Effect of Maturation with American Oak Chips on the Volatile and Sensory Profile of a Cabernet Sauvignon Rosé Wine and Its Comparison with Commercial Wines
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Description of the Experiment
2.1.1. Variety and Origin of the Grapes
2.1.2. Winemaking Process
2.2. Physicochemical Characterization of Wines
2.2.1. pH
2.2.2. Titratable Acidity
2.2.3. Reducing Sugars
2.2.4. Free and Total Sulfur Dioxide
2.2.5. Volatile Acidity
2.2.6. Total Anthocyanins Content
2.2.7. Alcohol Content
2.2.8. Total Phenolics Content Determination
2.3. Volatile Compounds Analysis
2.3.1. Headspace Solid-Phase Micro Extraction Procedure (HS-SPME)
2.3.2. Volatile Compounds Identification
2.4. Sensory Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties Analysis
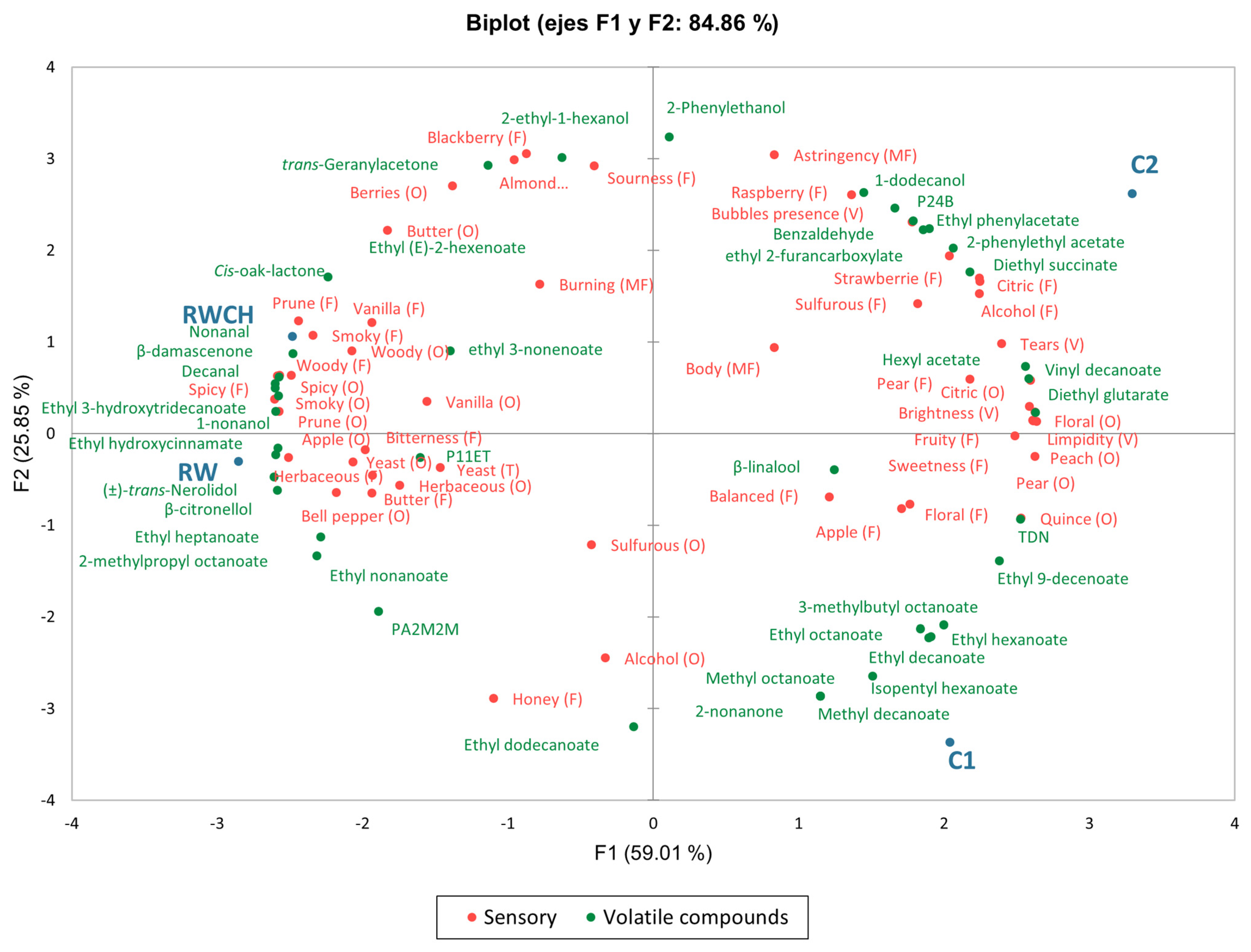
3.2. Volatile Analysis
3.3. Sensory Analysis Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bevan, A. Mediterranean containerization. Curr. Anthr. 2014, 55, 387–418. [Google Scholar] [CrossRef]
- Chira, K.; González-Centeno, M.R.; Teissedre, P.L. Wine Ageing in Oak Barrel: Effect of Toasting Process. Agri. Res. Technol. Open Access J. 2017, 12, 555847. [Google Scholar] [CrossRef]
- Návojská, J.; Brandes, W.; Nauer, S.; Eder, R.; Frančáková, H. Influence of different oak chips on aroma compounds in wine. J. Microbiol. Biotechnol. Food Sci. 2012, 1, 957–971. Available online: https://www.researchgate.net/publication/266348739_Influence_of_different_oak_chips_on_aroma_compounds_in_wine (accessed on 2 April 2023).
- Rodríguez-Rodríguez, P.; Gómez-Plaza, E. Differences in the extraction of volatile compounds from oak chips in wine and model solutions. Am. J. Enol. Vitic. 2011, 62, 127–132. [Google Scholar] [CrossRef]
- Rubio-Bretón, P.; Garde-Cerdán, T.; Martínez, J. Use of Oak Fragments during the Aging of Red Wines. Effect on the Phenolic, Aromatic, and Sensory Composition of Wines as a Function of the Contact Time with the Wood. Beverages 2018, 4, 102. [Google Scholar] [CrossRef]
- Hidalgo, T. Elaboración de vinos blancos y rosados. In Tratado de Enología, 3rd ed.; Mundi-Prensa: Madrid, Spain, 2018; p. 916. [Google Scholar]
- OIV Focus. The Rosé Wine Market. 2015. Available online: https://www.oiv.int/public/medias/3103/focus-2015-les-vins-roses-en.pdf (accessed on 2 April 2023).
- Amerine, M.; Singleton, V. Wine: An Introduction for Americans; University of California Press: Berkeley, CA, USA, 1965; p. 125. [Google Scholar]
- Koussissi, E.; Dourtoglou, V.G.; Ageloussis, G.; Paraskevopoulos, Y.; Dourtoglou, T.; Paterson, A.; Chatzilazarou, A. Influence of toasting of oak chips on red wine maturation from sensory and gas chromatographic headspace analysis. Food Chem. 2009, 114, 1503–1509. [Google Scholar] [CrossRef]
- Cano-López, M.; Bautista-Ortín, A.B.; Pardo-Mínguez, F.; López-Roca, J.M.; Gómez-Plaza, E. Sensory descriptive analysis of a red wine aged with oak chips in stainless steel tanks or used barrels: Effect of the contact time and size of the oak chips. J. Food Qual. 2008, 31, 645–660. [Google Scholar] [CrossRef]
- Stegăruș, D.I.; Călugăr, A.; Tanase, C.; Muscă, A.; Botoran, O.R.; Manolache, M.; Babeș, A.C.; Bunea, C.; Gál, E.; Bunea, A.; et al. Influence of Oak Chips and Oak Barrel Ageing on Volatile Profile in Chardonnay Wine of Romania. Appl. Sci. 2021, 11, 3691. [Google Scholar] [CrossRef]
- International Organization of Vineyard and Wine: World Statistics. Available online: https://www.oiv.int/what-we-do/global-report?oiv (accessed on 2 April 2023).
- Rosé Wines World Tracking. Available online: https://www.rosewinesworldtracking.com/ (accessed on 2 April 2023).
- Lorey, T. The Success of Rosé Wine in France: The Millennial Revolution. Cornell Hosp. Q. 2021, 62, 357–370. [Google Scholar] [CrossRef]
- Análisis del Consumidor de Vino en México. 2021. Available online: https://www.lamarcalab.com/wp-content/uploads/dlm_uploads/2021/10/Analisis-del-consumo-de-vino-en-Mexico-2021-lamarcalab.com-3.pdf (accessed on 3 April 2023).
- Fenómeno Hidrometeorológico. DOLORES HIDALGO C.I.N. Available online: https://servicios-ssp.guanajuato.gob.mx/atlas/hm/hm_dolores_hidalgo.pdf (accessed on 15 August 2023).
- Normales Climatológicas Por Estado. Available online: https://smn.conagua.gob.mx/tools/RESOURCES/Normales_Climatologicas/Mensuales/gto/mes11051.TXT (accessed on 15 August 2023).
- OIV Focus 2017: Distribution of the World’s Grapevine Varieties. Available online: https://www.oiv.int/public/medias/5888/en-distribution-of-the-worlds-grapevine-varieties.pdf (accessed on 3 August 2023).
- Eglinton, J.M.; McWilliam, S.J.; Fogarty, M.W.; Francis, I.L.; Kwiatkowski, M.J.; Høj, P.B.; Henschke, P.A. The effect of Saccharomyces bayanus-mediated fermentation on the chemical composition and aroma profile of Chardonnay wine. Aust. J. Grape Wine Res. 2000, 6, 190–196. [Google Scholar] [CrossRef]
- Espitia-López, J. Estudio de los Compuestos Formados Durante el Añejamiento en Madera del Vino Tino Merlot. Ph.D. Thesis, Universidad Autónoma Metropolitana, Mexico City, Mexico, 2023. [Google Scholar]
- Jacobson, J. Analytical procedures. In Introduction to Wine Laboratory Practices and Procedures; Springer Science Business Media, Inc.: New York, NY, USA, 2006; pp. 271, 273, 280, 292. Available online: https://www.tau.ac.il/~chemlaba/Files/jean-l-jacobson-introduction-to-wine-laboratory-practices-and-procedures.pdf (accessed on 8 February 2016).
- ASBC. Beer 12-B: Reducing sugars: Lane-Eynon volumetric method. In American Society of Brewing Chemists, Methods of Analysis; American Society of Brewing Chemists: St. Paul, MN, USA, 2004. [Google Scholar]
- Giusti, M.; Wrolstad, R. Characterization and measurement of anthocyanins by UV-Visible spectroscopy. In Current Protocols in Food Analytical Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001; pp. F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- ASBC. Beer 4-A: Alcohol: Beer and distillate measured volumetrically. In American Society of Brewing Chemists, Methods of Analysis; American Society of Brewing Chemists: St. Paul, MN, USA, 2004. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagent. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Hernández-Carapia, M.A. Estudio de la Composición Volátil Y Perfil Sensorial de un Vino Rosado Elaborado Con Uvas Cabernet Sauvignon, Madurado en Botella Con la Adición de Chips de Madera. Master’s Thesis, Universidad Autónoma Metropolitana, Mexico City, Mexico, 2007. [Google Scholar]
- Gomide, A.I.; Silva, R.d.C.d.S.N.; Nascimento, M.; Minim, L.A.; Minim, V.P.R. Study of the influence of line scale length (9 and 15 cm) on the sensory evaluations of two descriptive methods. J. Food Sci. Technol. 2021, 58, 2815–2824. [Google Scholar] [CrossRef] [PubMed]
- NOM-199-SCFI-2017. Bebidas Alcohólicas-Denominación, Especificaciones Fisicoquímicas, Información Comercial y Métodos de Prueba. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5502882&fecha=30/10/2017#gsc.tab=0 (accessed on 7 April 2023).
- Maximum Acceptable Limits of Various Substances Contained in Wine. Available online: https://www.oiv.int/es/standards/compendium-of-international-methods-of-wine-and-must-analysis/annex-c/annex-c-maximum-acceptable-limits-of-various-substances/maximum-acceptable (accessed on 18 April 2023).
- International Standard for the Labelling of Wines. Available online: https://www.oiv.int/public/medias/8175/en-oiv-wine-labelling-standard-2022.pdf (accessed on 20 April 2023).
- Călugăr, A.; Coldea, T.E.; Pop, C.R.; Pop, T.I.; Babeș, A.C.; Bunea, C.I.; Manolache, M.; Gal, E. Evaluation of Volatile Compounds during Ageing with Oak Chips and Oak Barrel of Muscat Ottonel Wine. Processes 2020, 8, 1000. [Google Scholar] [CrossRef]
- Călugăr, A.; Pop, T.I.; Coldea, T.E.; Mureșan, I.; Gal, E.; Manolache, M. Eonological parameters of Fetească regală wines subjected to aging with oak chips of and barrel during three periods of time. J. Hortic. Sci. Biotechnol. 2019, 23, 78–83. [Google Scholar]
- Jordão, A.M.; Lozano, V.; González-SanJosé, M.L. Influence of Different Wood Chip Extracts Species on Color Changes and Anthocyanin Content in Synthetic Wine Solutions. Foods 2019, 8, 254. [Google Scholar] [CrossRef] [PubMed]
- Barrera-García, V.D.; Gougeon, R.D.; Majo, D.D.; Aguirre, C.; Voilley, A.; Chassagne, D. Different sorption behaviours for wine polyphenols in contact with oak wood. J. Agric. Food Chem. 2007, 55, 7021–7027. [Google Scholar] [CrossRef]
- Ugliano, M.; Slaghenaufi, D.; Picariello, L.; Olivieri, G. Oxygen and SO2 Consumption of Different Enological Tannins in Relationship to Their Chemical and Electrochemical Characteristics. J. Agric. Food Chem. 2020, 68, 13418–13425. [Google Scholar] [CrossRef] [PubMed]
- Psarra, C.; Gortzi, O.; Makris, D.P. Kinetics of polyphenol extraction from wood chips in wine model solutions: Effect of chip amount and botanical species. J. Inst. Brew. 2015, 121, 207–212. [Google Scholar] [CrossRef]
- Santos, F.; Correia, A.C.; Ortega-Heras, M.; García-Lomillo, J.; González-San José, M.L.; Jordão, A.M.; Ricardo-da-Silva, J.M. Acacia, cherry, and oak wood chips used on a short aging period of rosé wines: Effects on general phenolic parameters, volatile composition, and sensory profile. J. Sci. Food. Agric. 2019, 99, 3588–3603. [Google Scholar] [CrossRef]
- Romano, P.; Braschi, G.; Siesto, G.; Patrignani, F.; Lanciotti, R. Role of Yeasts on the Sensory Component of Wines. Foods 2022, 11, 1921. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Seo, M.; Riu, M.; Cotta, J.; Block, D.; Dokoozlian, N.; Ebeler, S. Vine Microclimate and Norisoprenoid Concentration in Cabernet Sauvignon Grapes and Wines. Am. J. Enol. Vitic. 2007, 58, 291–301. [Google Scholar] [CrossRef]
- Slaghenaufi, D.; Vanzo, L.; Luzzini, G.; Arapitsas, P.; Marangon, M.; Curioni, A.; Mattivi, F.; Piombino, P.; Moio, L.; Versari, A.; et al. Monoterpenoids and norisoprenoids in Italian red wines. OENO One 2022, 56, 185–193. [Google Scholar] [CrossRef]
- Waterhouse, A.; Sacks, G.; Jeffery, D. Esters. In Understanding Wine Chemistry; John Wiley & Sons: Chichester, UK, 2016; pp. 57–67. [Google Scholar] [CrossRef]
- Saerens, S.M.; Delvaux, F.R.; Verstrepen, K.J.; Thevelein, J.M. Production and biological function of volatile esters in Saccharomyces cerevisiae. Microb. Biotechnol. 2010, 3, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Daviet, L.; Schalk, M.; Siewers, V.; Nielsen, J. Establishing a platform cell factory through engineering of yeast acetyl-CoA metabolism. Metab Eng. 2013, 15, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Vilanova, M.; Siebert, T.E.; Varela, C.; Pretorius, I.S.; Henschke, P.A. Effect of ammonium nitrogen supplementation of grape juice on wine volatiles and non-volatiles composition of the aromatic grape variety Albarino. Food Chem. 2012, 133, 124–131. [Google Scholar] [CrossRef]
- Prusova, B.; Humaj, J.; Sochor, J.; Baron, M. Formation, Losses, Preservation and Recovery of Aroma Compounds in the Winemaking Process. Fermentation 2022, 8, 93. [Google Scholar] [CrossRef]
- Molina, A.M.; Guadalupe, V.; Varela, C.; Swiegers, J.H.; Pretorius, I.S.; Agosin, E. Differential synthesis of fermentative aroma compounds of two related commercial wine yeast strains. Food Chem. 2009, 117, 189–195. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, C.; Gonçalves, C.; Castillo, M.; Câmara, J.S. An Approach of the Madeira Wine Chemistry. Beverages 2020, 6, 12. [Google Scholar] [CrossRef]
- Guchu, E.; Díaz-Maroto, M.C.; Díaz-Maroto, I.J.; Vila-Lameiro, P.; Pérez-Coello, M.S. Influence of the species and geographical location on volatile composition of Spanish oak wood (Quercus petraea Liebl. and Quercus robur L.). J. Agric. Food Chem. 2006, 54, 3062–3066. [Google Scholar] [CrossRef]
- Ortega-Heras, M.; González-Huerta, C.; Herrera, P.; González-Sanjosé, M.L. Changes in wine volatile compounds of varietal wines during ageing in wood barrels. Anal. Chim. Acta 2004, 513, 341–350. [Google Scholar] [CrossRef]
- Abbott, N.; Puech, J.L. Determination of the Aroma Threshold of the cis and trans Racemic Forms of β-Methyl-γ-Octalactone by Gas Chromatography-Sniffing Analysis. Am. J. Enol. Vitic. 1995, 46, 292–294. [Google Scholar] [CrossRef]
- Lee-Rangel, H.A.; Mendoza-Martinez, G.D.; Diaz de León-Martínez, L.; Relling, A.E.; Vazquez-Valladolid, A.; Palacios-Martínez, M.; Hernández-García, P.A.; Chay-Canul, A.J.; Flores-Ramirez, R.; Roque-Jiménez, J.A. Application of an Electronic Nose and HS-SPME/GC-MS to Determine Volatile Organic Compounds in Fresh Mexican Cheese. Foods 2022, 11, 1887. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Yang, W.; Qian, X.; Zhang, X.; Ling, M.; Yang, L.; Shi, Y.; Duan, C.; Lan, Y. Comparison of Chemical and Sensory Profiles between Cabernet Sauvignon and Marselan Dry Red Wines in China. Foods 2023, 12, 1110. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Xie, S.; Guan, X.; Song, C.; Zhang, Z.; Meng, J. Methoxypyrazines Biosynthesis and Metabolism in Grape: A review. Food Chem. 2018, 245, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.S.; Lacey, M.J. Methoxypyrazines of Grapes and Wines. In Chemistry of Wine Flavor; Waterhouse, A.L., Ebeler, S., Eds.; Oxford University Press: Washington, DC, USA, 1998; pp. 31–38. [Google Scholar]
- PubChem. Nonanal. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Nonanal#section=Color-Form (accessed on 7 May 2023).
- Pons, A.; Lavigne, V.; Eric, F.; Darriet, P.; Dubourdieu, D. Identification of Volatile Compounds Responsible for Prune Aroma in Prematurely Aged Red Wines. J. Agric. Food Chem. 2008, 56, 5285–5290. [Google Scholar] [CrossRef] [PubMed]
- Perestrelo, R.; Silva, C.; Câmara, J.S. Madeira Wine Volatile Profile. A Platform to Establish Madeira Wine Aroma Descriptors. Molecules 2019, 24, 3028. [Google Scholar] [CrossRef]
- Martínez-Avila, O.; Sánchez, A.; Font, X.; Barrena, R. Bioprocesses for 2-phenylethanol and 2-phenylethyl acetate production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 9991–10004. [Google Scholar] [CrossRef]
- PubChem. 1-Dodecanol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/1-Dodecanol#section=Experimental-Properties (accessed on 7 May 2023).
- Hu, L.; Liu, R.; Wang, X.; Zhang, X. The Sensory Quality Improvement of Citrus Wine through Co-Fermentations with Selected Non-Saccharomyces Yeast Strains and Saccharomyces cerevisiae. Microorganisms 2020, 8, 323. [Google Scholar] [CrossRef]
- Cao, W.; Shu, N.; Wen, J.; Yang, Y.; Jin, Y.; Lu, W. Characterization of the Key Aroma Volatile Compounds in Nine Different Grape Varieties Wine by Headspace Gas Chromatography–Ion Mobility Spectrometry (HS-GC-IMS), Odor Activity Values (OAV) and Sensory Analysis. Foods 2022, 11, 2767. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, Z. Volatile Compounds of Young Wines from Cabernet Sauvignon, Cabernet Gernischet and Chardonnay Varieties Grown in the Loess Plateau Region of China. Molecules 2010, 15, 9184–9196. [Google Scholar] [CrossRef]
- Raigón, M.D.; García-Martínez, M.D.; Chiriac, O.P. Nutritional Characterization of a Traditional Cultivar of Tomato Grown Under Organic Conditions—Cv. “Malacara”. Front. Nutr. 2022, 8, 810812. [Google Scholar] [CrossRef] [PubMed]
- Tai-Xin, Y.; Ming, C.; Chang-Zheng, S.; Mei-Ying, L.; Jiang-Fei, M.; Zhen-Wen, Z.; Mei-Hua, L. Aroma Characterization of Cabernet Sauvignon Wine from the Plateau of Yunnan (China) with Different Altitudes Using SPME-GC/MS. Int. J. Food Prop. 2015, 18, 1584–1596. [Google Scholar] [CrossRef]
- Schreier, P.; Jennings, W.G. Flavour composition of wines: A review. Crit. Rev. Food Sci. Nutr. 1979, 12, 59–111. [Google Scholar] [CrossRef] [PubMed]


| Property | Rosé Wine without Chips | Rosé Wine Aged with Chips | C1 | C2 |
|---|---|---|---|---|
| pH | 3.37 ± 0.03 a | 3.36 ± 0.03 a | 3.22 ± 0.01 b | 2.98 ± 0.01 c |
| Alcohol (% v/v) | 12.47 ± 0.23 a | 12.76 ± 0.29 a | 12.41 ± 0.47 a | 12.38 ± 0.29 a |
| Reducing sugars (g/L) | 6.33 ± 0.17 a | 6.34 ± 0.30 a | 4.63 ± 0.11 b | 4.46 ± 0.06 b |
| Titratable acidity (g of tartaric acid/L) | 5.77 ± 0.15 b | 5.47 ± 0.05 c | 6.4 ± 0.03 a | 5.74 ± 0.03 b |
| Volatile acidity (g of acetic acid/L) | 0.76 ±0.03 a | 0.70 ± 0.04 a | 0.28 ± 0.01 c | 0.34 ± 0.01 b |
| Total SO₂ (mg/L) | 61.46 ± 1.76 b | 52.76 ± 0.87 c | 95.57 ± 1.47 a | 104.96 ± 6.77 a |
| Free SO₂ (mg/L) | 29.79 ± 0.88 b | 23.58 ± 1.76 d | 26.02 ± 1.95 c | 34.08 ± 1.52 a |
| Total anthocyanin (mg of malvidin-3-glucoside/L) | 62.78 ± 2.11 a | 58.16 ± 1.49 b | 4.55 ± 0.13 d | 6.34 ± 0.08 c |
| Total phenolics (mg/L) | 349.4 ± 5.0 b | 394.2 ± 9.0 a | 168.5 ± 0.98 c | 161.44 ± 2.67 d |
| Volatile Compound | RWCH | RW | C1 | C2 |
|---|---|---|---|---|
| Esters | ||||
| Ethyl 2-furancarboxylate | 38.71 b | 27.02 b | 41.05 b | 100 a |
| Propanoic acid, 2-methyl-, 1-(1,1-dimethylethyl)-2-methyl-1,3-propanediyl ester | 100 a | 92.49 a | 94.50 a | 63.08 a |
| Ethyl hexanoate | 64.18 a | 65.08 a | 100 a | 77.16 a |
| Hexyl acetate | 32.63 b | 31.79 b | 68.36 a | 100 a |
| Ethyl (E)-2-hexenoate | 100 a | 99.45 a | 49.46 c | 72.20 b |
| Ethyl heptanoate | 97.47 a | 100 a | 42.27 b | N.D. |
| Diethyl succinate | 16.21 c | 15.01 c | 31.80 b | 100 a |
| Methyl octanoate | N.D. | N.D. | 100 a | N.D. |
| Ethyl octanoate | 38.49 c | 38.31 c | 100 a | 63.01 b |
| Isopentyl hexanoate | 74.72 b | 76.77 b | 100 a | 79.81 b |
| 2-phenylethyl acetate | 42.37 b | 34.08 b | 39.73 b | 100 a |
| Ethyl nonanoate | 100 a | 87.90 a | 65.02 b | N.D. |
| Ethyl 3-nonenoate | 76.40 a | 100 a | N.D. | N.D. |
| Ethyl decanoate | 28.65 c | 29.22 c | 100 a | 53.50 b |
| Methyl decanoate | N.D. | N.D. | 100 a | N.D. |
| 2-methylpropyl octanoate | 100 a | 85.68 a | 75.11 a | 40.57 b |
| 3-methylbutyl octanoate | 82.57 a | 78.70 a | 100 a | 86.81 a |
| Ethyl hydroxycinnamate | 100 a | 84.34 a | 29.00 b | N.D. |
| Ethyl dodecanoate | 69.44 b | 72.42 b | 100 a | 50.09 b |
| Ethyl phenylacetate | 42.13 b | 40.60 b | 39.06 b | 100 a |
| Ethyl 9-decenoate | N.D. | N.D. | 100 a | 66.35 b |
| Ethyl 3-hydroxytridecanoate | 89.52 a | 100 a | N.D. | N.D. |
| Diethyl glutarate | N.D. | N.D. | 69.70 b | 100 a |
| Vinyl decanoate | N.D. | N.D. | 57.41 b | 100 a |
| Alcohols | ||||
| 2-ethyl-1-hexanol | 75.21 a | 84.86 a | N.D. | 100 a |
| 2-Phenylethanol | 76.08 b | 63.49 b | 25.63 c | 100 a |
| 1-nonanol | 100 a | 85.14 b | 15.27 c | N.D. |
| 1-dodecanol | 65.46 b | 65.38 b | 57.43 b | 100 a |
| Phenol, 2,4-bis(1,1-dimethylethyl)- | 11.86 b | 8.22 b | N.D. | 100 a |
| Terpenes | ||||
| β-citronellol | 92.84 a | 100 a | 35.87 b | N.D. |
| (±)-trans-Nerolidol | 100 a | 88.74 a | 31.14 b | N.D. |
| β-linalool | N.D. | N.D. | 81.80 a | 100 a |
| Aldehydes | ||||
| Nonanal | 100 a | 78.85 a | 19.96 b | 25.51 b |
| Decanal | 98.08 a | 100 a | N.D. | N.D. |
| Benzaldehyde | N.D. | N.D. | N.D. | 100 a |
| Lactones | ||||
| Cis-oak-lactone | 100 a | N.D. | N.D. | N.D. |
| Norisoprenoids | ||||
| β-damascenone | 100 a | 87.17 a | N.D. | N.D. |
| trans-Geranylacetone | 100 a | 86.69 a | 42.28 b | 92.38 a |
| Naphthalene, 1,2-dihydro-1,1,6-trimethyl- | N.D. | N.D. | 100 a | 83.77 a |
| Ketones | ||||
| 2-nonanone | N.D. | N.D. | 100 | N.D. |
| Acetals | ||||
| 1-(1-ethoxyethoxy)-pentane | N.D. | 100 | N.D. | N.D. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Carapia, M.Á.; Verde-Calvo, J.R.; Escalona-Buendía, H.B.; Peña-Álvarez, A. Effect of Maturation with American Oak Chips on the Volatile and Sensory Profile of a Cabernet Sauvignon Rosé Wine and Its Comparison with Commercial Wines. Beverages 2023, 9, 72. https://doi.org/10.3390/beverages9030072
Hernández-Carapia MÁ, Verde-Calvo JR, Escalona-Buendía HB, Peña-Álvarez A. Effect of Maturation with American Oak Chips on the Volatile and Sensory Profile of a Cabernet Sauvignon Rosé Wine and Its Comparison with Commercial Wines. Beverages. 2023; 9(3):72. https://doi.org/10.3390/beverages9030072
Chicago/Turabian StyleHernández-Carapia, Miguel Ángel, José Ramón Verde-Calvo, Héctor Bernardo Escalona-Buendía, and Araceli Peña-Álvarez. 2023. "Effect of Maturation with American Oak Chips on the Volatile and Sensory Profile of a Cabernet Sauvignon Rosé Wine and Its Comparison with Commercial Wines" Beverages 9, no. 3: 72. https://doi.org/10.3390/beverages9030072
APA StyleHernández-Carapia, M. Á., Verde-Calvo, J. R., Escalona-Buendía, H. B., & Peña-Álvarez, A. (2023). Effect of Maturation with American Oak Chips on the Volatile and Sensory Profile of a Cabernet Sauvignon Rosé Wine and Its Comparison with Commercial Wines. Beverages, 9(3), 72. https://doi.org/10.3390/beverages9030072