Association between Milk Electrical Conductivity Biomarkers with Lameness in Dairy Cows
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Animal and Experimental Design
2.2. Measurements
2.3. Treatment of Lameness
2.4. Statistical Analysis
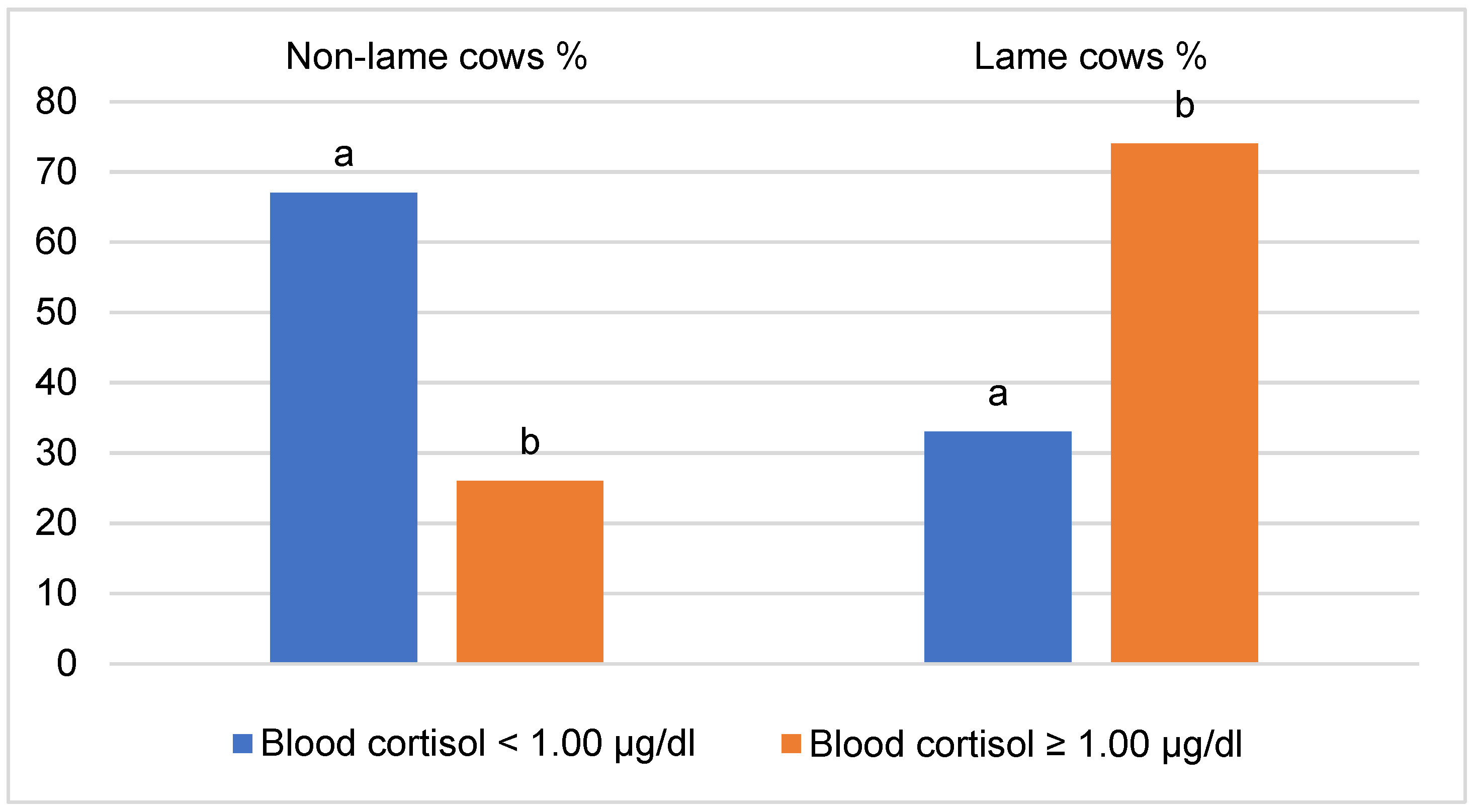
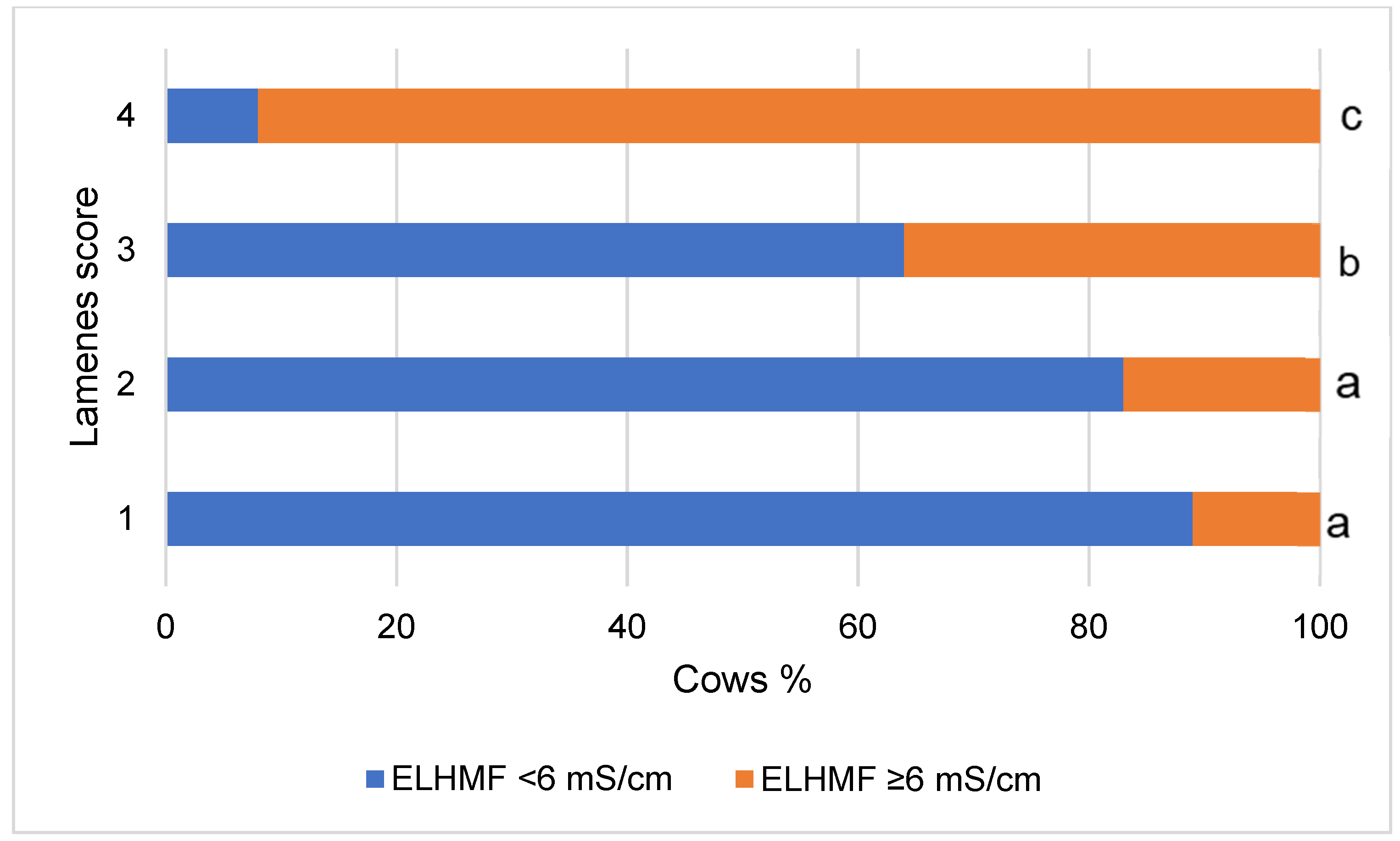
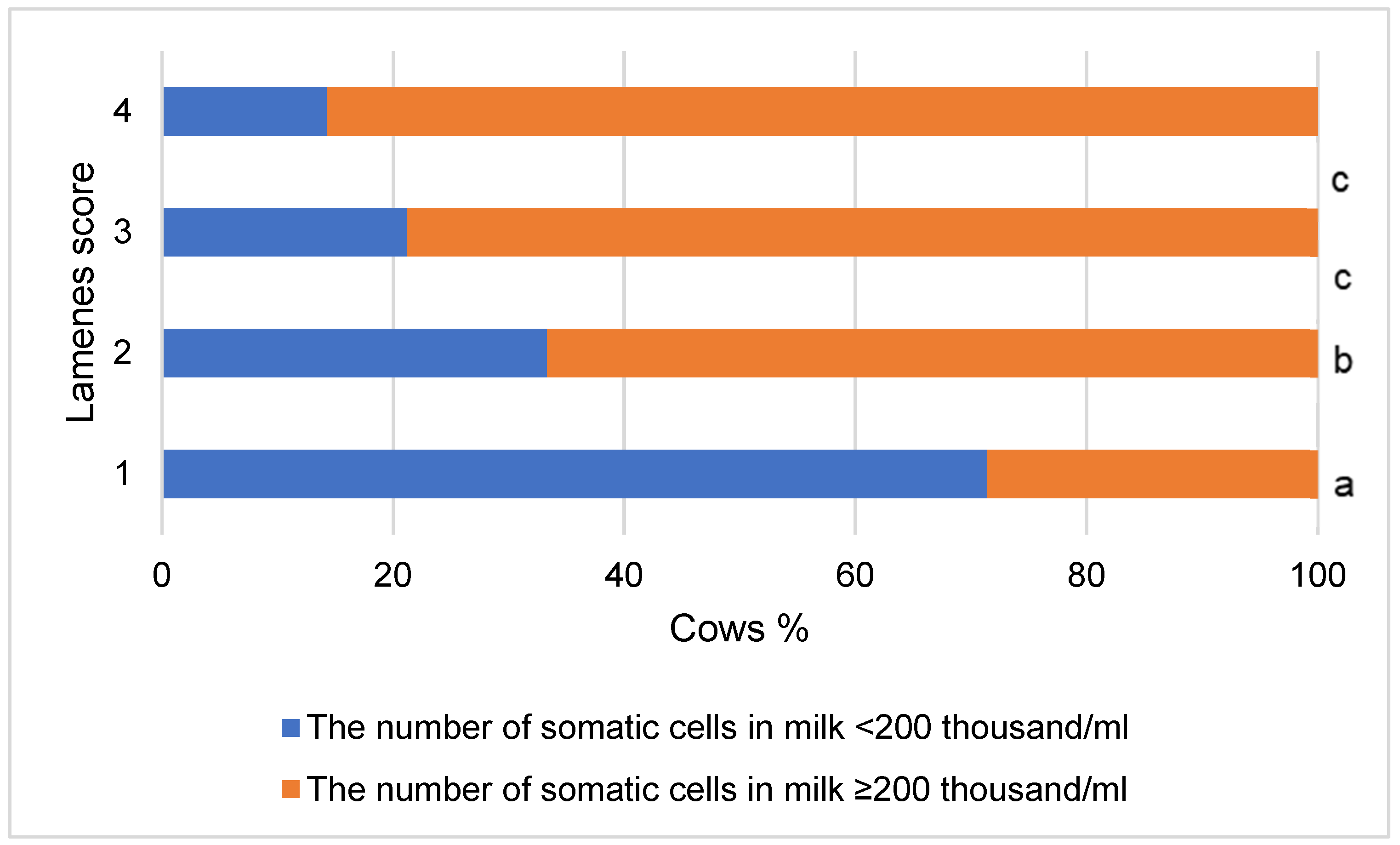
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thompson, A.J.; Weary, D.M.; Bran, J.A.; Daros, R.R.; Hötzel, M.J.; von Keyserlingk, M.A.G. Lameness and Lying Behavior in Grazing Dairy Cows. J. Dairy Sci. 2019, 102, 6373–6382. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.B. Prevalence of Lameness among Dairy Cattle in Wisconsin as a Function of Housing Type and Stall Surface. J. Am. Vet. Med. Assoc. 2003, 223, 1324–1328. [Google Scholar] [CrossRef]
- Barker, Z.E.; Leach, K.A.; Whay, H.R.; Bell, N.J.; Main, D.C.J. Assessment of Lameness Prevalence and Associated Risk Factors in Dairy Herds in England and Wales. J. Dairy Sci. 2010, 93, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Bicalho, R.C.; Vokey, F.; Erb, H.N.; Guard, C.L. Visual Locomotion Scoring in the First Seventy Days in Milk: Impact on Pregnancy and Survival. J. Dairy Sci. 2007, 90, 4586–4591. [Google Scholar] [CrossRef] [PubMed]
- Espejo, L.A.; Endres, M.I. Herd-Level Risk Factors for Lameness in High-Producing Holstein Cows Housed in Freestall Barns. J. Dairy Sci. 2007, 90, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Solano, L.; Barkema, H.W.; Pajor, E.A.; Mason, S.; LeBlanc, S.J.; Zaffino Heyerhoff, J.C.; Nash, C.G.R.; Haley, D.B.; Vasseur, E.; Pellerin, D.; et al. Prevalence of Lameness and Associated Risk Factors in Canadian Holstein-Friesian Cows Housed in Freestall Barns. J. Dairy Sci. 2015, 98, 6978–6991. [Google Scholar] [CrossRef] [PubMed]
- Espejo, L.A.; Endres, M.I.; Salfer, J.A. Prevalence of Lameness in High-Producing Holstein Cows Housed in Freestall Barns in Minnesota. J. Dairy Sci. 2006, 89, 3052–3058. [Google Scholar] [CrossRef]
- Sprecher, D.J.; Hostetler, D.E.; Kaneene, J.B. A Lameness Scoring System That Uses Posture and Gait to Predict Dairy Cattle Reproductive Performance. Theriogenology 1997, 47, 1179–1187. [Google Scholar] [CrossRef]
- Whay, H.R.; Main, D.C.J.; Green, L.E.; Webster, A.J.F. Assessment of the Welfare of Dairy Caftle Using Animal-Based Measurements: Direct Observations and Investigation of Farm Records. Vet. Rec. 2003, 153, 197–202. [Google Scholar] [CrossRef]
- Grimm, K.; Haidn, B.; Erhard, M.; Tremblay, M.; Döpfer, D. New Insights into the Association between Lameness, Behavior, and Performance in Simmental Cows. J. Dairy Sci. 2019, 102, 2453–2468. [Google Scholar] [CrossRef]
- Reader, J.D.; Green, M.J.; Kaler, J.; Mason, S.A.; Green, L.E. Effect of Mobility Score on Milk Yield and Activity in Dairy Cattle. J. Dairy Sci. 2011, 94, 5045–5052. [Google Scholar] [CrossRef]
- Pavlenko, A.; Bergsten, C.; Ekesbo, I.; Kaart, T.; Aland, A.; Lidfors, L. Influence of Digital Dermatitis and Sole Ulcer on Dairy Cow Behaviour and Milk Production. Animal 2011, 5, 1259–1269. [Google Scholar] [CrossRef] [PubMed]
- Charfeddine, N.; Pérez-Cabal, M.A. Effect of Claw Disorders on Milk Production, Fertility, and Longevity, and Their Economic Impact in Spanish Holstein Cows. J. Dairy Sci. 2017, 100, 653–665. [Google Scholar] [CrossRef]
- Jacobs, J.A.; Siegford, J.M. Invited Review: The Impact of Automatic Milking Systems on Dairy Cow Management, Behavior, Health, and Welfare. J. Dairy Sci. 2012, 95, 2227–2247. [Google Scholar] [CrossRef] [PubMed]
- King, M.T.M.; LeBlanc, S.J.; Pajor, E.A.; DeVries, T.J. Cow-Level Associations of Lameness, Behavior, and Milk Yield of Cows Milked in Automated Systems. J. Dairy Sci. 2017, 100, 4818–4828. [Google Scholar] [CrossRef]
- Underwood, W. Pain and Distress in Agricultural Animals. J. Am. Vet. Med. Assoc. 2002, 221, 208–211. [Google Scholar] [CrossRef]
- Möstl, E.; Palme, R. Hormones as Indicators of Stress. Domest. Anim. Endocrinol. 2002, 23, 67–74. [Google Scholar] [CrossRef]
- O’Driscoll, K.; McCabe, M.; Earley, B. Leukocyte Profile, Gene Expression, Acute Phase Response, and Metabolite Status of Cows with Sole Hemorrhages. J. Dairy Sci. 2017, 100, 9382–9391. [Google Scholar] [CrossRef] [PubMed]
- Comin, A.; Peric, T.; Corazzin, M.; Veronesi, M.C.; Meloni, T.; Zufferli, V.; Cornacchia, G.; Prandi, A. Hair Cortisol as a Marker of Hypothalamic-Pituitary-Adrenal Axis Activation in Friesian Dairy Cows Clinically or Physiologically Compromised. Livest. Sci. 2013, 152, 36–41. [Google Scholar] [CrossRef]
- Fischer-Tenhagen, C.; Ladwig-Wiegard, M.; Heuwieser, W.; Thöne-Reineke, C. Short Communication: Is Hair Cortisol a Potential Indicator for Stress Caused by Chronic Lameness in Dairy Cows? J. Dairy Sci. 2018, 101, 5439–5443. [Google Scholar] [CrossRef]
- Urbonavicius, G.; Antanaitis, R.; Zilaitis, V.; Tusas, S.; Kajokiene, L.; Zymantiene, J.; Spancerniene, U.; Gavelis, A.; Juskiene, V.; Juozaitienė, V. The Influence of Lameness on Several Automatic Milking System Variables and Reproductive Performance Indicators in Dairy Cows. Pol. J. Vet. Sci. 2020, 23, 383–390. [Google Scholar] [CrossRef]
- Miguel-Pacheco, G.G.; Kaler, J.; Remnant, J.; Cheyne, L.; Abbott, C.; French, A.P.; Pridmore, T.P.; Huxley, J.N. Behavioural Changes in Dairy Cows with Lameness in an Automatic Milking System. Appl. Anim. Behav. Sci. 2014, 150, 1–8. [Google Scholar] [CrossRef]
- Mazrier, H.; Tal, S.; Aizinbud, E.; Bargai, U. A Field Investigation of the Use of the Pedometer for the Early Detection of Lameness in Cattle. Can. Vet. J. 2006, 47, 883–886. [Google Scholar] [PubMed]
- González, L.A.; Tolkamp, B.J.; Coffey, M.P.; Ferret, A.; Kyriazakis, I. Changes in Feeding Behavior as Possible Indicators for the Automatic Monitoring of Health Disorders in Dairy Cows. J. Dairy Sci. 2008, 91, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, J.P.; Kelly, K.A.; Locker, A.R.; Miller, D.B.; Lasley, S.M. Corticosterone Primes the Neuroinflammatory Response to DFP in Mice: Potential Animal Model of Gulf War Illness. J. Neurochem. 2015, 133, 708–721. [Google Scholar] [CrossRef] [PubMed]
- Daros, R.R.; Eriksson, H.K.; Weary, D.M.; von Keyserlingk, M.A.G. The Relationship between Transition Period Diseases and Lameness, Feeding Time, and Body Condition during the Dry Period. J. Dairy Sci. 2020, 103, 649–665. [Google Scholar] [CrossRef]
- Silva, S.R.; Araujo, J.P.; Guedes, C.; Silva, F.; Almeida, M.; Cerqueira, J.L. Precision Technologies to Address Dairy Cattle Welfare: Focus on Lameness, Mastitis and Body Condition. Animals 2021, 11, 2253. [Google Scholar] [CrossRef]
- Garvey, M. Lameness in Dairy Cow Herds: Disease Aetiology, Prevention and Management. Dairy 2022, 3, 199–210. [Google Scholar] [CrossRef]
- Council, N.R. Nutrient Requirements of Dairy Cattle: Seventh Revised Edition, 2001; National Academies Press: Washington, DC, USA, 2001; ISBN 978-0-309-06997-7. [Google Scholar]
- Higgs, P.; Costa, M.; Freke, A.; Papasouliotis, K. Measurement of Thyroxine and Cortisol in Canine and Feline Blood Samples Using Two Immunoassay Analysers. J. Small Anim. Pract. 2014, 55, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Archer, S.; Bell, N.; Huxley, J. Lameness in UK Dairy Cows: A Review of the Current Status. In Pract. 2010, 32, 492–504. [Google Scholar] [CrossRef]
- Rushen, J.; Pombourcq, E.; Passillé, A.M. de Validation of Two Measures of Lameness in Dairy Cows. Appl. Anim. Behav. Sci. 2007, 106, 173–177. [Google Scholar] [CrossRef]
- Warnick, L.D.; Janssen, D.; Guard, C.L.; Gröhn, Y.T. The Effect of Lameness on Milk Production in Dairy Cows. J. Dairy Sci. 2001, 84, 1988–1997. [Google Scholar] [CrossRef] [PubMed]
- Booth, C.J.; Warnick, L.D.; Gröhn, Y.T.; Maizon, D.O.; Guard, C.L.; Janssen, D. Effect of Lameness on Culling in Dairy Cows. J. Dairy Sci. 2004, 87, 4115–4122. [Google Scholar] [CrossRef] [PubMed]
- Bicalho, R.C.; Machado, V.S.; Caixeta, L.S. Lameness in Dairy Cattle: A Debilitating Disease or a Disease of Debilitated Cattle? A Cross-Sectional Study of Lameness Prevalence and Thickness of the Digital Cushion. J. Dairy Sci. 2009, 92, 3175–3184. [Google Scholar] [CrossRef]
- Almeida, P.E.; Weber, P.S.D.; Burton, J.L.; Zanella, A.J. Depressed DHEA and Increased Sickness Response Behaviors in Lame Dairy Cows with Inflammatory Foot Lesions. Domest. Anim. Endocrinol. 2008, 34, 89–99. [Google Scholar] [CrossRef]
- Walker, S.; Smith, R.; Jones, D.; Routly, J.; Morris, M.; Dobson, H. The Effect of a Chronic Stressor, Lameness, on Detailed Sexual Behaviour and Hormonal Profiles in Milk and Plasma of Dairy Cattle. Reprod. Domest. Anim. 2010, 45, 109–117. [Google Scholar] [CrossRef]
- Bustamante, H.A.; Rodríguez, A.R.; Herzberg, D.E.; Werner, M.P. Stress and Pain Response after Oligofructose Induced-Lameness in Dairy Heifers. J. Vet. Sci. 2015, 16, 405–411. [Google Scholar] [CrossRef]
- Hannibal, K.E.; Bishop, M.D. Chronic Stress, Cortisol Dysfunction, and Pain: A Psychoneuroendocrine Rationale for Stress Management in Pain Rehabilitation. Phys. Ther. 2014, 94, 1816–1825. [Google Scholar] [CrossRef]
- Ito, K.; von Keyserlingk, M.A.G.; LeBlanc, S.J.; Weary, D.M. Lying Behavior as an Indicator of Lameness in Dairy Cows. J. Dairy Sci. 2010, 93, 3553–3560. [Google Scholar] [CrossRef]
- Singh, A.; Singh, S.; Gupta, D.K.; Bansal, B.K. Relationship of Lameness to Body Condition Score, Udder Health and Milk Quality in Crossbred Dairy Cattle. Vet. Arh. 2018, 88, 179–190. [Google Scholar] [CrossRef]
| Indicator | Description |
|---|---|
| ELHMF | Electrical conductivity at the maximum milk flow (mS/cm) |
| ELAP | Electrical conductivity during the first few minutes of milking (mS/cm) (beginning peak level of the electrical conductivity) |
| ELMAX | Maximum electrical conductivity after reaching the highest milking speed (mS/cm) |
| ELMNG | Maximum electrical conductivity following main milking (mS/cm) |
| ELAD | Beginning of the electrical conductivity peak difference (mS/cm) |
| Group of Cows | ELHMF | ELAP | ELMAX | ELMNG | ELAD | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| mS/cm | ||||||||||
| <6 | ≥6 | <6 | ≥6 | <6 | ≥6 | <6 | ≥6 | <6 | ≥6 | |
| Non-lame | 90.8 | 9.2 | 55.4 | 44.6 | 75.4 | 24.6 | 92.3 | 7.7 | 92.0 | 8.0 |
| Lame | 61.8 | 38.2 | 27.3 | 72.7 | 50.9 | 49.1 | 74.5 | 25.5 | 72.5 | 27.5 |
| χ2 statistic | χ2 = 14.320, p < 0.001 | χ2 = 9.634, p = 0.002 | χ2 = 7.762, p = 0.005 | χ2 = 14.310, p < 0.001 | χ2 = 14.300, p < 0.001 | |||||
| Indicator | Cortisol | Lameness Score |
|---|---|---|
| Cortisol | - | 0.457 * |
| ELHMF | 0.704 | 0.339 * |
| ELAP | 0.519 | 0.404 * |
| ELMAX | 0.633 | 0.264 * |
| ELMNG | 0.081 | 0.153 |
| ELAD | 0.060 | 0.284 * |
| Factor | p | OR | 95% C.I. for OR | |
|---|---|---|---|---|
| Lower | Upper | |||
| ELHMF (class) | <0.001 | 6.074 | 2.233 | 16.52 |
| Cortisol (class) | <0.001 | 5.704 | 2.422 | 13.431 |
| Constant | 0.010 | 0.576 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paulauskas, A.; Juozaitienė, V.; Džermeikaitė, K.; Bačėninaitė, D.; Urbonavičius, G.; Tušas, S.; Šlyžius, E.; Baumgartner, W.; Rutkauskas, A.; Antanaitis, R. Association between Milk Electrical Conductivity Biomarkers with Lameness in Dairy Cows. Vet. Sci. 2023, 10, 47. https://doi.org/10.3390/vetsci10010047
Paulauskas A, Juozaitienė V, Džermeikaitė K, Bačėninaitė D, Urbonavičius G, Tušas S, Šlyžius E, Baumgartner W, Rutkauskas A, Antanaitis R. Association between Milk Electrical Conductivity Biomarkers with Lameness in Dairy Cows. Veterinary Sciences. 2023; 10(1):47. https://doi.org/10.3390/vetsci10010047
Chicago/Turabian StylePaulauskas, Algimantas, Vida Juozaitienė, Karina Džermeikaitė, Dovilė Bačėninaitė, Gediminas Urbonavičius, Saulius Tušas, Evaldas Šlyžius, Walter Baumgartner, Arūnas Rutkauskas, and Ramūnas Antanaitis. 2023. "Association between Milk Electrical Conductivity Biomarkers with Lameness in Dairy Cows" Veterinary Sciences 10, no. 1: 47. https://doi.org/10.3390/vetsci10010047
APA StylePaulauskas, A., Juozaitienė, V., Džermeikaitė, K., Bačėninaitė, D., Urbonavičius, G., Tušas, S., Šlyžius, E., Baumgartner, W., Rutkauskas, A., & Antanaitis, R. (2023). Association between Milk Electrical Conductivity Biomarkers with Lameness in Dairy Cows. Veterinary Sciences, 10(1), 47. https://doi.org/10.3390/vetsci10010047








