Patterns of Lymphocytic Infiltrates Can Differentiate Feline Hepatic Lymphoma from Lymphocytic Portal Hepatitis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Histopathology
2.3. Immunohistochemistry
2.4. DNA Preparation and PCR
2.5. Case Evaluation
2.6. Statistical Analysis
3. Results
3.1. Clonality
3.2. Case Evaluation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warren, A.; Center, S.; McDonough, S.; Chiotti, R.; Goldstein, R.; Meseck, E.; Jacobsen, M.; Rowland, P.; Simpson, K. Histopathologic Features, Immunophenotyping, Clonality, and Eubacterial Fluorescence In Situ Hybridization in Cats With Lymphocytic Cholangitis/Cholangiohepatitis. Vet. Pathol. 2010, 48, 627–641. [Google Scholar] [CrossRef]
- Weiss, D.J.; Gagne, J.M.; Armstrong, P.J. Relationship between inflammatory hepatic disease and inflammatory bowel disease, pancreatitis, and nephritis in cats. J. Am. Vet. Med. Assoc. 1996, 209, 1114–1116. [Google Scholar] [PubMed]
- Zawie, D.A.; Garvey, M.S. Feline hepatic disease. Vet. Clin. N. Am. Small Anim. Pract. 1984, 14, 1201–1230. [Google Scholar] [CrossRef] [PubMed]
- Norsworthy, G.D.; Estep, J.S.; Hollinger, C.; Steiner, J.M.; Lavallee, J.O.; Gassler, L.N.; Restine, L.M.; Kiupel, M. Prevalence and underlying causes of histologic abnormalities in cats suspected to have chronic small bowel disease: 300 cases (2008–2013). J. Am. Vet. Med. Assoc. 2015, 247, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Moore, P.F.; Rodriguez-Bertos, A.; Kass, P.H. Feline gastrointestinal lymphoma: Mucosal architecture, immunophenotype, and molecular clonality. Vet. Pathol. 2012, 49, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Smedby, K.E.; Baecklund, E.; Askling, J. Malignant lymphomas in autoimmunity and inflammation: A review of risks, risk factors, and lymphoma characteristics. Cancer Epidemiol. Biomark. Prev. 2006, 15, 2069–2077. [Google Scholar] [CrossRef]
- Li, P.; Zhang, D.; Zhou, J.; Li, P.; Shen, Y.; Pan, Z.; Evans, A.G.; Liao, X. Hepatic involvement by T-cell neoplasms: A clinicopathologic study of 40 cases. Hum. Pathol. 2020, 106, 1–12. [Google Scholar] [CrossRef]
- Loddenkemper, C.; Longerich, T.; Hummel, M.; Ernestus, K.; Anagnostopoulos, I.; Dienes, H.-P.; Schirmacher, P.; Stein, H. Frequency and diagnostic patterns of lymphomas in liver biopsies with respect to the WHO classification. Virchows Arch. 2007, 450, 493–502. [Google Scholar] [CrossRef]
- Baumhoer, D.; Tzankov, A.; Dirnhofer, S.; Tornillo, L.; Terracciano, L.M. Patterns of liver infiltration in lymphoproliferative disease. Histopathology 2008, 53, 81–90. [Google Scholar] [CrossRef]
- Walz-Mattmüller, R.; Horny, H.-P.; Ruck, P.; Kaiserling, E. Incidence and Pattern of Liver Involvement in Haematological Malignancies. Pathol.-Res. Pract. 1998, 194, 781–789. [Google Scholar] [CrossRef]
- Kemp, S.; Zimmerman, K.; Panciera, D.; Monroe, W.; Leib, M.; Lanz, O. A Comparison of Liver Sampling Techniques in Dogs. J. Vet. Intern. Med. 2014, 29, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Kiupel, M.; Teske, E.; Bostock, D. Prognostic Factors for Treated Canine Malignant Lymphoma. Vet. Pathol. 1999, 36, 292–300. [Google Scholar] [CrossRef]
- Moore, P.F.; Woo, J.C.; Vernau, W.; Kosten, S.; Graham, P.S. Characterization of feline T cell receptor gamma (TCRG) variable region genes for the molecular diagnosis of feline intestinal T cell lymphoma. Vet. Immunol. Immunopathol. 2005, 106, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Kiupel, M.; Smedley, R.C.; Pfent, C.; Xie, Y.; Xue, Y.; Wise, A.G.; DeVaul, J.M.; Maes, R.K. Diagnostic Algorithm to Differentiate Lymphoma From Inflammation in Feline Small Intestinal Biopsy Samples. Vet. Pathol. 2010, 48, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Werner, J.A.; Woo, J.C.; Vernau, W.; Graham, P.S.; Grahn, R.A.; Lyons, L.A.; Moore, P.F. Characterization of Feline Immunoglobulin Heavy Chain Variable Region Genes for the Molecular Diagnosis of B-cell Neoplasia. Vet. Pathol. 2005, 42, 596–607. [Google Scholar] [CrossRef]
- Andrews, C.; Operacz, M.; Maes, R.; Kiupel, M. Cross Lineage Rearrangement in Feline Enteropathy-Associated T-cell Lymphoma. Vet. Pathol. 2015, 53, 559–562. [Google Scholar] [CrossRef]
- Berkvens, D.; Speybroeck, N.; Praet, N.; Adel, A.; Lesaffre, E. Estimating Disease Prevalence in a Bayesian Framework Using Probabilistic Constraints. Epidemiology 2006, 17, 145–153. [Google Scholar] [CrossRef]
- Radtanakatikanon, A.; Moore, P.F.; Keller, S.M.; Vernau, W. Novel clonality assays for T cell lymphoma in cats targeting the T cell receptor beta, T cell receptor delta, and T cell receptor gamma loci. J. Vet. Intern. Med. 2021, 35, 2865–2875. [Google Scholar] [CrossRef]
- Hui, S.L.; Walter, S.D. Estimating the Error Rates of Diagnostic Tests. Biometrics 1980, 36, 167. [Google Scholar] [CrossRef]
- Toft, N.; Jørgensen, E.; Højsgaard, S. Diagnosing diagnostic tests: Evaluating the assumptions underlying the estimation of sensitivity and specificity in the absence of a gold standard. Prev. Vet. Med. 2005, 68, 19–33. [Google Scholar] [CrossRef]
- Economu, L.; Stell, A.; O’Neill, D.G.; Schofield, I.; Stevens, K.; Brodbelt, D. Incidence and risk factors for feline lymphoma in UK primary-care practice. J. Small Anim. Pract. 2020, 62, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Gagne, J.M.; Weiss, D.J.; Armstrong, P.J. Histopathologic Evaluation of Feline Inflammatory Liver Disease. Vet. Pathol. 1996, 33, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Rothuizen, J.; Bunch, S.E.; Charles, J.A.; Cullen, J.M.; Desmet, V.J.; Szatmári, V.; Twedt, D.C.; Ingh, T.S.V.D.; Van Winkle, T.; Washabau, R.J. Morphological Classification of Parenchymal Disorders of the Canine and Feline Liver. In WSAVA Standards for Clinical and Histological Diagnosis of Canine and Feline Liver Diseases; Elsevier: Amsterdam, The Netherlands, 2009; pp. 85–101. [Google Scholar]
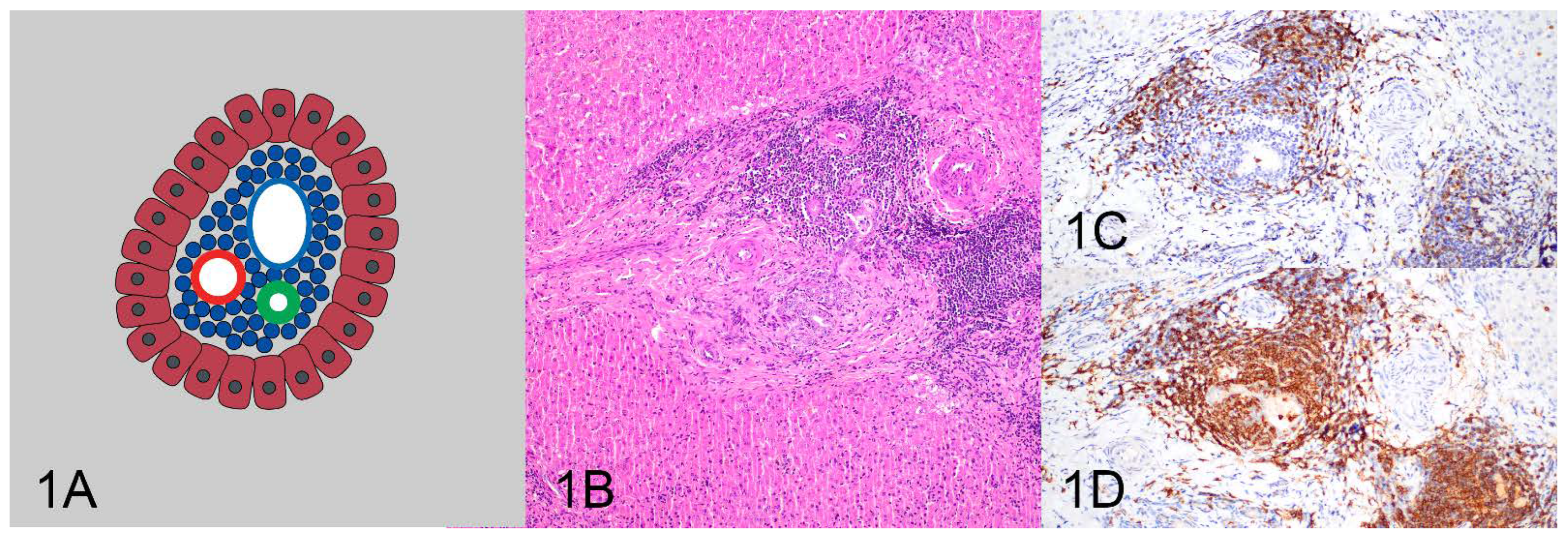
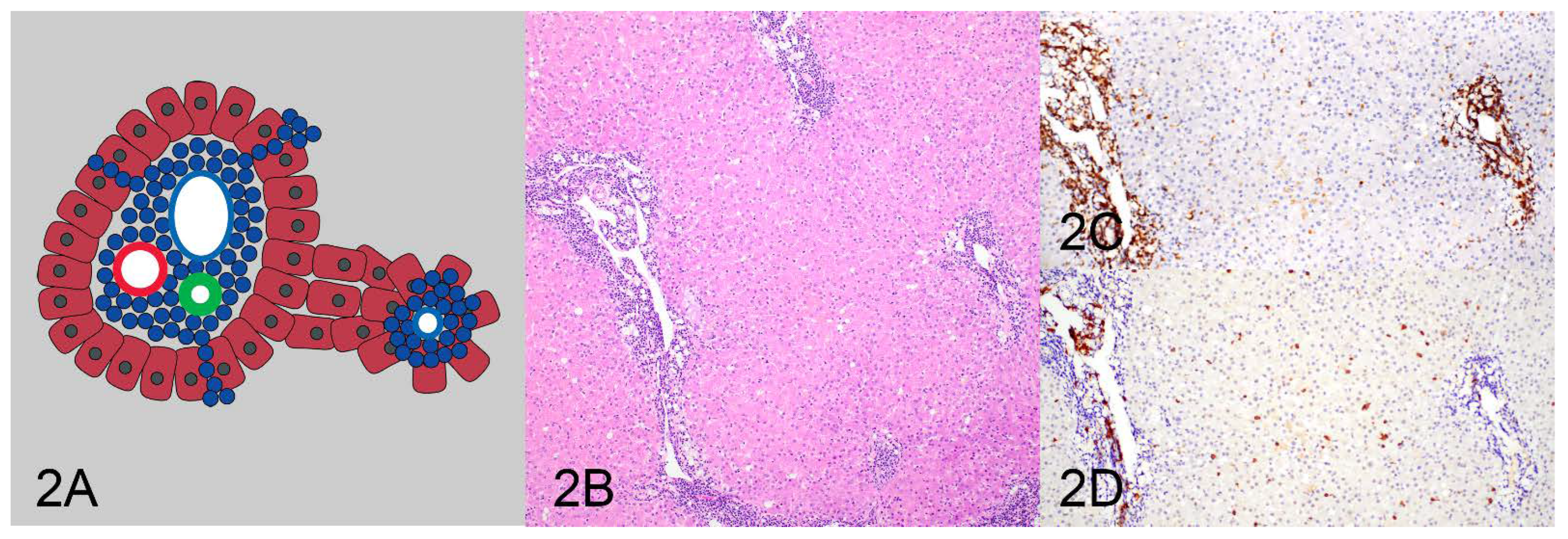
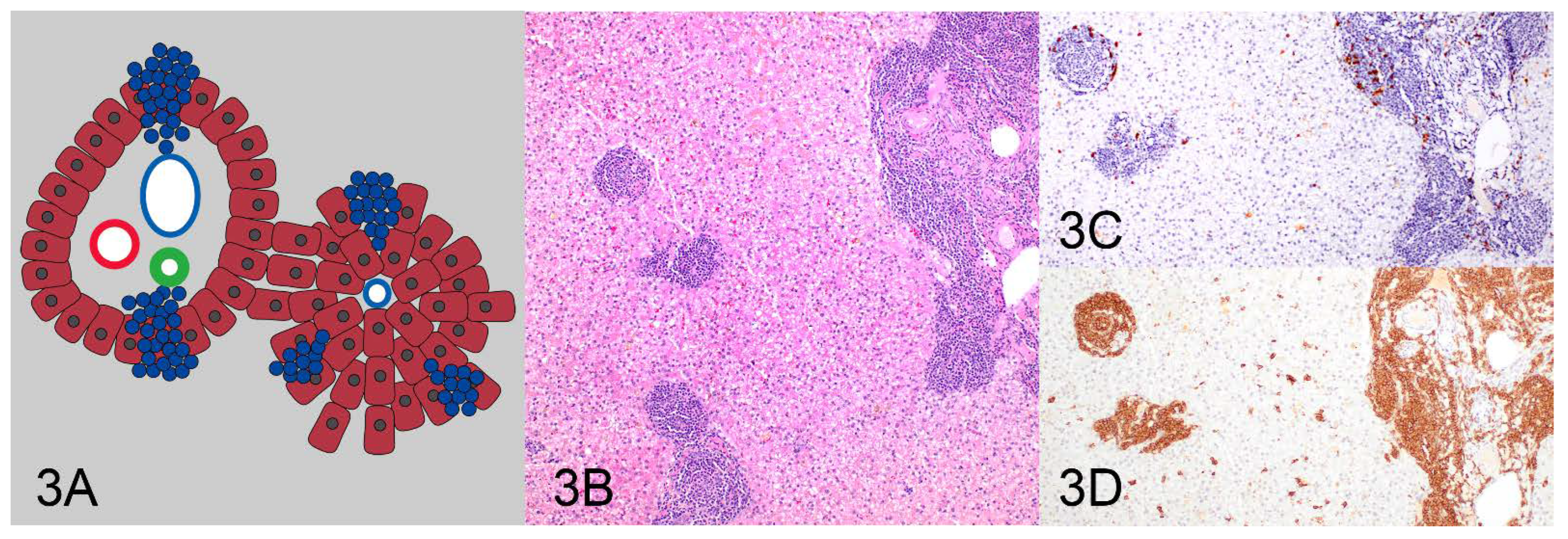
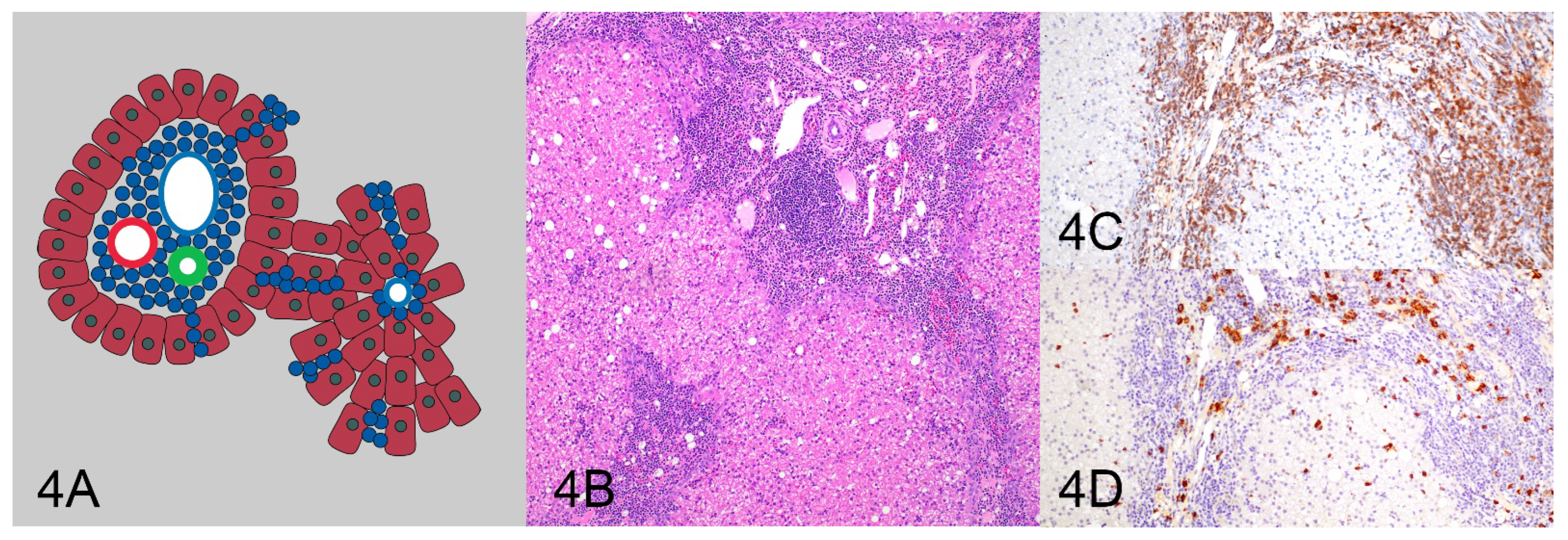

| Pattern | Clonality Negative | Clonality Positive | Total |
|---|---|---|---|
| Pattern 1: Tightly periportal | 15 (83.3%) | 3 (16.7%) | 18 (100%) |
| Pattern 2: Periportal and centrilobular | 0 (0%) | 1 (100%) | 1 (100%) |
| Pattern 3: Nodular | 0 (0%) | 2 (100%) | 2 (100%) |
| Pattern 4: Periportal with sinusoidal extension | 8 (38.1%) | 13 (61.9%) | 21 (100%) |
| Pattern 2 and 4: Periportal and centrilobular and sinusoidal extension | 0 (0%) | 2 (100%) | 2 (100%) |
| Total | 23 (52.3%) | 21 (47.7%) | 44 (100%) |
| Parameter | Flat Prior Model | Weakly Informative Prior Model |
|---|---|---|
| Pattern sensitivity | 82% (65–96%) | 83% (67–96%) |
| Pattern specificity | 77% (54–100%) | 79% (57–100%) |
| Clonality sensitivity | 78% (57–100%) | 79% (59–100%) |
| Clonality specificity * | 100% (100–100%) | 100% (100–100%) |
| Prevalence male | 66% (39–90%) | 65% (40–90%) |
| Prevalence female | 54% (30–79%) | 54% (31–78%) |
| Clonality Negative | Clonality Positive | |||||
|---|---|---|---|---|---|---|
| Histology | Cases | n | Percent (95% CI) | Cases | n | Percent (95% CI) |
| Bile duct targetting | 23 | 4 | 17.4 (7.0–37.1) | 21 | 5 | 23.8 (10.6–45.1) |
| EMH * | 23 | 1 | 4.3 (0.8–21.0) | 21 | 4 | 19.0 (7.7–40.0) |
| Fibrosis | 23 | 6 | 26.1 (12.5–46.5) | 21 | 3 | 14.3 (5.0–34.6) |
| Lipogranulomas | 23 | 8 | 34.8 (18.8–55.1) | 21 | 5 | 23.8 (10.6–45.1) |
| Lymphonodular aggregates | 23 | 13 | 56.5 (36.8–74.4) | 21 | 12 | 57.1 (36.5–75.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sebastian, K.; Smedley, R.C.; Bartel, A.; Kiupel, M. Patterns of Lymphocytic Infiltrates Can Differentiate Feline Hepatic Lymphoma from Lymphocytic Portal Hepatitis. Vet. Sci. 2023, 10, 127. https://doi.org/10.3390/vetsci10020127
Sebastian K, Smedley RC, Bartel A, Kiupel M. Patterns of Lymphocytic Infiltrates Can Differentiate Feline Hepatic Lymphoma from Lymphocytic Portal Hepatitis. Veterinary Sciences. 2023; 10(2):127. https://doi.org/10.3390/vetsci10020127
Chicago/Turabian StyleSebastian, Kimberley, Rebecca C. Smedley, Alexander Bartel, and Matti Kiupel. 2023. "Patterns of Lymphocytic Infiltrates Can Differentiate Feline Hepatic Lymphoma from Lymphocytic Portal Hepatitis" Veterinary Sciences 10, no. 2: 127. https://doi.org/10.3390/vetsci10020127
APA StyleSebastian, K., Smedley, R. C., Bartel, A., & Kiupel, M. (2023). Patterns of Lymphocytic Infiltrates Can Differentiate Feline Hepatic Lymphoma from Lymphocytic Portal Hepatitis. Veterinary Sciences, 10(2), 127. https://doi.org/10.3390/vetsci10020127






