Tylvalosin Tartrate Improves the Health Status of Swine Herds during Immunization with Porcine Reproductive and Respiratory Syndrome Virus-Inactivated Vaccine
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Animal Experimental Design
2.3. Blood Sample Collection
2.4. Serum Cytokine Detection
2.5. Detection of the Proportion of Peripheral Blood Leukocytes and Lymphocytes
2.6. Statistical Analysis
3. Results
3.1. Clinical Presentation
3.2. Dynamic Changes in White Blood Cell Counts
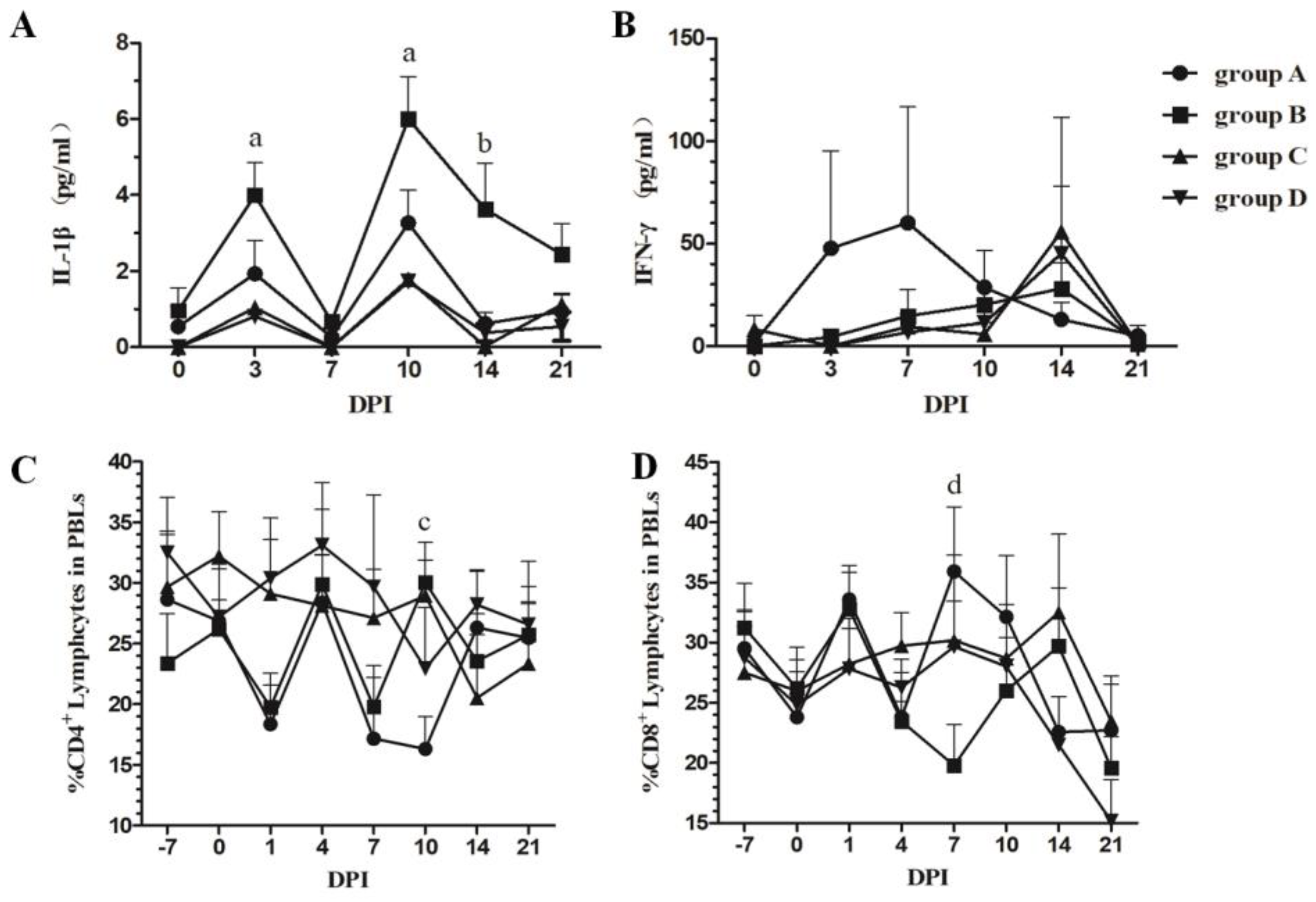
3.3. Dynamic Changes in Serum IL-1β and IFN-γ
3.4. Changes in the Number of CD4+CD8− and CD4−CD8+ T Cells in PBLs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Infromed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rossow, K.D. Porcine Reproductive and Respiratory Syndrome. Vet. Pathol. 1998, 35, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Yoon, I.J.; Joo, H.S.; Christianson, W.T.; Kim, H.S.; Collins, J.E.; Carlson, J.H.; Dee, S.A. Isolation of a Cytopathic Virus from Weak Pigs on Farms with a History of Swine Infertility and Respiratory Syndrome. J. Vet. Diagn. Investig. 1992, 4, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuis, N.; Duinhof, T.F.; van Nes, A. Economic analysis of outbreaks of porcine reproductive and respiratory syndrome virus in nine sow herds. Vet. Rec. 2012, 170, 225. [Google Scholar] [CrossRef] [PubMed]
- Neumann, E.J.; Kliebenstein, J.B.; Johnson, C.D.; Mabry, J.W.; Bush, E.J.; Seitzinger, A.H.; Green, A.L.; Zimmerman, J.J. Assessment of the economic impact of porcine reproductive and respiratory syndrome on swine production in the United States. J. Am. Vet. Med Assoc. 2005, 227, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Z.; Li, H.; Yang, S.; Ren, F.; Bian, T.; Sun, L.; Zhou, B.; Zhou, L.; Qu, X. The economic impact of porcine reproductive and respiratory syndrome outbreak in four Chinese farms: Based on cost and revenue analysis. Front. Vet. Sci. 2022, 9, 1024720. [Google Scholar] [CrossRef]
- Goyal, S.M. Porcine Reproductive and Respiratory Syndrome. J. Vet. Diagn. Investing. 1993, 5, 656–664. [Google Scholar] [CrossRef]
- Halbur, P.G.; Paul, P.S.; Frey, M.L.; Landgraf, J.; Eernisse, K.; Meng, X.-J.; Lum, M.A.; Andrews, J.J.; Rathje, J.A. Comparison of the Pathogenicity of Two US Porcine Reproductive and Respiratory Syndrome Virus Isolates with that of the Lelystad Virus. Vet. Pathol. 1995, 32, 648–660. [Google Scholar] [CrossRef]
- Tian, K.; Yu, X.; Zhao, T.; Feng, Y.; Cao, Z.; Wang, C.; Hu, Y.; Chen, X.; Hu, D.; Tian, X.; et al. Emergence of Fatal PRRSV Variants: Unparalleled Outbreaks of Atypical PRRS in China and Molecular Dissection of the Unique Hallmark. PLoS ONE 2007, 2, e526. [Google Scholar] [CrossRef]
- Lunney, J.K.; Fang, Y.; Ladinig, A.; Chen, N.; Li, Y.; Rowland, B.; Renukaradhya, G.J. Porcine Reproductive and Respiratory Syndrome Virus (PRRSV): Pathogenesis and Interaction with the Immune System. Annu. Rev. Anim. Biosci. 2016, 4, 129–154. [Google Scholar] [CrossRef]
- Mengeling, W.L.; Vorwald, A.C.; Lager, K.M.; Brockmeier, S.L. Comparison among strains of porcine reproductive and respiratory syndrome virus for their ability to cause reproductive failure. Am. J. Vet. Res. 1996, 57, 834–839. [Google Scholar]
- Guo, X.; Zhang, Q.; Hou, S.; Zhai, G.; Zhu, H.; Sánchez-Vizcaíno, J. Plasmid containing CpG motifs enhances the efficacy of porcine reproductive and respiratory syndrome live attenuated vaccine. Vet. Immunol. Immunopathol. 2011, 144, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.-H.; Wu, G.-J.; Liu, Y.-G.; Liu, G.-Q.; Shi, W.-D.; Wang, S.-J.; Ma, P.; Li, C.-J.; Han, W.-Y. Attenuation of Virulent Porcine Reproductive and Respiratory Syndrome Virus Strain CH-1a and Genetic Variation of ORF5 Gene. J. Integr. Agric. 2012, 11, 2035–2042. [Google Scholar] [CrossRef]
- Tong, G.Z.; Zhou, Y.J.; Hao, X.F.; Tian, Z.J.; An, T.Q.; Qiu, H.J. Highly pathogenic porcine reproductive and respiratory syndrome, China. Emerg. Infect. Dis. 2007, 13, 1434–1436. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Bo, K.; Wang, X.; Tang, B.; Yang, B.; Jiang, W.; Jiang, P. Emergence of a highly pathogenic porcine reproductive and respiratory syndrome virus in the Mid-Eastern region of China. Vet. J. 2007, 174, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Sun, E.; Huang, L.; Zhang, X.; Zhang, J.; Shen, D.; Zhang, Z.; Wang, Z.; Huo, H.; Wang, W.; Huangfu, H.; et al. Genotype I African swine fever viruses emerged in domestic pigs in China and caused chronic infection. Emerg. Microbes Infect. 2021, 10, 2183–2193. [Google Scholar] [CrossRef] [PubMed]
- Sun, E.; Zhang, Z.; Wang, Z.; He, X.; Zhang, X.; Wang, L.; Wang, W.; Huang, L.; Xi, F.; Huangfu, H.; et al. Emergence and prevalence of naturally occurring lower virulent African swine fever viruses in domestic pigs in China in 2020. Sci. China Life Sci. 2021, 64, 752–765. [Google Scholar] [CrossRef]
- Zhao, K.; Ye, C.; Chang, X.-B.; Jiang, C.-G.; Wang, S.-J.; Cai, X.-H.; Tong, G.-Z.; Tian, Z.-J.; Shi, M.; An, T.-Q. Importation and Recombination Are Responsible for the Latest Emergence of Highly Pathogenic Porcine Reproductive and Respiratory Syndrome Virus in China. J. Virol. 2015, 89, 10712–10716. [Google Scholar] [CrossRef]
- Zhang, H.-L.; Zhang, W.-L.; Xiang, L.-R.; Leng, C.-L.; Tian, Z.-J.; Tang, Y.-D.; Cai, X.-H. Emergence of novel porcine reproductive and respiratory syndrome viruses (ORF5 RFLP 1-7-4 viruses) in China. Vet. Microbiol. 2018, 222, 105–108. [Google Scholar] [CrossRef]
- Liu, J.; Lai, L.; Xu, Y.; Yang, Y.; Li, J.; Liu, C.; Hunag, C.; Wei, C. Evolutionary Analysis of Four Recombinant Viruses of the Porcine Reproductive and Respiratory Syndrome Virus From a Pig Farm in China. Front. Vet. Sci. 2022, 9, 933896. [Google Scholar] [CrossRef]
- Bassaganya-Riera, J.; Thacker, B.J.; Yu, S.; Strait, E.; Wannemuehler, M.J.; Thacker, E.L. Impact of Immunizations with Porcine Reproductive and Respiratory Syndrome Virus on Lymphoproliferative Recall Responses of CD8+ T Cells. Viral Immunol. 2004, 17, 25–37. [Google Scholar] [CrossRef]
- Ding, Y.; Wubshet, A.K.; Ding, X.; Zhang, Z.; Li, Q.; Dai, J.; Hou, Q.; Hu, Y.; Zhang, J. Evaluation of Four Commercial Vaccines for the Protection of Piglets against the Highly Pathogenic Porcine Reproductive and Respiratory Syndrome Virus (hp-PRRSV) QH-08 Strain. Vaccines 2021, 9, 1020. [Google Scholar] [CrossRef] [PubMed]
- Renukaradhya, G.J.; Meng, X.-J.; Calvert, J.G.; Roof, M.; Lager, K.M. Inactivated and subunit vaccines against porcine reproductive and respiratory syndrome: Current status and future direction. Vaccine 2015, 33, 3065–3072. [Google Scholar] [CrossRef] [PubMed]
- Vanhee, M.; Delputte, P.L.; Delrue, I.; Geldhof, M.F.; Nauwynck, H.J. Development of an experimental inactivated PRRSV vaccine that induces virus-neutralizing antibodies. Vet. Res. 2009, 40, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zuckermann, F.A.; Garcia, E.A.; Luque, I.D.; Christopher-Hennings, J.; Doster, A.; Brito, M.; Osorio, F. Assessment of the efficacy of commercial porcine reproductive and respiratory syndrome virus (PRRSV) vaccines based on measurement of serologic response, frequency of gamma-IFN-producing cells and virological parameters of protection upon challenge. Vet. Microbiol. 2007, 123, 69–85. [Google Scholar] [CrossRef] [PubMed]
- CVMP European Public Assessment Report [EPAR]. For Aivlosin. 2013. Available online: https://www.ema.europa.eu/en/documents/variation-report/aivlosin-v-c-83-x-0051-epar-assessment-report-extension_en.pdf (accessed on 10 October 2013).
- Rodriguez, A.L.; Berge, A.C.; Ramage, C.; Saltzman, R.; Domangue, R.J.; Gnozzio, M.J.; Muller, A.; Sierra, P.; Benchaoui, H.A. Evaluation of the clinical efficacy of a water soluble formulation of tylvalosin in the control of enzootic pneumonia associated with Mycoplasma hyopneumoniae and Pasteurella multocida in pigs. Porc. Heal. Manag. 2020, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Z.; Deng, X.; Li, J.; Li, T.; Lv, Y. Tylvalosin administration in pregnant sows attenuates the enlargement and bluish coloration of inguinal lymph nodes in newborn piglets. Res. Vet. Sci. 2019, 125, 148–152. [Google Scholar] [CrossRef]
- Zhang, J.; Bai, J.; Sun, Y.; Liu, X.; Gao, Y.; Wang, X.; Yang, Y.; Jiang, P. Comparison of pathogenicity of different subgenotype porcine reproductive and respiratory syndrome viruses isolated in China. Microb. Pathog. 2022, 168, 105607. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, Z.; Ding, Y.; Ge, X.; Guo, X.; Yang, H. NADC30-like Strain of Porcine Reproductive and Respiratory Syndrome Virus, China. Emerg. Infect. Dis. 2015, 21, 2256–2257. [Google Scholar] [CrossRef]
- Wang, L.-J.; Xie, W.; Chen, X.-X.; Qiao, S.; Zhao, M.; Gu, Y.; Zhao, B.-L.; Zhang, G. Molecular epidemiology of porcine reproductive and respiratory syndrome virus in Central China since 2014: The prevalence of NADC30-like PRRSVs. Microb. Pathog. 2017, 109, 20–28. [Google Scholar] [CrossRef]
- Xu, H.; Song, S.; Zhao, J.; Leng, C.; Fu, J.; Li, C.; Tang, Y.; Xiang, L.; Peng, J.; Wang, Q.; et al. A potential endemic strain in China: NADC34-like porcine reproductive and respiratory syndrome virus. Transbound. Emerg. Dis. 2020, 67, 1730–1738. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Y.; Ma, Z.; Feng, W.H. Recombinant Kluyveromyces lactis expressing highly pathogenic porcine reproductive and respiratory syndrome virus GP5 elicits mucosal and cell-mediated immune responses in mice. J. Vet. Sci. 2014, 15, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Xu, H.; Li, C.; Tang, Y.-D.; An, T.-Q.; Li, Z.; Liu, C.; Song, S.; Zhao, J.; Leng, C.; et al. Long-Term Genome Monitoring Retraces the Evolution of Novel Emerging Porcine Reproductive and Respiratory Syndrome Viruses. Front. Microbiol. 2022, 13, 885015. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wang, J.; Bai, X.; Ji, G.; Yan, H.; Li, Y.; Wang, Y.; Tan, F.; Xiao, Y.; Li, X.; et al. Pathogenicity comparison between highly pathogenic and NADC30-like porcine reproductive and respiratory syndrome virus. Arch. Virol. 2016, 161, 2257–2261. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Xu, H.; Zhao, J.; Leng, C.; Xiang, L.; Li, C.; Fu, J.; Tang, Y.-D.; Peng, J.; Wang, Q.; et al. Pathogenicity of NADC34-like PRRSV HLJDZD32-1901 isolated in China. Vet. Microbiol. 2020, 246, 108727. [Google Scholar] [CrossRef]
- Zhenzhong, W.; Chuanxiang, Q.; Shengqiang, G.; Jinming, L.; Yongxin, H.; Xiaoyue, Z.; Yan, L.; Naijun, H.; Xiaodong, W.; Zhiliang, W.; et al. Genetic variation and evolution of attenuated African swine fever virus strain isolated in the field: A review. Virus Res. 2022, 319, 198874. [Google Scholar] [CrossRef]
- Guedes, R.M.C.; França, S.A.; Machado, G.S.; Blumer, M.A.; Cruz, E.C.D.C. Use of tylvalosin-medicated feed to control porcine proliferative enteropathy. Vet. Rec. 2009, 165, 342–345. [Google Scholar] [CrossRef]
- Zhao, Z.; Tang, X.; Zhao, X.; Zhang, M.; Zhang, W.; Hou, S.; Yuan, W.; Zhang, H.; Shi, L.; Jia, H.; et al. Tylvalosin exhibits anti-inflammatory property and attenuates acute lung injury in different models possibly through suppression of NF-kappaB activation. Biochem. Pharmacol. 2014, 90, 73–87. [Google Scholar] [CrossRef]
- Shinkai, M.; Henke, M.O.; Rubin, B.K. Macrolide antibiotics as immunomodulatory medications: Proposed mechanisms of action. Pharmacol. Ther. 2008, 117, 393–405. [Google Scholar] [CrossRef]
- Notkins, A.L.; Mergenhagen, S.E.; Howard, R.J. Effect of Virus Infections on the Function of the Immune System. Annu. Rev. Microbiol. 1970, 24, 525–538. [Google Scholar] [CrossRef]
- Ding, X.; Yan, Y.; Li, X.; Li, K.; Ciric, B.; Yang, J.; Zhang, Y.; Wu, S.; Xu, H.; Chen, W.; et al. Silencing IFN-γ Binding/Signaling in Astrocytes versus Microglia Leads to Opposite Effects on Central Nervous System Autoimmunity. J. Immunol. 2015, 194, 4251–4264. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Cui, C.; Zhang, S.; Deng, X.; Cai, X.; Wang, G. Tylvalosin Tartrate Improves the Health Status of Swine Herds during Immunization with Porcine Reproductive and Respiratory Syndrome Virus-Inactivated Vaccine. Vet. Sci. 2023, 10, 12. https://doi.org/10.3390/vetsci10010012
Zhang Q, Cui C, Zhang S, Deng X, Cai X, Wang G. Tylvalosin Tartrate Improves the Health Status of Swine Herds during Immunization with Porcine Reproductive and Respiratory Syndrome Virus-Inactivated Vaccine. Veterinary Sciences. 2023; 10(1):12. https://doi.org/10.3390/vetsci10010012
Chicago/Turabian StyleZhang, Qianru, Chenchen Cui, Siyu Zhang, Xiaohong Deng, Xuehui Cai, and Gang Wang. 2023. "Tylvalosin Tartrate Improves the Health Status of Swine Herds during Immunization with Porcine Reproductive and Respiratory Syndrome Virus-Inactivated Vaccine" Veterinary Sciences 10, no. 1: 12. https://doi.org/10.3390/vetsci10010012
APA StyleZhang, Q., Cui, C., Zhang, S., Deng, X., Cai, X., & Wang, G. (2023). Tylvalosin Tartrate Improves the Health Status of Swine Herds during Immunization with Porcine Reproductive and Respiratory Syndrome Virus-Inactivated Vaccine. Veterinary Sciences, 10(1), 12. https://doi.org/10.3390/vetsci10010012




