Obesity during Pregnancy in the Horse: Effect on Term Placental Structure and Gene Expression, as Well as Colostrum and Milk Fatty Acid Concentration
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Experimental Design, Management, and Feeding of Mares and Foals
2.3. Measurements and Sampling
2.3.1. Measurements and Sampling at Foaling
2.3.2. Measurements and Sampling during Lactation
2.4. Placental Analyses
2.4.1. Histology and Stereology
2.4.2. Functional Analyses
2.5. Fatty Acid Analyses
2.6. Statistical Analyses
- A principal component analysis (PCA) was used for each point of measurement separately using the FactoMineR package.
- Non-parametric permutations ANOVA (package lmPerm, function aovp) considering the group (N or O), the age of the mare for plasma of mares and milk, and the sex of the foal for plasma of foals. The seed was set at 1234, and p-values were corrected using the fdr method. A volcano plot was drawn using the log2(fold-change) and the −10log(adjusted p-value) to summarise the linear model results. Boxplots and volcano plots were drawn using the ggplot2 package.
- –
- The individual factor map is a plot of the Principal Component Scores for individuals on the first two principal components. Confidence ellipses (0.95, around the barycenter) were added to the figure. A permutational multivariate analysis of variance (PERMANOVA, package vegan, function adonis2) was used to evaluate the difference between the barycenters of both groups, which was considered significant when p < 0.05.
- –
- The variable factor map presents a view of the projection of the observed variables projected into the plane spanned by the first two principal components. Also named a Correlation circle, it can help to visualise the most correlated variables (i.e., variables that group together) and to show the structural relationship between the variables and the components.
- –
- The projection of a variable vector onto the component axis allows us to directly read the correlation between the variable and the component. To simplify the reading of the figures, we merged the individuals and the variable factor maps into one biplot using the factoextra package (function fviz_pca_biplot).
3. Results
3.1. Placental Analyses
3.1.1. Neonate and Placental Biometry
3.1.2. Placental Stereology
3.1.3. Placental Gene Expression
3.2. Fatty Acid Concentrations
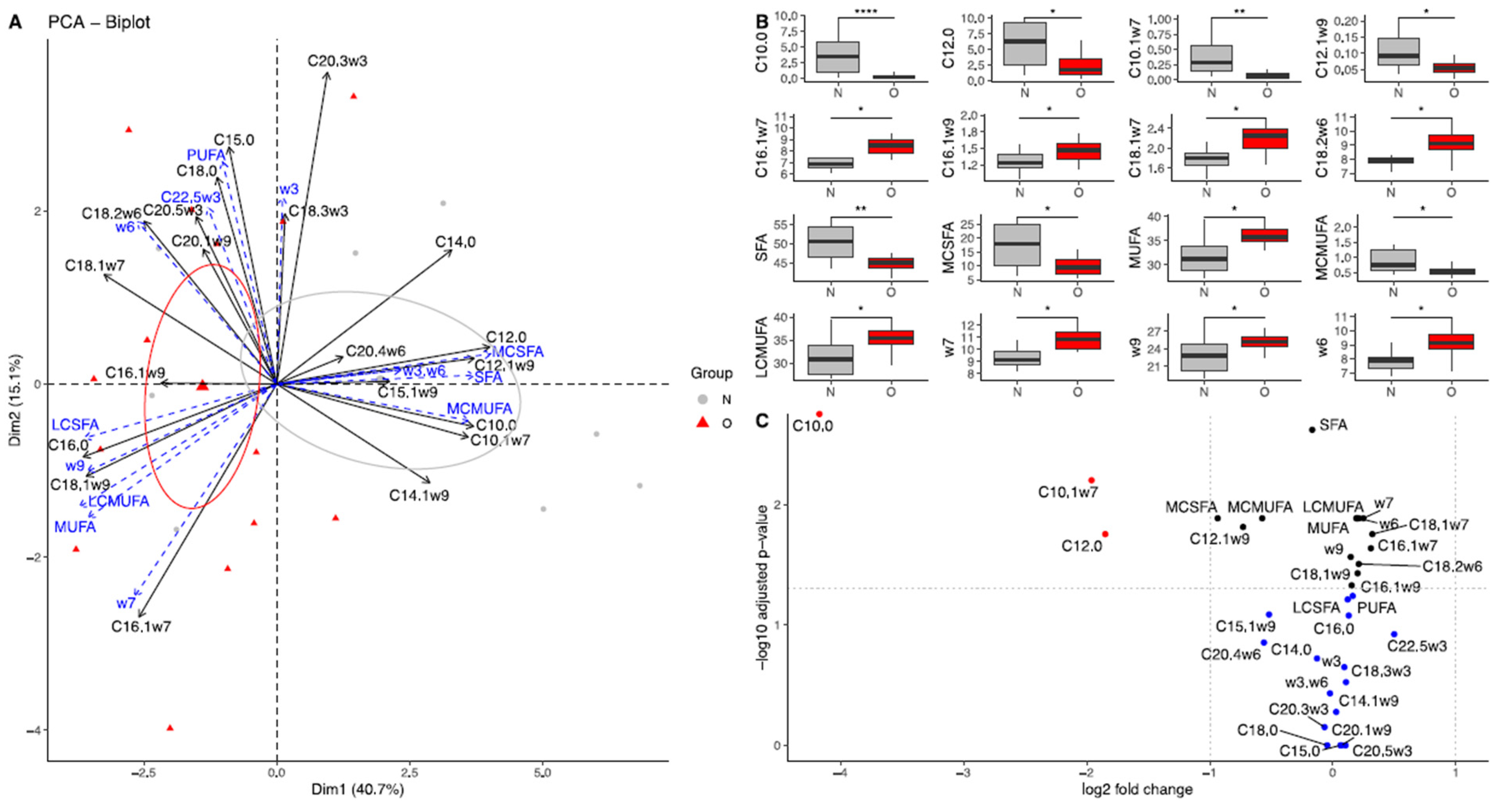
3.2.1. Fatty Acid Composition in Plasma of Mares
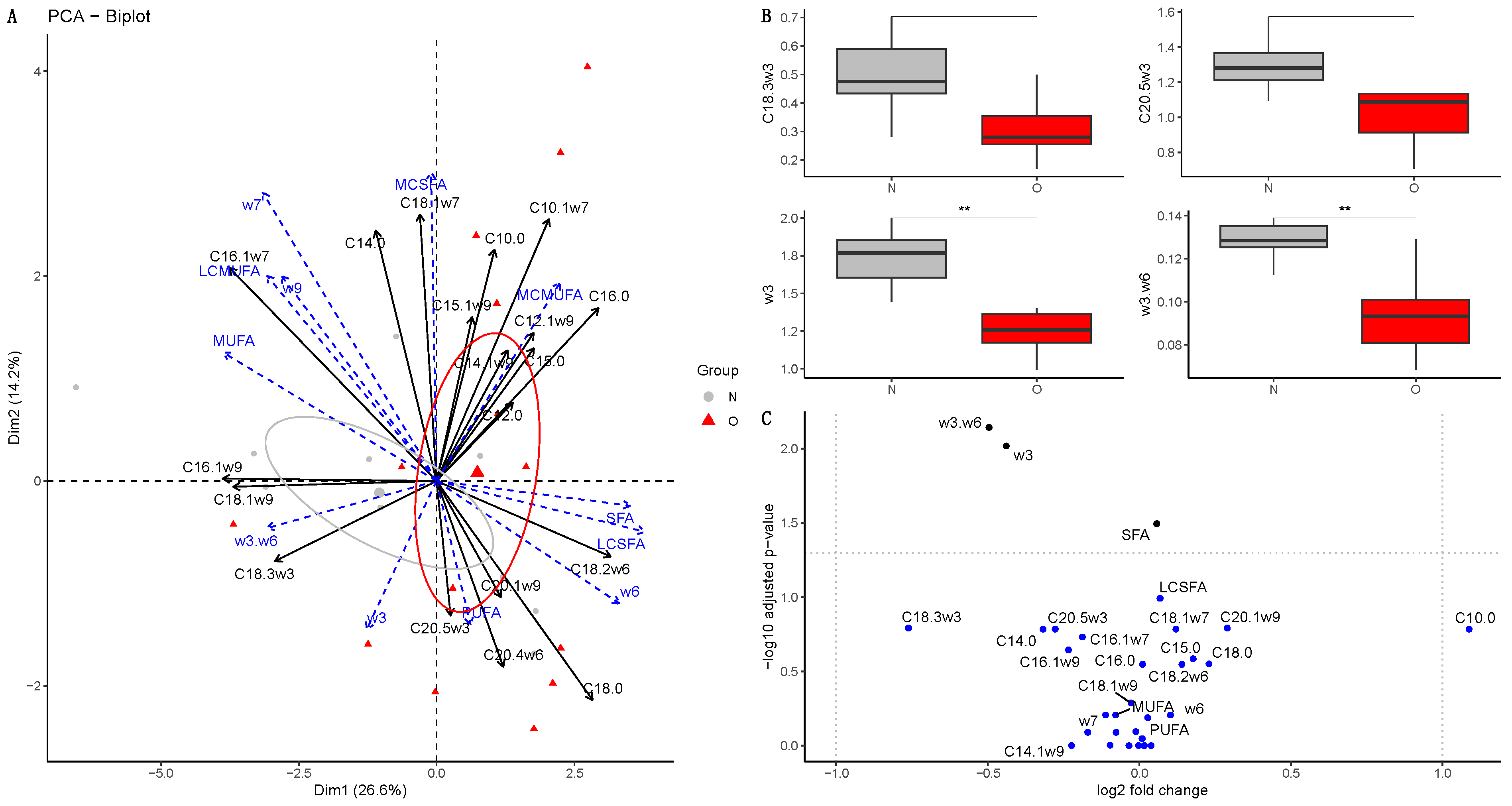
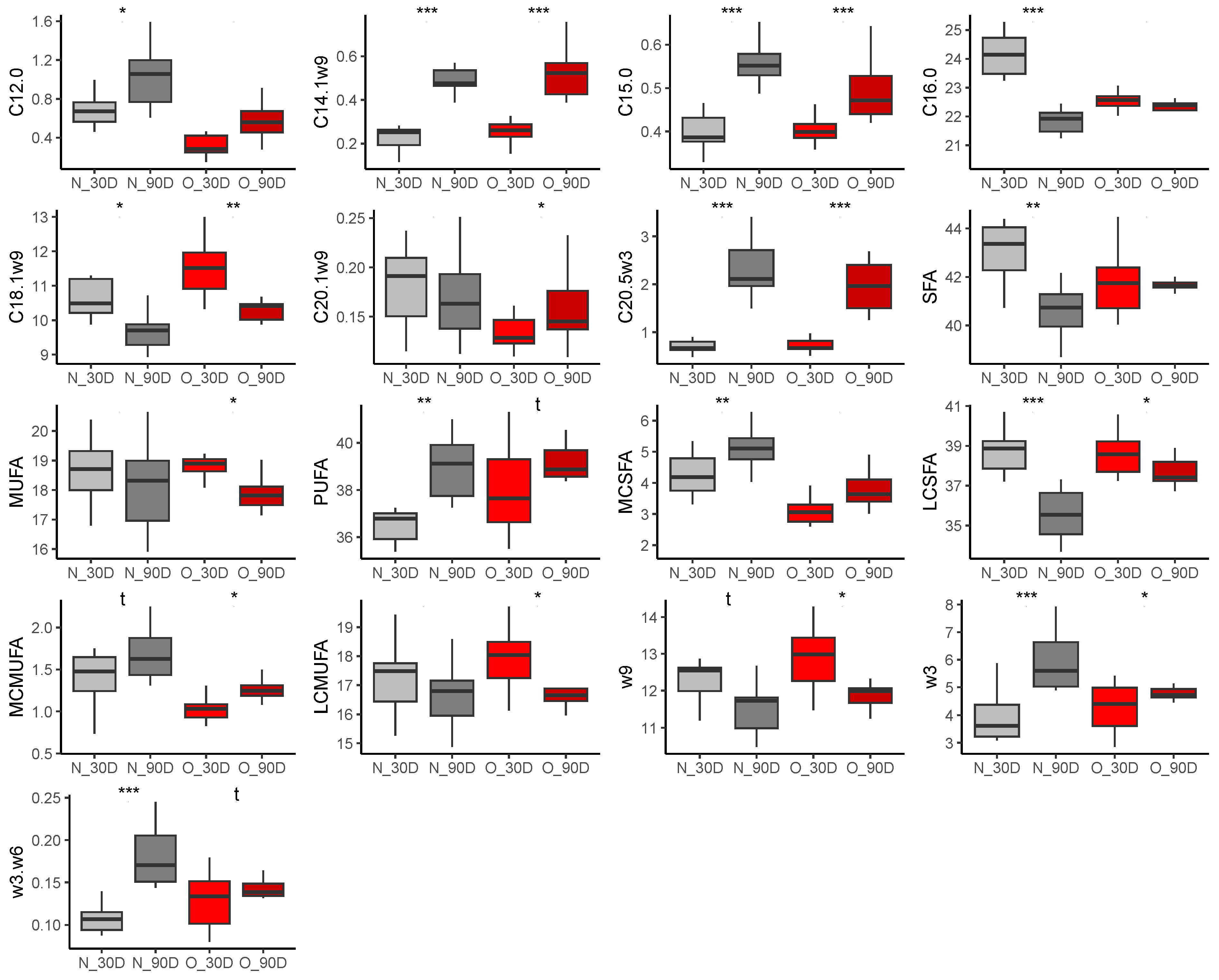
3.2.2. Fatty Acid Composition in Milk
Colostrum
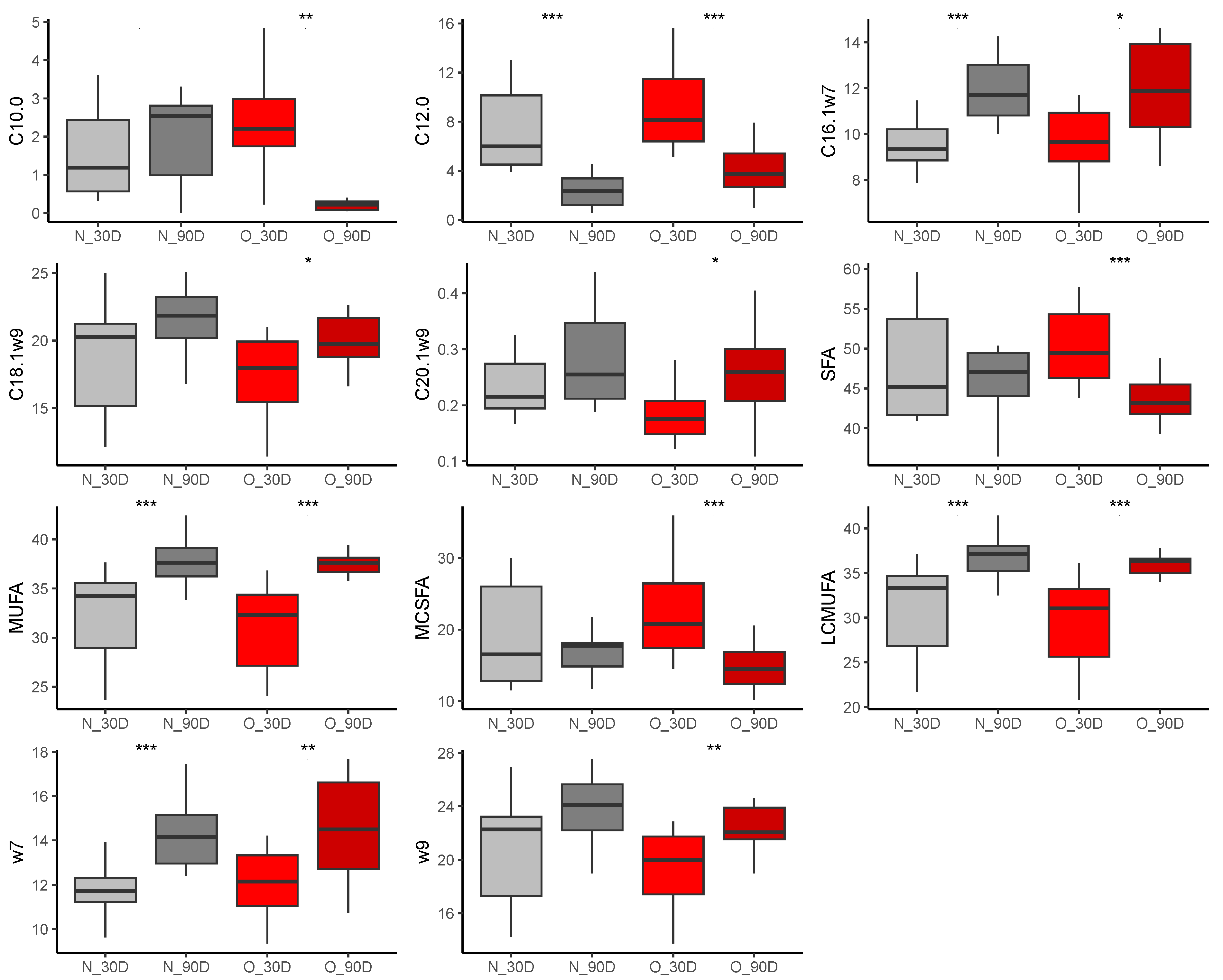
At 30 Days of Lactation
At 90 Days of Lactation
3.2.3. Fatty Acid Composition in Plasma of Foals
At Birth
At 30 Days of Age
At 90 Days of Age
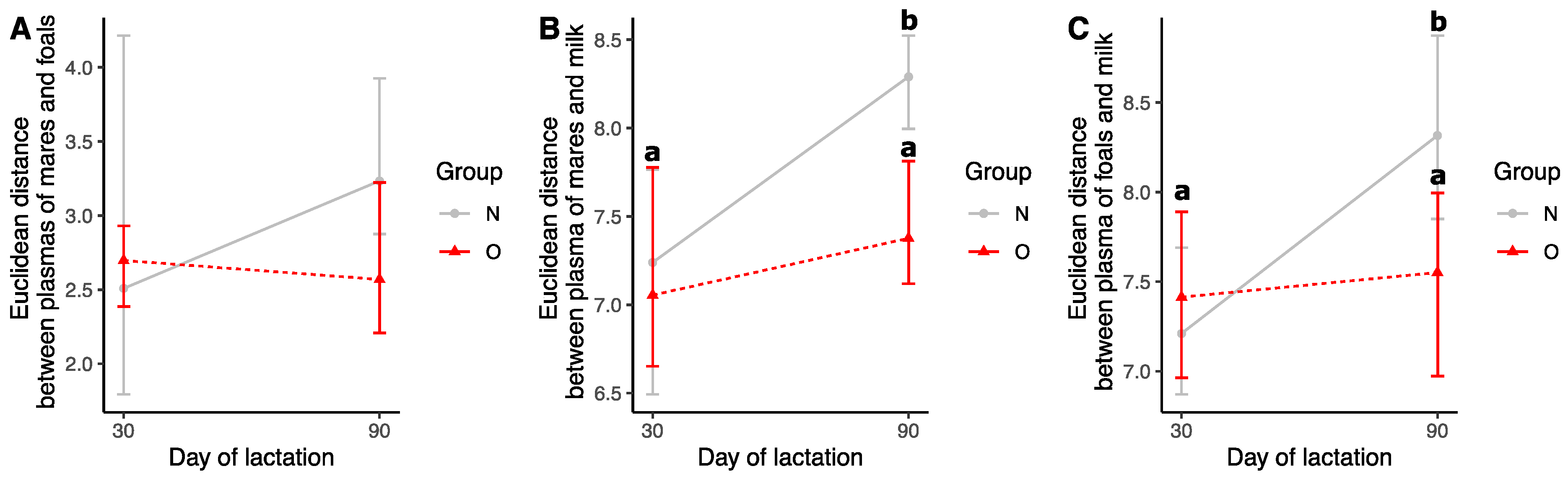
3.2.4. Comparison of Samples during Lactation
Euclidean Distances
Effect of Time of Lactation on Plasma Fatty Acid Profiles from Mares
Effect of Time of Lactation on Milk Fatty Acid Profiles
Effect of Time of Lactation on Plasma Fatty Acid Profiles from Foals
4. Discussion
4.1. Placental Measurements and Plasma from Foals at Birth
4.2. Fatty Acid Profiles
4.2.1. Plasma from Mares
4.2.2. Colostrum
- –
- An increase in SCD1 expression and/or activity in colostrum from obese mares. In goat and buffalo mammary epithelial cells in vitro, an overexpression of SCD1 leads to an increased production of oleic and palmitoleic acids [92]. We showed previously that O mares were insulin resistant in late gestation [17]. In bovine mammary epithelial cells in vitro, insulin was shown to increase SCD1 promoter activity [93], and SCD1 activity was shown to be increased in insulin-resistant individuals in humans and rodents [94]. These differences in glucose metabolism may therefore affect the expression and/or activity of SCD1 in the mammary glands of obese mares.
- –
- An increase in long-chain fatty acid transport in the mammary gland. PPARG1 has been shown in goats to upregulate the expression of LPL and CD36, which are involved in long-chain fatty acid transport [95]. However, there is no information on the effect of obesity on the mammary gland gene expression of SCD1, LPL, CD36, and PPARG1. More research is needed to understand the physiology of the mammary gland in equids and the effect of obesity on mammary gland gene and protein expression.
4.2.3. Milk and Plasma of Foals
4.3. Study Limitations
- –
- Sample size: Ten mares were in the Obese group and 14 in the Normal group. This sample size is considered low and may have led to low statistical power (especially with the multiple test correction). This could have inflated the risk of false negatives. Therefore, this study needs to be replicated in order to confirm the absence of differences in placental structure and function between obese and non-obese mares, as well as differences in fatty acids in the analysed tissues between groups.
- –
- Pasture quality information: No information was available for pasture quality or pasture consumption by mares and foals during the lactation period. Therefore, the intensity of the drought could not be measured directly on the fields. The fact that, despite having unlimited access to hay, mares of both groups lost body condition during the heat stress episode highlighted the severity of this event.
- –
- Method of milk sampling: Milk was sampled after a 3 h waiting period, with foals muzzled to prevent them from suckling their mother. Our results may therefore differ from studies in which milk was sampled directly. This waiting period may have decreased the fatty acid concentration and altered the udder’s fatty acid metabolism. Because all mares and foals were sampled using the exact same protocol, our results are comparable to each other’s but may not be comparable with other studies.
4.4. Study Perspectives for the Equine Industry
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peugnet, P.; Robles, M.; Wimel, L.; Tarrade, A.; Chavatte-Palmer, P. Management of the Pregnant Mare and Long-Term Consequences on the Offspring. Theriogenology 2016, 86, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Robles, M.; Hammer, C.; Staniar, B.; Chavatte-Palmer, P. Nutrition of Broodmares. Vet. Clin. Equine Pract. 2021, 37, 177–205. [Google Scholar] [CrossRef] [PubMed]
- Chavatte-Palmer, P.; Derisoud, E.; Robles, M. Pregnancy and Placental Development in Horses: An Update. Domest. Anim. Endocrinol. 2022, 79, 106692. [Google Scholar] [CrossRef] [PubMed]
- Derisoud, E.; Auclair-Ronzaud, J.; Palmer, E.; Robles, M.; Chavatte-Palmer, P.; Derisoud, E.; Auclair-Ronzaud, J.; Palmer, E.; Robles, M.; Chavatte-Palmer, P. Female Age and Parity in Horses: How and Why Does It Matter? Reprod. Fertil. Dev. 2021, 34, 52–116. [Google Scholar] [CrossRef] [PubMed]
- Peugnet, P.; Wimel, L.; Duchamp, G.; Sandersen, C.; Camous, S.; Guillaume, D.; Dahirel, M.; Dubois, C.; Jouneau, L.; Reigner, F.; et al. Enhanced or Reduced Fetal Growth Induced by Embryo Transfer into Smaller or Larger Breeds Alters Post-Natal Growth and Metabolism in Pre-Weaning Horses. PLoS ONE 2014, 9, e102044. [Google Scholar] [CrossRef] [PubMed]
- Peugnet, P.; Mendoza, L.; Wimel, L.; Duchamp, G.; Dubois, C.; Reigner, F.; Caudron, I.; Deliège, B.; Toquet, M.; Richard, E.; et al. Longitudinal Study of Growth and Osteoarticular Status in Foals Born to Between-Breed Embryo Transfers. J. Equine Vet. Sci. 2016, 37, 24–38. [Google Scholar] [CrossRef]
- Giussani, D.; Forhead, A.; Gardner, D.; Fletcher, A.; Allen, W.; Fowden, A. Postnatal Cardiovascular Function after Manipulation of Fetal Growth by Embryo Transfer in the Horse. J. Physiol. 2003, 547, 67–76. [Google Scholar] [CrossRef]
- Forhead, A.; Ousey, J.; Allen, W.; Fowden, A. Postnatal Insulin Secretion and Sensitivity after Manipulation of Fetal Growth by Embryo Transfer in the Horse. J. Endocrinol. 2004, 181, 459–467. [Google Scholar] [CrossRef][Green Version]
- Tischner, M.; Klimczak, M. The Development of Polish Ponies Born after Embryo Transfer to Large Recipients. Equine Vet. J. 1989, 21, 62–63. [Google Scholar] [CrossRef]
- Kubiak, J.; Evans, J.; Potter, G.; Harms, P.; Jenkins, W. Parturition in the Multiparous Mare Fed to Obesity. Equine Vet. Sci. 1988, 8, 135–140. [Google Scholar] [CrossRef]
- Kubiak, J.; Evans, J.; Potter, G.; Harms, P.; Jenkins, W. Milk Yield Composition in the Multiparous Mare Fed to Obesity. Equine Vet. Sci. 1991, 11, 158–162. [Google Scholar] [CrossRef]
- Henneke, D.; Potter, G.; Kreider, J. Body Condition during Pregnancy and Lactation and Reproductive Efficiency of Mares. Theriogenology 1984, 21, 897–909. [Google Scholar] [CrossRef]
- Bradbery, A.N.; Coverdale, J.A.; Hammer, C.J.; Dunlap, K.A.; Leatherwood, J.L.; Satterfield, M.C. Effect of Maternal Overnutrition on Predisposition to Insulin Resistance in the Foal: Foal Skeletal Muscle Development and Insulin Signaling. Domest. Anim. Endocrinol. 2021, 77, 106648. [Google Scholar] [CrossRef]
- Wilsher, S.; Allen, W. Effects of a Streptococcus Equi Infection—Mediated Nutritional Insult during Mid-Gestation in Primiparous Thoroughbred Fillies. Part 1: Placental and Fetal Development. Equine Vet. J. 2006, 38, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Peugnet, P.; Robles, M.; Mendoza, L.; Wimel, L.; Dubois, C.; Dahirel, M.; Guillaume, D.; Camous, S.; Berthelot, V.; Toquet, M.-P.; et al. Effects of Moderate Amounts of Barley in Late Pregnancy on Growth, Glucose Metabolism and Osteoarticular Status of Pre-Weaning Horses. PLoS ONE 2015, 10, e0122596. [Google Scholar] [CrossRef] [PubMed]
- Robles, M.; Gautier, C.; Mendoza, L.; Peugnet, P.; Dubois, C.; Dahirel, M.; Lejeune, J.; Caudron, I.; Guenon, I.; Camous, S.; et al. Maternal Nutrition during Pregnancy Affects Testicular and Bone Development, Glucose Metabolism and Response to Overnutrition in Weaned Horses up to Two Years. PLoS ONE 2017, 12, e0169295. [Google Scholar] [CrossRef] [PubMed]
- Robles, M.; Nouveau, E.; Gautier, C.; Mendoza, L.; Dubois, C.; Dahirel, M.; Lagofun, B.; Aubrière, M.; Lejeune, J.; Caudron, I.; et al. Maternal Obesity Increases Insulin Resistance, Low-Grade Inflammation and Osteochondrosis Lesions in Foals and Yearlings until 18 Months of Age. PLoS ONE 2018, 13, e0190309. [Google Scholar] [CrossRef] [PubMed]
- Giles, S.; Rands, S.; Nicol, C.; Harris, P. Obesity Prevalence and Associated Risk Factors in Outdoor Living Domestic Horses and Ponies. PeerJ 2014, 2, e299. [Google Scholar] [CrossRef]
- Potter, S.; Bamford, N.; Harris, P.; Bailey, S. Prevalence of Obesity and Owners’ Perceptions of Body Condition in Pleasure Horses and Ponies in South-Eastern Australia. Aust. Vet. J. 2016, 94, 427–432. [Google Scholar] [CrossRef]
- Hoffman, C.; Costa, L.; Freeman, L. Survey of Feeding Practices, Supplement Use, and Knowledge of Equine Nutrition among a Subpopulation of Horse Owners in New England. J. Equine Vet. Sci. 2009, 29, 719–726. [Google Scholar] [CrossRef]
- Furtado, T.; Perkins, E.; Pinchbeck, G.; McGowan, C.; Watkins, F.; Christley, R. Exploring Horse Owners’ Understanding of Obese Body Condition and Weight Management in UK Leisure Horses. Equine Vet. J. 2021, 53, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R.M.; Boston, R.C.; Stefanovski, D.; Kronfeld, D.S.; Harris, P.A. Obesity and Diet Affect Glucose Dynamics and Insulin Sensitivity in Thoroughbred Geldings. J. Anim. Sci. 2003, 81, 2333–2342. [Google Scholar] [CrossRef] [PubMed]
- Siwinska, N.; Janus, I.; Zak-Bochenek, A.; Noszczyk-Nowak, A. Influence of Obesity on Histological Tissue Structure of the Cardiovascular System in Horses. Animals 2022, 12, 732. [Google Scholar] [CrossRef] [PubMed]
- Lovett, A.L.; Gilliam, L.L.; Sykes, B.W.; McFarlane, D. Thromboelastography in Obese Horses with Insulin Dysregulation Compared to Healthy Controls. J. Vet. Intern. Med. 2022, 36, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Pearson, W.; Wood, K.; Stanley, S.; Macnicol, J. Exploring Relationships between Body Condition Score, Body Fat, Activity Level and Inflammatory Biomarkers. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1062–1068. [Google Scholar] [CrossRef]
- Salinas, C.; Espinosa, G.; Morales, N.; Henríquez, C.; Morán, G.; Gajardo, G.; Burgos, R.A.; Uberti, B. Evaluation of Peripheral Blood Polymorphonuclear Cell Functions after an Oral Carbohydrate Overload in Obese and Insulin Dysregulated Horses. Vet. Immunol. Immunopathol. 2022, 250, 110455. [Google Scholar] [CrossRef] [PubMed]
- Frank, N. Equine Metabolic Syndrome. Vet. Clin. North Am. Equine Pract. 2011, 27, 73–92. [Google Scholar] [CrossRef]
- Alford, P.; Geller, S.; Richrdson, B.; Slater, M.; Honnas, C.; Foreman, J.; Robinson, J.; Messer, M.; Roberts, M.; Goble, D.; et al. A Multicenter, Matched Case-Control Study of Risk Factors for Equine Laminitis. Prev. Vet. Med. 2001, 49, 209–222. [Google Scholar] [CrossRef]
- Garber, A.; Hastie, P.; Murray, J.-A. Factors Influencing Equine Gut Microbiota: Current Knowledge. J. Equine Vet. Sci. 2020, 88, 102943. [Google Scholar] [CrossRef]
- Roth, P.; Stanley, J.; Chamoun-Emanuelli, A.; Whitfield-Cargile, C.; Coleman, M. Fecal Extract from Obese Horses Induces an Inflammatory Response by Murine Macrophages in Vitro. Am. J. Vet. Res. 2022, 83, 419–425. [Google Scholar] [CrossRef]
- Pratt-Phillips, S.; Munjizun, A. Impacts of Adiposity on Exercise Performance in Horses. Anim. Open Access J. 2023, 13, 666. [Google Scholar] [CrossRef] [PubMed]
- Jansson, A.; Gunnarsson, V.Þ.; Ringmark, S.; Ragnarsson, S.; Söderroos, D.; Ásgeirsson, E.; Jóhannsdóttir, T.R.; Liedberg, C.; Stefánsdóttir, G.J. Increased Body Fat Content in Horses Alters Metabolic and Physiological Exercise Response, Decreases Performance, and Increases Locomotion Asymmetry. Physiol. Rep. 2021, 9, e14824. [Google Scholar] [CrossRef] [PubMed]
- Jaqueth, A.L.; Iwaniuk, M.E.; Burk, A.O. Characterization of the Prevalence and Management of Over-Conditioned Ponies and Horses in Maryland. J. Equine Vet. Sci. 2018, 68, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, N.; Ezechukwu, H.C.; Holland, O.J.; Hryciw, D.H. Developmental Programming of Peripheral Diseases in Offspring Exposed to Maternal Obesity during Pregnancy. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2020, 319, R507–R516. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.C.; Powell, T.L.; Jansson, T. Placental Function in Maternal Obesity. Clin. Sci. 2020, 134, 961–984. [Google Scholar] [CrossRef]
- Schoonejans, J.M.; Ozanne, S.E. Developmental Programming by Maternal Obesity: Lessons from Animal Models. Diabet. Med. 2021, 38, e14694. [Google Scholar] [CrossRef]
- Lecoutre, S.; Maqdasy, S.; Breton, C. Maternal Obesity as a Risk Factor for Developing Diabetes in Offspring: An Epigenetic Point of View. World J. Diabetes 2021, 12, 366–382. [Google Scholar] [CrossRef]
- Kislal, S.; Shook, L.L.; Edlow, A.G. Perinatal Exposure to Maternal Obesity: Lasting Cardiometabolic Impact on Offspring. Prenat. Diagn. 2020, 40, 1109–1125. [Google Scholar] [CrossRef]
- Rodgers, A.; Sferruzzi-Perri, A.N. Developmental Programming of Offspring Adipose Tissue Biology and Obesity Risk. Int. J. Obes. 2021, 45, 1170–1192. [Google Scholar] [CrossRef]
- Kong, L.; Chen, X.; Gissler, M.; Lavebratt, C. Relationship of Prenatal Maternal Obesity and Diabetes to Offspring Neurodevelopmental and Psychiatric Disorders: A Narrative Review. Int. J. Obes. 2020, 44, 1981–2000. [Google Scholar] [CrossRef]
- Menting, M.D.; van de Beek, C.; Mintjens, S.; Wever, K.E.; Korosi, A.; Ozanne, S.E.; Limpens, J.; Roseboom, T.J.; Hooijmans, C.; Painter, R.C. The Link between Maternal Obesity and Offspring Neurobehavior: A Systematic Review of Animal Experiments. Neurosci. Biobehav. Rev. 2019, 98, 107–121. [Google Scholar] [CrossRef]
- Derisoud, E.; Auclair-Ronzaud, J.; Rousseau-Ralliard, D.; Philau, S.; Aujean, E.; Durand, A.; Dahirel, M.; Charlier, M.; Boutinaud, M.; Wimel, L.; et al. Maternal Age, Parity and Nursing Status at Fertilization Affects Postpartum Lactation Up to Weaning in Horses. J. Equine Vet. Sci. 2023, 128, 104868. [Google Scholar] [CrossRef] [PubMed]
- Robles, M.; Loux, S.C.; de Mestre, A.M.; Chavatte-Palmer, P. Environmental Constraints and Pathologies That Modulate Equine Placental Genes and Development. Reprod. Camb. Engl. 2022, 163, R25–R38. [Google Scholar] [CrossRef] [PubMed]
- Allen, W.; Wilsher, S.; Turnbull, C.; Stewart, F.; Ousey, J.; Rossdale, P.; Fowden, A.; Turnbull, C.; Stewart, F.; Ousey, J.; et al. Influence of Maternal Size on Placental, Fetal and Postnatal Growth in the Horse. I. Development in Utero. Reproduction 2002, 172, 445–453. [Google Scholar] [CrossRef]
- Robles, M.; Peugnet, P.; Dubois, C.; Piumi, F.; Jouneau, L.; Bouchez, O.; Aubrière, M.; Dahirel, M.; Aioun, J.; Wimel, L.; et al. Placental Function and Structure at Term Is Altered in Broodmares Fed with Cereals from Mid-Gestation. Placenta 2018, 64, 44–52. [Google Scholar] [CrossRef]
- Auclair-Ronzaud, J.; Jaffrézic, F.; Wimel, L.; Dubois, C.; Laloë, D.; Chavatte-Palmer, P. Estimation of Milk Production in Suckling Mares and Factors Influencing Their Milk Yield. Animal 2022, 16, 100498. [Google Scholar] [CrossRef]
- Reed, M.; Howard, C.; DE Yanés, G. One-Stop Stereology: The Estimation of 3D Parameters Using Isotropic Rulers. J. Microsc. 2010, 239, 54–65. [Google Scholar] [CrossRef]
- Chomczynski, P.; Sacchi, N. Single-Step Method of RNA Isolation by Acid Guanidinium Thiocyanate-Phenol-Chloroform Extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Tarrade, A.; Rousseau-Ralliard, D.; Aubrière, M.; Peynot, N.; Dahirel, M.; Bertrand-Michel, J.; Aguirre-Lavin, T.; Morel, O.; Beaujean, N.; Duranthon, V.; et al. Sexual Dimorphism of the Feto-Placental Phenotype in Response to a High Fat and Control Maternal Diets in a Rabbit Model. PLoS ONE 2013, 8, e83458. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.S. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Morrison, W.R.; Smith, L.M. Preparation of Fatty Acid Methyl Esters and Dimethylacetals from Lipids with Boron Fluoride–Methanol. J. Lipid Res. 1964, 5, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, D.; Héliès-Toussaint, C.; Moreau, D.; Raederstorff, D.; Grynberg, A. Dietary N-3 PUFAs Affect the Blood Pressure Rise and Cardiac Impairments in a Hyperinsulinemia Rat Model in Vivo. Am. J. Physiol.-Heart Circ. Physiol. 2003, 285, H1294–H1302. [Google Scholar] [CrossRef] [PubMed]
- Dubé, E.; Gravel, A.; Martin, C.; Desparois, G.; Moussa, I.; Ethier-Chiasson, M.; Forest, J.-C.; Giguère, Y.; Masse, A.; Lafond, J. Modulation of Fatty Acid Transport and Metabolism by Maternal Obesity in the Human Full-Term Placenta1. Biol. Reprod. 2012, 87, 14. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Dong, S.S.; Hu, J.; Yao, J.J.; Yan, P.S. The Effect of Maternal Obesity on Fatty Acid Transporter Expression and Lipid Metabolism in the Full-Term Placenta of Lean Breed Swine. J. Anim. Physiol. Anim. Nutr. 2018, 102, e242–e253. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Wang, N.; Peng, Y.; Sun, B.; Cui, W. Placental Lipid Transport and Content in Response to Maternal Overweight and Gestational Diabetes Mellitus in Human Term Placenta. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Nogues, P.; Dos Santos, E.; Couturier-Tarrade, A.; Berveiller, P.; Arnould, L.; Lamy, E.; Grassin-Delyle, S.; Vialard, F.; Dieudonne, M.-N. Maternal Obesity Influences Placental Nutrient Transport, Inflammatory Status, and Morphology in Human Term Placenta. J. Clin. Endocrinol. Metab. 2020, 12, dgaa660. [Google Scholar] [CrossRef] [PubMed]
- Rasool, A.; Mahmoud, T.; Mathyk, B.; Kaneko-Tarui, T.; Roncari, D.; White, K.O.; O’Tierney-Ginn, P. Obesity Downregulates Lipid Metabolism Genes in First Trimester Placenta. Sci. Rep. 2022, 12, 19368. [Google Scholar] [CrossRef] [PubMed]
- Napso, T.; Lean, S.C.; Lu, M.; Mort, E.J.; Desforges, M.; Moghimi, A.; Bartels, B.; El-Bacha, T.; Fowden, A.L.; Camm, E.J.; et al. Diet-induced Maternal Obesity Impacts Feto-placental Growth and Induces Sex-specific Alterations in Placental Morphology, Mitochondrial Bioenergetics, Dynamics, Lipid Metabolism and Oxidative Stress in Mice. Acta Physiol. 2022, 234, e13795. [Google Scholar] [CrossRef]
- Powell, T.L.; Barner, K.; Madi, L.; Armstrong, M.; Manke, J.; Uhlson, C.; Jansson, T.; Ferchaud-Roucher, V. Sex-Specific Responses in Placental Fatty Acid Oxidation, Esterification and Transfer Capacity to Maternal Obesity. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2021, 1866, 158861. [Google Scholar] [CrossRef]
- Bucher, M.; Montaniel, K.R.C.; Myatt, L.; Weintraub, S.; Tavori, H.; Maloyan, A. Dyslipidemia, Insulin Resistance, and Impairment of Placental Metabolism in the Offspring of Obese Mothers. J. Dev. Orig. Health Dis. 2021, 12, 738–747. [Google Scholar] [CrossRef]
- Jarvie, E.; Hauguel-de-Mouzon, S.; Nelson, S.M.; Sattar, N.; Catalano, P.M.; Freeman, D.J. Lipotoxicity in Obese Pregnancy and Its Potential Role in Adverse Pregnancy Outcome and Obesity in the Offspring. Clin. Sci. 2010, 119, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Saben, J.; Lindsey, F.; Zhong, Y.; Thakali, K.; Badger, T.M.; Andres, A.; Gomez-Acevedo, H.; Shankar, K. Maternal Obesity Is Associated with a Lipotoxic Placental Environment. Placenta 2014, 35, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Stewart, F.M.; Freeman, D.J.; Ramsay, J.E.; Greer, I.A.; Caslake, M.; Ferrell, W.R. Longitudinal Assessment of Maternal Endothelial Function and Markers of Inflammation and Placental Function throughout Pregnancy in Lean and Obese Mothers. J. Clin. Endocrinol. Metab. 2007, 92, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Challier, J.C.; Basu, S.; Bintein, T.; Minium, J.; Hotmire, K.; Catalano, P.M.; Hauguel-de Mouzon, S. Obesity in Pregnancy Stimulates Macrophage Accumulation and Inflammation in the Placenta. Placenta 2008, 29, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.A.; Riley, S.C.; Reynolds, R.M.; Barr, S.; Evans, M.; Statham, A.; Hor, K.; Jabbour, H.N.; Norman, J.E.; Denison, F.C. Placental Structure and Inflammation in Pregnancies Associated with Obesity. Placenta 2011, 32, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Aye, I.L.M.H.; Lager, S.; Ramirez, V.I.; Gaccioli, F.; Dudley, D.J.; Jansson, T.; Powell, T.L. Increasing Maternal Body Mass Index Is Associated with Systemic Inflammation in the Mother and the Activation of Distinct Placental Inflammatory Pathways1. Biol. Reprod. 2014, 90, 129. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, T.; Wu, Y.; Wei, H.; Peng, J. Oxidative Stress and Inflammation in Sows with Excess Backfat: Up-Regulated Cytokine Expression and Elevated Oxidative Stress Biomarkers in Placenta. Animals 2019, 9, 796. [Google Scholar] [CrossRef]
- Pietro, L.; Daher, S.; Rudge, M.V.C.; Calderon, I.M.P.; Damasceno, D.C.; Sinzato, Y.K.; Bandeira, C.; Bevilacqua, E. Vascular Endothelial Growth Factor (VEGF) and VEGF-Receptor Expression in Placenta of Hyperglycemic Pregnant Women. Placenta 2010, 31, 770–780. [Google Scholar] [CrossRef]
- Pazinato, F.M.; Curcio, B.D.R.; Fernandes, C.G.; Santos, C.A.; Feijó, L.S.; Varela, A.S.; Nogueira, C.E.W. Histomorphometry of the Placental Vasculature and Microcotyledons in Thoroughbred Mares with Chronic Laminitis. Theriogenology 2017, 91, 77–81. [Google Scholar] [CrossRef]
- Sessions-Bresnahan, D.R.; Heuberger, A.L.; Carnevale, E.M. Obesity in Mares Promotes Uterine Inflammation and Alters Embryo Lipid Fingerprints and Homeostasis. Biol. Reprod. 2018, 99, 761–772. [Google Scholar] [CrossRef]
- Dunbar, B.; Bauer, J. Metabolism of Dietary Essential Fatty Acids and Their Conversion to Long-Chain Polyunsaturated Metabolites. J. Am. Vet. Med. Assoc. 2002, 220, 1621–1626. [Google Scholar] [CrossRef]
- King, S.; AbuGhazaleh, A.; Webel, S.; Jones, K. Circulating Fatty Acid Profiles in Response to Three Levels of Dietary Omega-3 Fatty Acid Supplementation in Horses. J. Anim. Sci. 2007, 86, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Hodge, L.B.; Rude, B.J.; Dinh, T.N.; Lemley, C.O. Effect of ω-3 Fatty Acid Supplementation to Gestating and Lactating Mares: On Milk IgG, Mare and Foal Blood Concentrations of IgG, Insulin and Glucose, Placental Efficiency, and Fatty Acid Composition of Milk and Serum from Mares and Foals. J. Equine Vet. Sci. 2017, 51, 70–78. [Google Scholar] [CrossRef]
- Wu, G. Chapter 6: Metabolism and Nutrition of Lipids. In Principles of Animal Nutrition; Animal nutrition|Metabolism; CRC Press: Boca Raton, FL, USA, 2018; p. 800. ISBN 978-1-03-209599-8. [Google Scholar]
- Islam, A.; Kodama, T.; Yamamoto, Y.; Ebrahimi, M.; Miyazaki, H.; Yasumoto, Y.; Kagawa, Y.; Sawada, T.; Owada, Y.; Tokuda, N. Omega-3 Fatty Acids Transport through the Placenta. Asian J. Med. Biol. Res. 2016, 2, 1–8. [Google Scholar] [CrossRef]
- Ortega-Senovilla, H.; Alvino, G.; Taricco, E.; Cetin, I.; Herrera, E. Gestational Diabetes Mellitus Upsets the Proportion of Fatty Acids in Umbilical Arterial but Not Venous Plasma. Diabetes Care 2009, 32, 120–122. [Google Scholar] [CrossRef] [PubMed]
- Elzinga, S.; Wood, P.; Adams, A.A. Plasma Lipidomic and Inflammatory Cytokine Profiles of Horses with Equine Metabolic Syndrome. J. Equine Vet. Sci. 2016, 40, 49–55. [Google Scholar] [CrossRef]
- O’Connor, C.I.; Lawrence, L.M.; Hayes, S.H. Dietary Fish Oil Supplementation Affects Serum Fatty Acid Concentrations in Horses1. J. Anim. Sci. 2007, 85, 2183–2189. [Google Scholar] [CrossRef]
- Dervishi, E.; Joy, M.; Sanz, A.; Alvarez-Rodriguez, J.; Molino, F.; Calvo, J.H. Forage Preservation (Grazing vs. Hay) Fed to Ewes Affects the Fatty Acid Profile of Milk and CPT1B Gene Expression in the Sheep Mammary Gland. BMC Vet. Res. 2012, 8, 106. [Google Scholar] [CrossRef]
- Pietrzak-Fiećko, R.; Kamelska-Sadowska, A.M. The Comparison of Nutritional Value of Human Milk with Other Mammals’ Milk. Nutrients 2020, 12, 1404. [Google Scholar] [CrossRef]
- Zentek, J.; Buchheit-Renko, S.; Ferrara, F.; Vahjen, W.; Van Kessel, A.G.; Pieper, R. Nutritional and Physiological Role of Medium-Chain Triglycerides and Medium-Chain Fatty Acids in Piglets. Anim. Health Res. Rev. 2011, 12, 83–93. [Google Scholar] [CrossRef]
- Takase, S.; Goda, T. Effects of Medium-Chain Triglycerides on Brush Border Membrane-Bound Enzyme Activity in Rat Small Intestine. J. Nutr. 1990, 120, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Dierick, N.A.; Decuypere, J.A.; Molly, K.; Van Beek, E.; Vanderbeke, E. The Combined Use of Triacylglycerols (TAGs) Containing Medium Chain Fatty Acids (MCFAs) and Exogenous Lipolytic Enzymes as an Alternative to Nutritional Antibiotics in Piglet Nutrition. Livest. Prod. Sci. 2002, 76, 1–16. [Google Scholar] [CrossRef]
- Kono, H.; Fujii, H.; Asakawa, M.; Maki, A.; Amemiya, H.; Hirai, Y.; Matsuda, M.; Yamamoto, M. Medium-Chain Triglycerides Enhance Secretory IgA Expression in Rat Intestine after Administration of Endotoxin. Am. J. Physiol.-Gastrointest. Liver Physiol. 2004, 286, G1081–G1089. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, X.; Simonavicius, N.; Tian, H.; Ling, L. Medium-Chain Fatty Acids as Ligands for Orphan G Protein-Coupled Receptor GPR84. J. Biol. Chem. 2006, 281, 34457–34464. [Google Scholar] [CrossRef] [PubMed]
- de la Garza Puentes, A.; Martí Alemany, A.; Chisaguano, A.M.; Montes Goyanes, R.; Castellote, A.I.; Torres-Espínola, F.J.; García-Valdés, L.; Escudero-Marín, M.; Segura, M.T.; Campoy, C.; et al. The Effect of Maternal Obesity on Breast Milk Fatty Acids and Its Association with Infant Growth and Cognition—The PREOBE Follow-Up. Nutrients 2019, 11, 2154. [Google Scholar] [CrossRef]
- Armand, M.; Bernard, J.Y.; Forhan, A.; Heude, B.; Charles, M.-A.; Annesi-Maesano, I.; Bernard, J.Y.; Botton, J.; Charles, M.-A.; Dargent-Molina, P.; et al. Maternal Nutritional Determinants of Colostrum Fatty Acids in the EDEN Mother-Child Cohort. Clin. Nutr. 2018, 37, 2127–2136. [Google Scholar] [CrossRef] [PubMed]
- Rydlewski, A.A.; Manin, L.P.; Pizzo, J.S.; Silva, P.D.; da Silveira, R.; Tavares, C.B.G.; de Paula, M.; Pereira, O.; Santos, O.O.; Visentainer, J.V. Lipid Profile by Direct Infusion ESI-MS and Fatty Acid Composition by GC-FID in Human Milk: Association with Nutritional Status of Donors. J. Food Compos. Anal. 2021, 100, 103797. [Google Scholar] [CrossRef]
- Tang, J. Palmitoleic Acid in Health and Disease. In Advances in Dietary Lipids and Human Health; Elsevier: Amsterdam, The Netherlands, 2022; pp. 293–302. ISBN 978-0-12-823914-8. [Google Scholar]
- Duckett, S.K.; Volpi Lagreca, G.; Alende, M.; Long, N. Palmitoleic Acid Reduces Intramuscular Lipid and Restores Insulin Sensitivity in Obese Sheep. Diabetes Metab. Syndr. Obes. Targets Ther. 2014, 7, 553–563. [Google Scholar] [CrossRef]
- de Souza, C.O.; Teixeira, A.A.S.; Biondo, L.A.; Lima Junior, E.A.; Batatinha, H.A.P.; Rosa Neto, J.C. Palmitoleic Acid Improves Metabolic Functions in Fatty Liver by PPARα-Dependent AMPK Activation: PALMITOLEATE CONTROLLING PPARα-AMPK ON LIVER. J. Cell. Physiol. 2017, 232, 2168–2177. [Google Scholar] [CrossRef]
- Yao, D.; Luo, J.; He, Q.; Shi, H.; Li, J.; Wang, H.; Xu, H.; Chen, Z.; Yi, Y.; Loor, J.J. SCD1 Alters Long-Chain Fatty Acid (LCFA) Composition and Its Expression Is Directly Regulated by SREBP-1 and PPARγ 1 in Dairy Goat Mammary Cells: SCD1 AND FATTY ACID METABOLISM. J. Cell. Physiol. 2017, 232, 635–649. [Google Scholar] [CrossRef]
- di Martino, O.; Troiano, A.; Addi, L.; Guarino, A.; Calabrò, S.; Tudisco, R.; Murru, N.; Cutrignelli, M.I.; Infascelli, F.; Calabrò, V. Regulation of Stearoyl Coenzyme A Desaturase 1 Gene Promoter in Bovine Mammary Cells. Anim. Biotechnol. 2015, 26, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Dobrzyn, P.; Jazurek, M.; Dobrzyn, A. Stearoyl-CoA Desaturase and Insulin Signaling—What Is the Molecular Switch? Biochim. Biophys. Acta BBA Bioenerg. 2010, 1797, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.B.; Zhao, W.S.; Luo, J.; Yao, D.W.; Sun, Y.T.; Li, J.; Shi, H.P.; Loor, J.J. Peroxisome Proliferator-Activated Receptor Γ1 and Γ2 Isoforms Alter Lipogenic Gene Networks in Goat Mammary Epithelial Cells to Different Extents. J. Dairy Sci. 2014, 97, 5437–5447. [Google Scholar] [CrossRef] [PubMed]
- Flint, D.J.; Travers, M.T.; Barber, M.C.; Binart, N.; Kelly, P.A. Diet-Induced Obesity Impairs Mammary Development and Lactogenesis in Murine Mammary Gland. Am. J. Physiol.-Endocrinol. Metab. 2005, 288, E1179–E1187. [Google Scholar] [CrossRef] [PubMed]
- Marko, R.C.; Branislava, B.; Bojan, T.; Aleksandar, P.; Milenko, S.; Branislav, L.; Ivan, R. Metabolic Acclimation to Heat Stress in Farm Housed Holstein Cows with Different Body Condition Scores. Afr. J. Biotechnol. 2011, 10, 10293–10303. [Google Scholar] [CrossRef]
- Liu, Z.; Ezernieks, V.; Wang, J.; Arachchillage, N.W.; Garner, J.B.; Wales, W.J.; Cocks, B.G.; Rochfort, S. Heat Stress in Dairy Cattle Alters Lipid Composition of Milk. Sci. Rep. 2017, 7, 961. [Google Scholar] [CrossRef] [PubMed]
- Bolzan, A.M.S.; Bonnet, O.J.F.; Wallau, M.O.; Basso, C.; Neves, A.P.; Carvalho, P.C.d.F. Foraging Behavior Development of Foals in Natural Grassland. Rangel. Ecol. Manag. 2020, 73, 243–251. [Google Scholar] [CrossRef]
- Contu, L.; Hawkes, C. A Review of the Impact of Maternal Obesity on the Cognitive Function and Mental Health of the Offspring. Int. J. Mol. Sci. 2017, 18, 1093. [Google Scholar] [CrossRef]
- Thompson, J.R.; Valleau, J.C.; Barling, A.N.; Franco, J.G.; De Capo, M.; Bagley, J.L.; Sullivan, E.L. Exposure to a High-Fat Diet during Early Development Programs Behavior and Impairs the Central Serotonergic System in Juvenile Non-Human Primates. Front. Endocrinol. 2017, 8, 164. [Google Scholar] [CrossRef]
- Muhlhausler, B.; Adam, C.; Findlay, P.; Duffield, J.; McMillen, I. Increased Maternal Nutrition Alters Development of the Appetite-Regulating Network in the Brain. FASEB J. 2006, 20, 1257–1259. [Google Scholar] [CrossRef]
- Purcell, R.; Sun, B.; Pass, L.; Power, M.; Moran, T.; Tamashiro, K. Maternal Stress and High-Fat Diet Effect on Maternal Behavior, Milk Composition, and Pup Ingestive Behavior. Physiol. Behav. 2011, 104, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Borengasser, S.J.; Lau, F.; Kang, P.; Blackburn, M.L.; Ronis, M.J.J.; Badger, T.M.; Shankar, K. Maternal Obesity during Gestation Impairs Fatty Acid Oxidation and Mitochondrial SIRT3 Expression in Rat Offspring at Weaning. PLoS ONE 2011, 6, e24068. [Google Scholar] [CrossRef] [PubMed]
- Isganaitis, E.; Woo, M.; Ma, H.; Chen, M.; Kong, W.; Lytras, A.; Sales, V.; De Coste-Lopez, J.; Lee, K.-J.; Leatherwood, C.; et al. Developmental Programming by Maternal Insulin Resistance: Hyperinsulinemia, Glucose Intolerance, and Dysregulated Lipid Metabolism in Male Offspring of Insulin-Resistant Mice. Diabetes 2014, 63, 688–700. [Google Scholar] [CrossRef] [PubMed]
- Boyle, K.E.; Patinkin, Z.W.; Shapiro, A.L.B.; Bader, C.; Vanderlinden, L.; Kechris, K.; Janssen, R.C.; Ford, R.J.; Smith, B.K.; Steinberg, G.R.; et al. Maternal Obesity Alters Fatty Acid Oxidation, AMPK Activity, and Associated DNA Methylation in Mesenchymal Stem Cells from Human Infants. Mol. Metab. 2017, 6, 1503–1516. [Google Scholar] [CrossRef]
- Musaev, A.; Sadykova, S.; Anambayeva, A.; Saizhanova, M.; Balkanay, G.; Kolbaev, M. Mare’s Milk: Composition, Properties, and Application in Medicine. Arch. Razi Inst. 2021, 76, 1125–1135. [Google Scholar] [CrossRef]
- World Meteorological Organization (WMO). State of the Climate in Europe 2022; WMO: Geneva, Switzerland, 2023; p. 40. [Google Scholar]





| Candidate Gene | Accession Number | Reverse and forward Primers | |
|---|---|---|---|
| Reference | GADPH | NM_001163856.1 | F 5′-AGTTGGGTGCCAAAACTTGTG-3′ R 5′-TGAAGGGGTCATTGATGGCG-3′ |
| SCAMP3 | Brosnahan et al. 2012 | F 5′-CTGTGCTGGGAATTGTGATG-3′ R 5′-ATTCTTGCTGGGCCTTCTG-3′ | |
| RPL32 | ENSECAG00000007201 | F 5′-TGAAGTGCTGCTCATGTGCA-3′ R 5′-GGGATTGGTGATTCTGATGGC-3′ | |
| Vascularization | ENG | XM_0011500078.5 | F 5′-ACAGTCGAACAGCGACTTCA-3′ R 5′-TTCTTCCCCAAATTCGATTCA-3′ |
| Flt1 | XM_003363176.1 | F 5′-AGTGTGAGCGGCTCCCTTATG-3′ R 5′-ATGCCAAATGCAGATGCTTG-3′ | |
| KDR | XM_001916946.2 | F 5′-CAGTGGGCTGATGACCAAGA-3′ R 5′-TCCACCGAAGATTCCATGCC-3′ | |
| Nutrient transport | SCL2A1 | NM_001163971.1 | F 5′-TGTGCTCATGACCATCGCC-3′ R 5′-AAGCCAAAGATGGCCACGAT-3′ |
| SLC2A3 | XM_001498757.2 | F 5′-CCGTTGGTGGTATGATTGGC-3′ R 5′-CAGAACCCCATAAGGCAGCC-3′ | |
| SlC38A2 | ENSECAT00000016020 | F 5′-ACAGCTCGAACAGCGACTTCA-3′ R 5′-TTCTTCCCCAAATTCGATTCA-3′ | |
| CD36 | ENSECAG00000015229 | F 5′-CCGTGCAGAAGCAGTGGTTA-3′ R 5′-CCGTGCAGAAGCAGTGGTTA-3′ | |
| LPL | XM_001489577.2 | F 5′-AGTTGGGGTGCCAAAACTTGTG-3′ R 5′-GCTTGGTGTACCCCGCAGAC-3′ | |
| Growth | H19 | NR_027326 | F 5′-GGACCCCAAGAACCCTCAAG-3′ R 5′-GGGACTTGAAGAAGTCCGGG-3′ |
| IGF-2 | NM_001114539 | F 5′-TTTCTTGGCTTTTGCCTCGT-3′ R 5′-CCTGCTGAAGTAAAAGCCGC-3′ | |
| IGF-1R | XM_001489765.2 | F 5′-CGAGAAGACCACCATCAACAAC-3′ R 5′-TGGCAGCACTCGTTTGTTCTC-3′ |
| Common Name | Systematic Name | Symbol Formula | Category |
|---|---|---|---|
| Saturated fatty acid (SFA) | |||
| Capric acid | Decanoic acid | C10:0 | Medium-chain fatty acid (MCFA) |
| Lauric acid | Dodecanoic acid | C12:0 | |
| Myristic acid | Tetradecanoic acid | C14:0 | |
| Pentadecylic acid | Pentadecanoic acid | C15:0 | Long-chain fatty acid (LCFA) |
| Palmitic acid | Hexadecanoic acid | C16:0 | |
| Stearic acid | Octadecanoic acid | C18:0 | |
| Mono-unsaturated fatty acid (MUFA) | |||
| Medium chain MUFA | |||
| Caproleic acid | decenoic acid H2C = CH(CH2)7CO2H | C10:1 | Medium-chain fatty acid (MCFA) |
| Lauroleic acid | 9-dodecenoic acid | C12:1ω3 | Medium-chain fatty acid (MCFA) |
| Myristoleic acid | 9-tetradecenoic acid | C14:1ω5 | |
| Omega-7 fatty acid | |||
| -Palmitoleic acid | 9-Hexadecenoic acid | C16:1ω7 | Long-chain fatty acid (LCFA) |
| Vaccenic acid | 11-Octadecenoic acid | C18:1ω7 | |
| Omega-9 fatty acid | |||
| 6-pentadecenoic acid | 6-pentadecenoic acid | C15:1ω9 | Long-chain fatty acid (LCFA) |
| Hypogeic acid | (Z)-hexadec-7-enoic acid | C16:1ω9 | |
| Oleic acid | 9Z-octadecenoic acid | C18:1ω9 | |
| Gondoic acid | 11Z-eicosenoic acid | C20:1ω9 | |
| Poly-unsaturated fatty acid (PUFA) | |||
| Omega-3 fatty acid | |||
| α-Linolenic Acid (ALA) | 9Z,12Z,15Z-octadecatrienoic acid | C18:3ω3 | Long-chain fatty acid (LCFA) |
| Eicosatrienoic acid (ETA) | 11,14,17-eicosatrienoic acid | C20:3ω3 | |
| Eicosapentaenoic acid (EPA) | 5Z,8Z,11Z,14Z,17Z-eicosapentaenoic acid | C20:5ω3 | |
| Docosapentaenoic acid | DPA | C22:5ω3 | |
| Omega-6 fatty acid | |||
| Linoleic acid (LA) | 9Z,12Z-octadecadienoic acid | C18:2ω6 | Long-chain fatty acid (LCFA) |
| Arachidonic acid (AA) | 5Z,8Z,11Z,14Z-eicosatetraenoic acid | C20:4ω6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robles, M.; Rousseau-Ralliard, D.; Dubois, C.; Josse, T.; Nouveau, É.; Dahirel, M.; Wimel, L.; Couturier-Tarrade, A.; Chavatte-Palmer, P. Obesity during Pregnancy in the Horse: Effect on Term Placental Structure and Gene Expression, as Well as Colostrum and Milk Fatty Acid Concentration. Vet. Sci. 2023, 10, 691. https://doi.org/10.3390/vetsci10120691
Robles M, Rousseau-Ralliard D, Dubois C, Josse T, Nouveau É, Dahirel M, Wimel L, Couturier-Tarrade A, Chavatte-Palmer P. Obesity during Pregnancy in the Horse: Effect on Term Placental Structure and Gene Expression, as Well as Colostrum and Milk Fatty Acid Concentration. Veterinary Sciences. 2023; 10(12):691. https://doi.org/10.3390/vetsci10120691
Chicago/Turabian StyleRobles, Morgane, Delphine Rousseau-Ralliard, Cédric Dubois, Tiphanie Josse, Émilie Nouveau, Michele Dahirel, Laurence Wimel, Anne Couturier-Tarrade, and Pascale Chavatte-Palmer. 2023. "Obesity during Pregnancy in the Horse: Effect on Term Placental Structure and Gene Expression, as Well as Colostrum and Milk Fatty Acid Concentration" Veterinary Sciences 10, no. 12: 691. https://doi.org/10.3390/vetsci10120691
APA StyleRobles, M., Rousseau-Ralliard, D., Dubois, C., Josse, T., Nouveau, É., Dahirel, M., Wimel, L., Couturier-Tarrade, A., & Chavatte-Palmer, P. (2023). Obesity during Pregnancy in the Horse: Effect on Term Placental Structure and Gene Expression, as Well as Colostrum and Milk Fatty Acid Concentration. Veterinary Sciences, 10(12), 691. https://doi.org/10.3390/vetsci10120691






