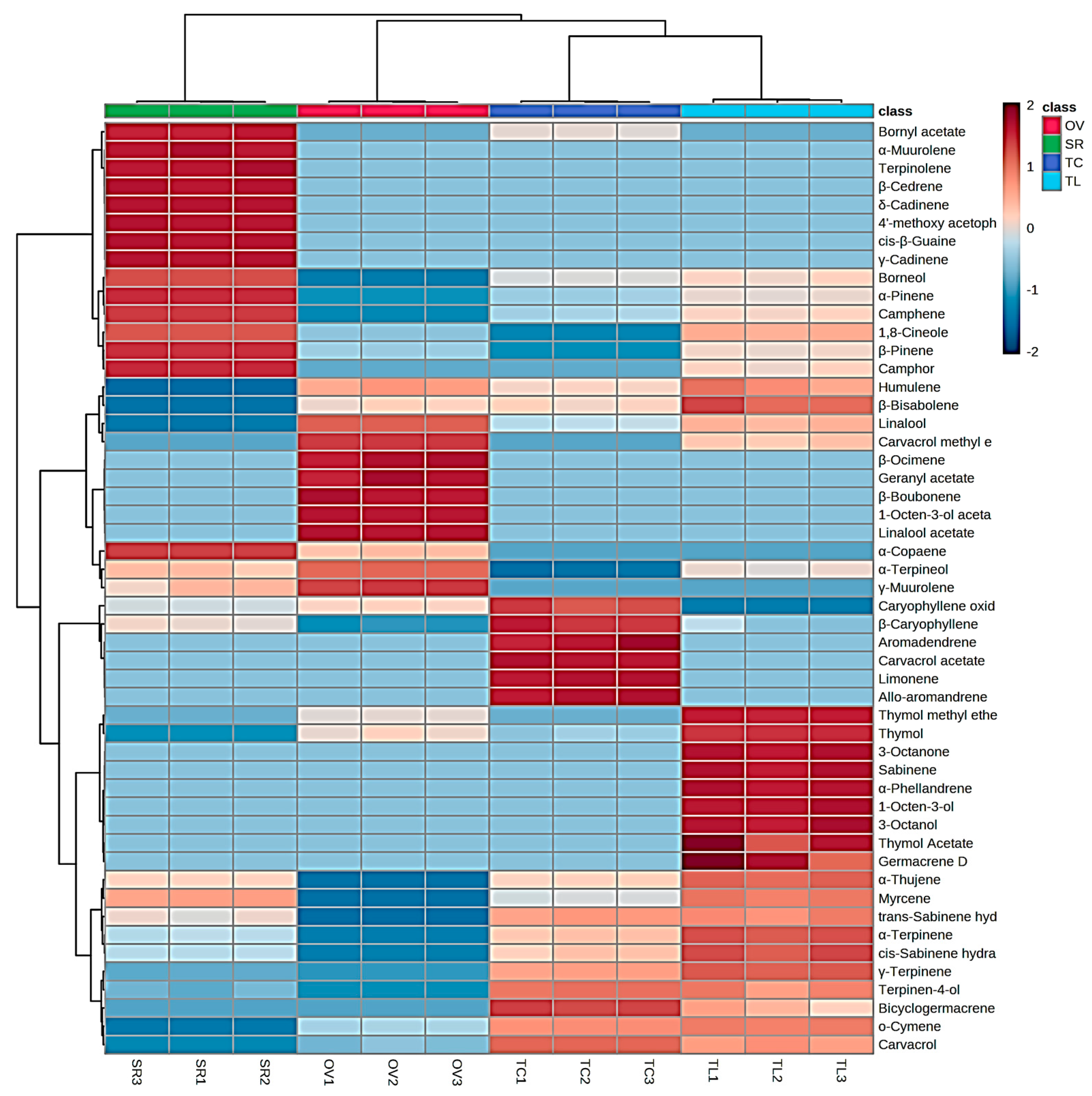
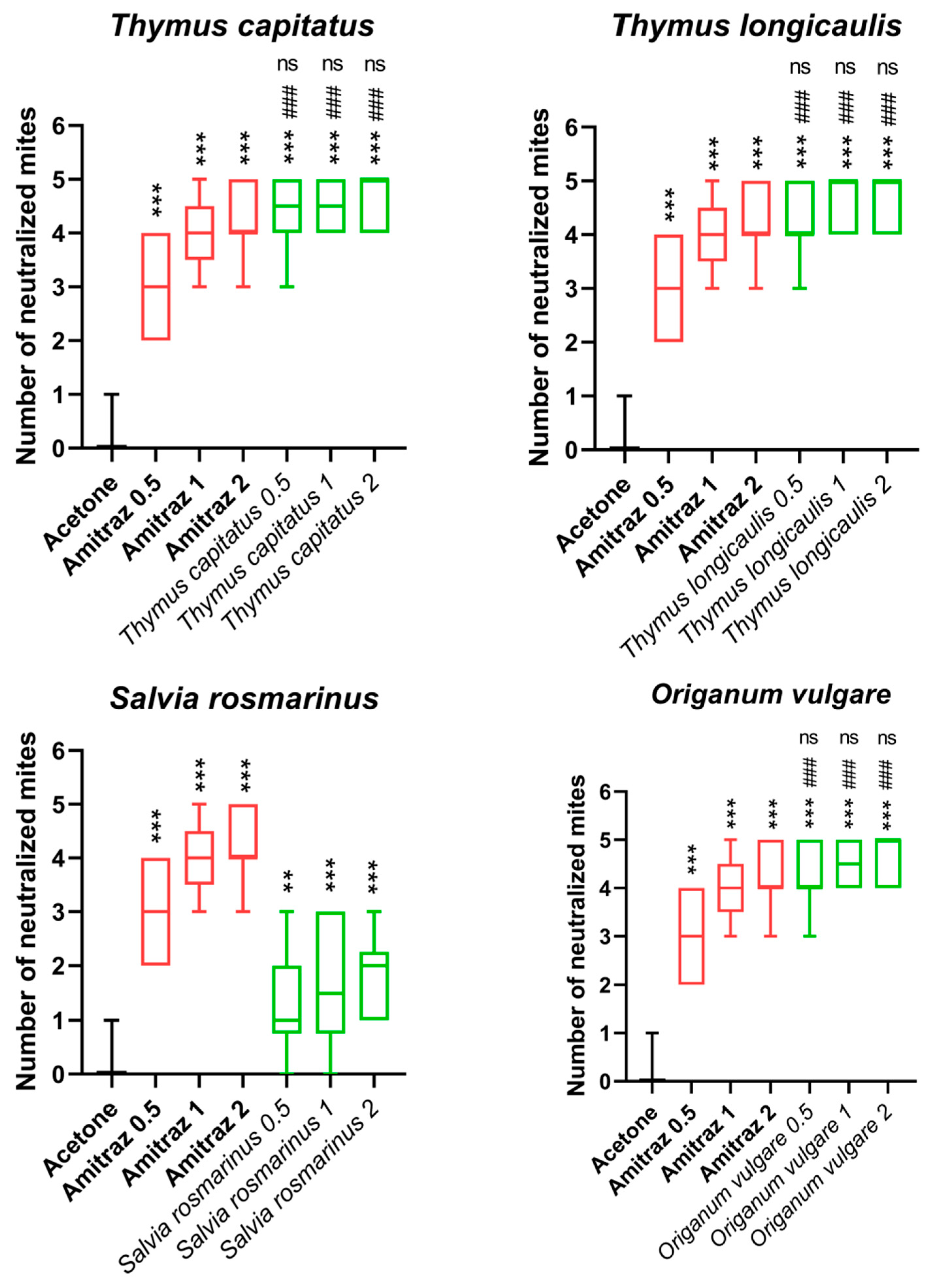
| No. | Compound 1 | KI 2 | KI 3 | % ± SD | i.m. 4 | |||
|---|---|---|---|---|---|---|---|---|
| OV | SR | TC | TL | |||||
| 1 | α-Thujene | 930 | 932 | - | 0.10 ± 0.00 | 0.10 ± 0.00 | 0.25 ± 0.01 | GC-MS |
| 2 | α-Pinene | 939 | 937 | - | 7.55 ± 0.03 | 0.09 ± 0.01 | 0.24 ± 0.01 | GC, GC-MS |
| 3 | Camphene | 954 | 955 | - | 2.54 ± 0.02 | 0.08 ± 0.01 | 0.21 ± 0.01 | GC, GC-MS |
| 4 | Sabinene | 975 | 970 | - | - | - | 0.20 ± 0.01 | GC, GC-MS |
| 5 | β-Pinene | 979 | 980 | 0.08 ± 0.00 | 6.48 ± 0.01 | - | 0.26 ± 0.01 | GC, GC-MS |
| 6 | 1-Octen-3-ol | 980 | 981 | - | - | - | 0.49 ± 0.02 | GC-MS |
| 7 | 3-Octanone | 986 | 984 | - | - | - | 2.22 ± 0.08 | GC-MS |
| 8 | Myrcene | 991 | 990 | - | 0.62 ± 0.02 | 0.27 ± 0.01 | 0.91 ± 0.06 | GC, GC-MS |
| 9 | 3-Octanol | 993 | 995 | - | - | - | 0.74 ± 0.05 | GC-MS |
| 10 | α-Phellandrene | 1002 | 1001 | - | - | - | 0.35 ± 0.02 | GC, GC-MS |
| 11 | α-Terpinene | 1018 | 1015 | - | 0.10 ± 0.01 | 0.23 ± 0.02 | 0.95 ± 0.06 | GC, GC-MS |
| 12 | o-Cymene | 1022 | 1021 | 0.39 ± 0.01 | - | 2.16 ± 0.09 | 2.68 ± 0.13 | GC, GC-MS |
| 13 | Limonene | 1029 | 1028 | - | - | 0.60 ± 0.02 | - | GC, GC-MS |
| 14 | 1,8-Cineole | 1031 | 1031 | 0.83 ± 0.02 | 46.92 ± 0.04 | - | 8.85 ± 0.50 | GC, GC-MS |
| 15 | β-Ocimene | 1050 | 1051 | 0.14 ± 0.01 | - | - | - | GC-MS |
| 16 | γ-Terpinene | 1062 | 1061 | 0.20 ± 0.00 | 0.28 ± 0.01 | 2.49 ± 0.09 | 5.86 ± 0.30 | GC, GC-MS |
| 17 | cis-Sabinene hydrate | 1068 | 1070 | 0.20 ± 0.00 | 0.56 ± 0.01 | 0.97 ± 0.08 | 2.55 ± 0.20 | GC, GC-MS |
| 18 | Terpinolene | 1088 | 1089 | - | 0.18 ± 0.01 | - | - | GC-MS |
| 19 | trans-Sabinene hydrate | 1097 | 1095 | - | 0.11 ± 0.01 | 0.22 ± 0.01 | 0.27 ± 0.03 | GC-MS |
| 20 | Linalool | 1098 | 1101 | 15.84 ± 0.00 | 0.28 ± 0.01 | 1.81 ± 0.12 | 5.14 ± 0.37 | GC, GC-MS |
| 21 | 1-Octen-3-ol acetate | 1112 | 1119 | 0.38 ± 0.00 | - | - | - | GC-MS |
| 22 | Camphor | 1146 | 1142 | - | 8.49 ± 0.04 | - | 0.68 ± 0.08 | GC, GC-MS |
| 23 | Borneol | 1169 | 1164 | - | 11.96 ± 0.07 | 2.17 ± 0.05 | 2.75 ± 0.19 | GC, GC-MS |
| 24 | Terpinen-4-ol | 1177 | 1175 | 0.17 ± 0.00 | 0.23 ± 0.01 | 0.79 ± 0.02 | 0.70 ± 0.09 | GC, GC-MS |
| 25 | α-Terpineol | 1188 | 1188 | 5.13 ± 0.10 | 1.80 ± 0.18 | 0.15 ± 0.01 | 1.15 ± 0.07 | GC, GC-MS |
| 26 | Thymol methyl ether | 1235 | 1237 | 0.18 ± 0.01 | - | - | 5.10 ± 0.25 | GC-MS |
| 27 | Carvacrol methyl ether | 1244 | 1247 | 1.53 ± 0.02 | - | - | 0.30 ± 0.02 | GC-MS |
| 28 | Linalool acetate | 1257 | 1256 | 65.27 ± 0.72 | - | - | - | GC, GC-MS |
| 29 | Bornyl acetate | 1288 | 1289 | - | 2.74 ± 0.19 | 0.10 ± 0.00 | - | GC, GC-MS |
| 30 | Thymol | 1290 | 1271 | 0.76 ± 0.18 | - | 0.18 ± 0.03 | 31.67 ± 0.97 | GC, GC-MS |
| 31 | Carvacrol | 1299 | 1307 | 0.23 ± 0.06 | - | 54.74 ± 0.78 | 14.39 ± 0.68 | GC, GC-MS |
| 32 | 4′-methoxy acetophenone | 1350 | 1349 | - | 0.09 ± 0.00 | - | - | GC-MS |
| 33 | Thymol Acetate | 1355 | 1357 | - | - | - | 1.63 ± 0.05 | GS-MS |
| 34 | Carvacrol acetate | 1372 | 1376 | - | - | 15.22 ± 0.38 | - | GC-MS |
| 35 | α-Copaene | 1376 | 1380 | 0.12 ± 0.01 | 0.48 ± 0.00 | - | - | GC-MS |
| 36 | Geranyl acetate | 1381 | 1385 | 0.25 ± 0.01 | - | - | - | GC, GC-MS |
| 37 | β-Boubonene | 1388 | 1388 | 0.15 ± 0.01 | - | - | - | GC-MS |
| 38 | β-Caryophyllene | 1408 | 1406 | 3.87 ± 0.09 | 6.46 ± 0.14 | 12.58 ± 0.59 | 5.18 ± 0.51 | GC, GC-MS |
| 39 | β-Cedrene | 1420 | 1417 | - | 0.11 ± 0.00 | - | - | GC-MS |
| 40 | Aromadendrene | 1441 | 1428 | - | - | 0.13 ± 0.01 | - | GC-MS |
| 41 | α-Humulene | 1454 | 1445 | 0.42 ± 0.04 | - | 0.25 ± 0.00 | 0.50 ± 0.11 | GC-MS |
| 42 | Allo-aromandrene | 1461 | 1453 | - | - | 0.10 ± 0.00 | - | GC-MS |
| 43 | γ-Muurolene | 1479 | 1476 | 2.36 ± 0.12 | 0.38 ± 0.02 | - | - | GC-MS |
| 44 | Germacrene D | 1480 | 1475 | - | - | - | 0.56 ± 0.02 | GC-MS |
| 45 | cis-β-Guaine | 1493 | 1491 | - | 0.15 ± 0.00 | - | - | GC-MS |
| 46 | Bicyclogermacrene | 1494 | 1492 | - | - | 1.32 ± 0.07 | 0.33 ± 0.01 | GC-MS |
| 47 | α-Muurolene | 1500 | 1499 | - | 0.09 ± 0.00 | - | - | GC-MS |
| 48 | β-Bisabolene | 1505 | 1511 | 0.99 ± 0.09 | - | 0.99 ± 0.06 | 2.90 ± 0.17 | GC-MS |
| 49 | γ-Cadinene | 1513 | 1510 | - | 0.28 ± 0.00 | - | - | GC-MS |
| 50 | δ-Cadinene | 1523 | 1521 | - | 0.68 ± 0.00 | - | - | GC-MS |
| 51 | Caryophyllene oxide | 1583 | 1569 | 0.50 ± 0.02 | 0.35 ± 0.00 | 2.27 ± 0.31 | - | GC-MS |
| Concentration mg/mL | O. vulgare viridulum Mortality % | T. capitatus Mortality % | T. longicaulis Mortality % | S. rosmarinus Mortality % | Acetone Mortality % | Amitraz Mortality % |
|---|---|---|---|---|---|---|
| 0.5 mg | 86 (±13) | 88 (±14) | 84 (±13) | 26 (±19) | 2 S (±6) | 60 (±14) |
| 1 mg | 90 (±11) | 88 (±11) | 94 (±10) | 34 (±25) | 67 (±3) | |
| 2 mg | 94 (±10) | 92 (±10) | 94 (±10) | 38 (±15) | 93 (±10) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bava, R.; Castagna, F.; Lupia, C.; Ruga, S.; Musella, V.; Conforti, F.; Marrelli, M.; Argentieri, M.P.; Britti, D.; Statti, G.; et al. Chemical Profile of Essential Oils of Selected Lamiaceae Plants and In Vitro Activity for Varroosis Control in Honeybees (Apis mellifera). Vet. Sci. 2023, 10, 701. https://doi.org/10.3390/vetsci10120701
Bava R, Castagna F, Lupia C, Ruga S, Musella V, Conforti F, Marrelli M, Argentieri MP, Britti D, Statti G, et al. Chemical Profile of Essential Oils of Selected Lamiaceae Plants and In Vitro Activity for Varroosis Control in Honeybees (Apis mellifera). Veterinary Sciences. 2023; 10(12):701. https://doi.org/10.3390/vetsci10120701
Chicago/Turabian StyleBava, Roberto, Fabio Castagna, Carmine Lupia, Stefano Ruga, Vincenzo Musella, Filomena Conforti, Mariangela Marrelli, Maria Pia Argentieri, Domenico Britti, Giancarlo Statti, and et al. 2023. "Chemical Profile of Essential Oils of Selected Lamiaceae Plants and In Vitro Activity for Varroosis Control in Honeybees (Apis mellifera)" Veterinary Sciences 10, no. 12: 701. https://doi.org/10.3390/vetsci10120701
APA StyleBava, R., Castagna, F., Lupia, C., Ruga, S., Musella, V., Conforti, F., Marrelli, M., Argentieri, M. P., Britti, D., Statti, G., & Palma, E. (2023). Chemical Profile of Essential Oils of Selected Lamiaceae Plants and In Vitro Activity for Varroosis Control in Honeybees (Apis mellifera). Veterinary Sciences, 10(12), 701. https://doi.org/10.3390/vetsci10120701













