Hemorrhage and Sudden Death in a Cat with Pancreatic Hemangiosarcoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
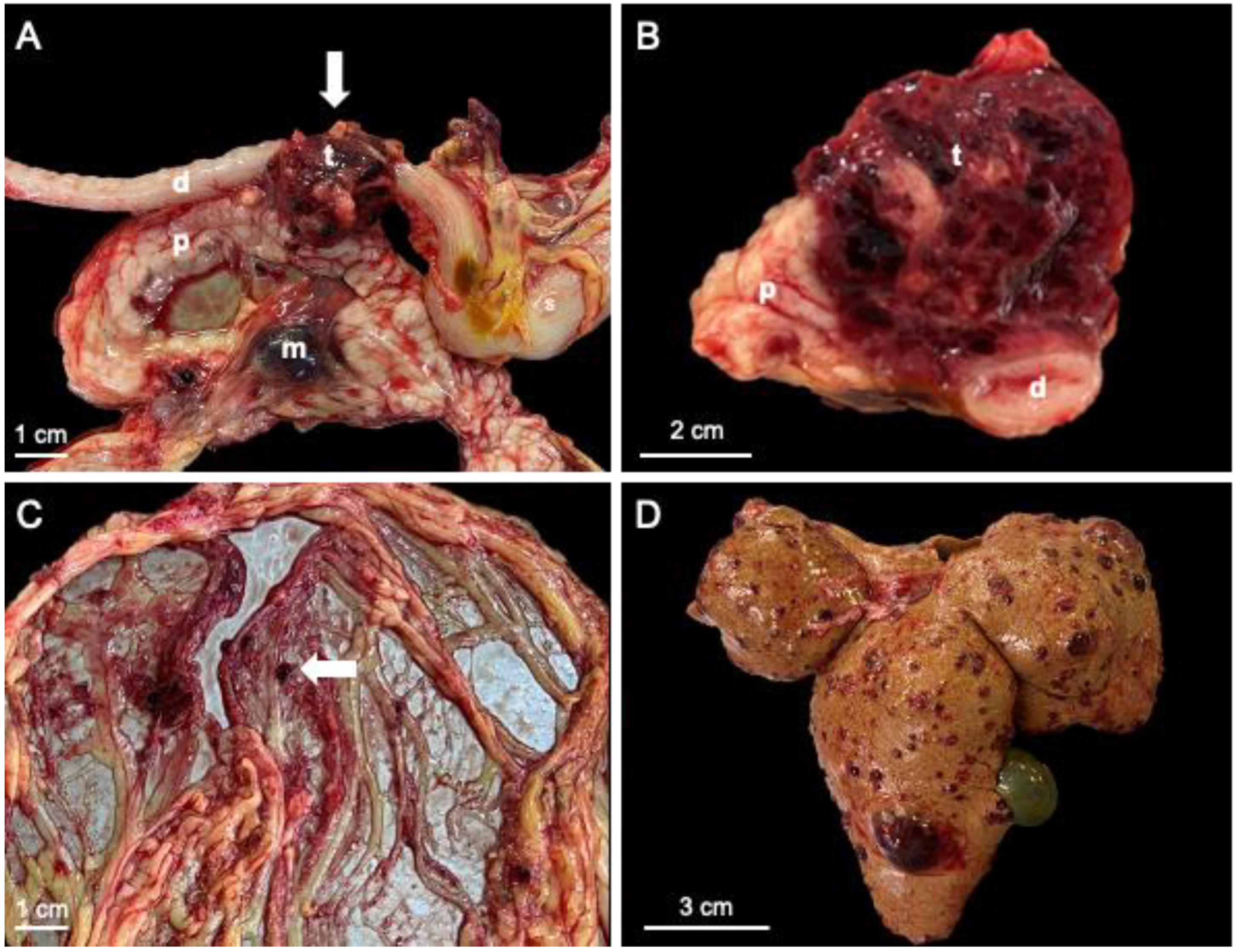
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scavelli, T.D.; Patnaik, A.K.; Mehlhaff, C.J.; Hayes, A.A. Hemangiosarcoma in the cat: Retrospective evaluation of 31 surgical cases. J. Am. Vet. Med. 1985, 187, 817–819. [Google Scholar] [PubMed]
- McAbee, K.P.; Ludwig, L.L.; Bergman, P.J.; Newman, S.J. Feline cutaneous hemangiosarcoma: A retrospective study of 18 cases (1998–2003). J. Am. Vet. Med. 2005, 41, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Culp, W.T.N.; Drobatz, K.J.; Glassman, M.M.; Baez, J.L.; Aronson, L.R. Feline visceral hemangiosarcoma. J. Vet. Intern. Med. 2008, 22, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.A.; Culp, W.T.; Rebhun, R.B. Canine and feline haemangiosarcoma. Vet. Rec. 2021, 189, e585. [Google Scholar] [CrossRef] [PubMed]
- Seaman, R.L. Exocrine pancreatic neoplasia in the cat: A case series. J. Am. Vet. Med. 2004, 40, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Engle, G.C.; Brodey, R.S. A retrospective study of 395 feline neoplasms. J. Am. Vet. Med. 1969, 5, 21–31. [Google Scholar]
- Ottenjann, M.; Kohn, B.; Weingart, C.; Rudolph, R.; Brunnberg, L. Ruptured splenic hemangiosarcoma causing hemoperitoneum in 4 cats. Kleintierpraxis 2003, 48, 345-+. [Google Scholar]
- Johannes, C.M.; Henry, C.J.; Turnquist, S.E.; Hamilton, T.A.; Smith, A.N.; Chun, R.; Tyler, J.W. Hemangiosarcoma in cats: 53 cases (1992–2002). J. Am. Vet. Med. 2007, 231, 1851–1856. [Google Scholar] [CrossRef] [PubMed]
- Culp, W.T.; Weisse, C.; Kellogg, M.E.; Gordon, I.K.; Clarke, D.L.; May, L.R.; Drobatz, K.J. Spontaneous hemoperitoneum in cats: 65 cases (1994–2006). J. Am. Vet. Med. 2010, 236, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Olsen, T.F.; Allen, A.L. Causes of sudden and unexpected death in cats: A 10-year retrospective study. Can. Vet. J. 2001, 42, 61–62. [Google Scholar] [PubMed]
- Törner, K.; Staudacher, M.; Steiger, K.; Aupperle-Lellbach, H. Clinical and pathological data of 17 non-epithelial pancreatic tumors in cats. Vet. Sci. 2020, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.S.; Ogilvie, G.K. Feline Oncology, 3rd ed.; Trenton: Veterinary Learning Systems; WB Saunders: Philadelphia, PA, USA, 2001; pp. 295–310. ISBN 978-1884254536. [Google Scholar]
- Meuten, D.J. Tumors in Domestic Animals, 5th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 159–163. ISBN 9780813821795. [Google Scholar]
- Ramírez, G.A.; Altimira, J.; García-González, B.; Vilafranca, M. Intrapancreatic ectopic splenic tissue in dogs and cats. J. Comp. Pathol. 2013, 148, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Vinayak, A.; Krahwinkel, D.J. Managing blunt trauma-induced hemoperitoneum in dogs and cats. Compendium 2004, 26, 276–290. [Google Scholar]
- Göritz, M.; Müller, K.; Krastel, D.; Staudacher, G.; Schmidt, P.; Kühn, M.; Nickel, R.; Schoon, H.A. Canine splenic haemangiosarcoma: Influence of metastases, chemotherapy and growth pattern on post-splenectomy survival and expression of angiogenic factors. J. comp. pathol. 2013, 149, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Avallone, G.; Rasotto, R.; Chambers, J.K.; Miller, A.D.; Behling-Kelly, E.; Monti, P.; Berlato, D.; Valenti, P.; Roccabianca, P. Review of histological grading systems in veterinary medicine. Vet. Pathol. 2021, 58, 809–828. [Google Scholar] [CrossRef] [PubMed]
- Jennings, R.N.; Miller, M.A.; Ramos-Vara, J.A. Comparison of CD34, CD31, and factor VIII–related antigen immunohistochemical expression in feline vascular neoplasms and CD34 expression in feline nonvascular neoplasms. Vet. Pathol. 2012, 49, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Maharani, A.; Aoshima, K.; Onishi, S.; Gulay, K.C.M.; Kobayashi, A.; Kimura, T. Cellular atypia is negatively correlated with immunohistochemical reactivity of CD31 and vWF expression levels in canine hemangiosarcoma. J. Vet. Med. Sci. 2018, 80, 213–218. [Google Scholar] [CrossRef] [PubMed]
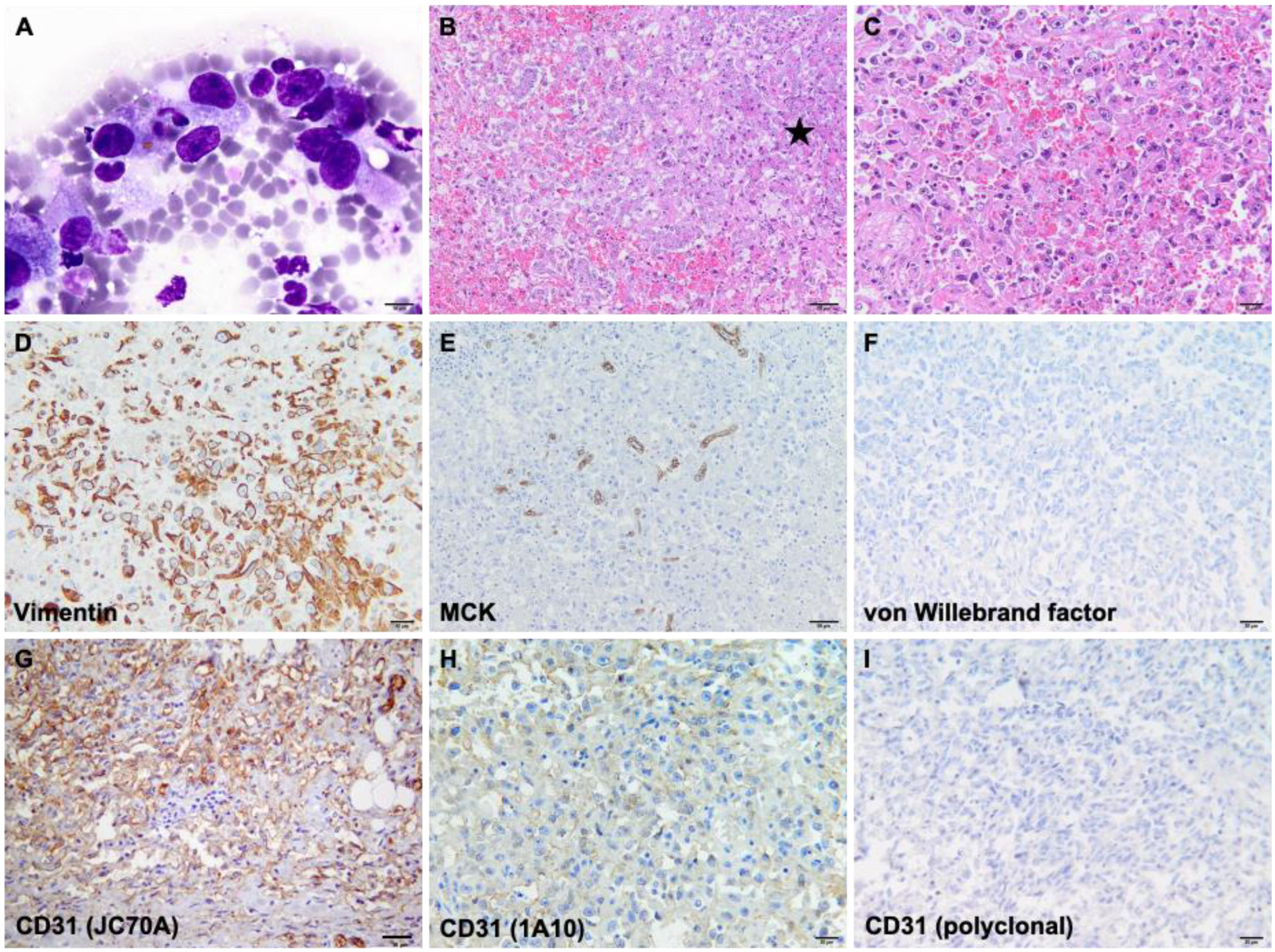
| Antibody | Clone | Company | Positive Control |
|---|---|---|---|
| Mouse anti-human monoclonal to vimentin | SRL33 | Leica Biosystems Newcastle Ltd., Newcastle upon Tyne, UK | Endothelial cells |
| Mouse anti-human to multi-cytokeratin | AE1/AE3 | Leica Biosystems Newcastle Ltd., Newcastle upon Tyne, UK | Pancreatic acinar cells |
| Rabbit polyclonal to CD31 | polyclonal | Abcam, Cambridge, UK | Endothelial cells |
| Mouse anti-human monoclonal to CD31 | JC70A | Leica Biosystems Newcastle Ltd., Newcastle upon Tyne, UK | Endothelial cells |
| Mouse anti-human monoclonal to CD31 | 1A10 | Leica Biosystems Newcastle Ltd., Newcastle upon Tyne, UK | Endothelial cells |
| Mouse anti-human monoclonal to von Willebrand factor | 36B11 | Leica Biosystems Newcastle Ltd., Newcastle upon Tyne, UK | Endothelial cells |
| Case | Source | Clinical findings |
|---|---|---|
| 1 | Engle and Brodey, 1969 [6] | Not reported |
| 2 | Ottenjann et al., 2003 [7] | Not reported |
| 3 | Culp et al., 2008 [3] | Not reported |
| 4 | Culp et al., 2008 [3] | Not reported |
| 5 | Törner et al., 2020 [11] | Palpable abdominal mass |
| 6 | Törner et al., 2020 [11] | Not reported |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toma, C.; Popa, R.; Haralambie, M.-G.; Haralambie, O.-R.; Marica, R. Hemorrhage and Sudden Death in a Cat with Pancreatic Hemangiosarcoma. Vet. Sci. 2023, 10, 8. https://doi.org/10.3390/vetsci10010008
Toma C, Popa R, Haralambie M-G, Haralambie O-R, Marica R. Hemorrhage and Sudden Death in a Cat with Pancreatic Hemangiosarcoma. Veterinary Sciences. 2023; 10(1):8. https://doi.org/10.3390/vetsci10010008
Chicago/Turabian StyleToma, Corina, Roxana Popa, Mara-Georgiana Haralambie, Oana-Roxana Haralambie, and Raluca Marica. 2023. "Hemorrhage and Sudden Death in a Cat with Pancreatic Hemangiosarcoma" Veterinary Sciences 10, no. 1: 8. https://doi.org/10.3390/vetsci10010008
APA StyleToma, C., Popa, R., Haralambie, M.-G., Haralambie, O.-R., & Marica, R. (2023). Hemorrhage and Sudden Death in a Cat with Pancreatic Hemangiosarcoma. Veterinary Sciences, 10(1), 8. https://doi.org/10.3390/vetsci10010008







