Advances in Extraction Techniques for Beer Flavor Compounds
Abstract
1. Introduction
2. Liquid-Phase Extraction Techniques
2.1. Liquid–Liquid Extraction (LLE)
2.2. Liquid-Phase Microextraction (LPME)
3. Solid-Phase Extraction Techniques
3.1. Solid-Phase Extraction (SPE)
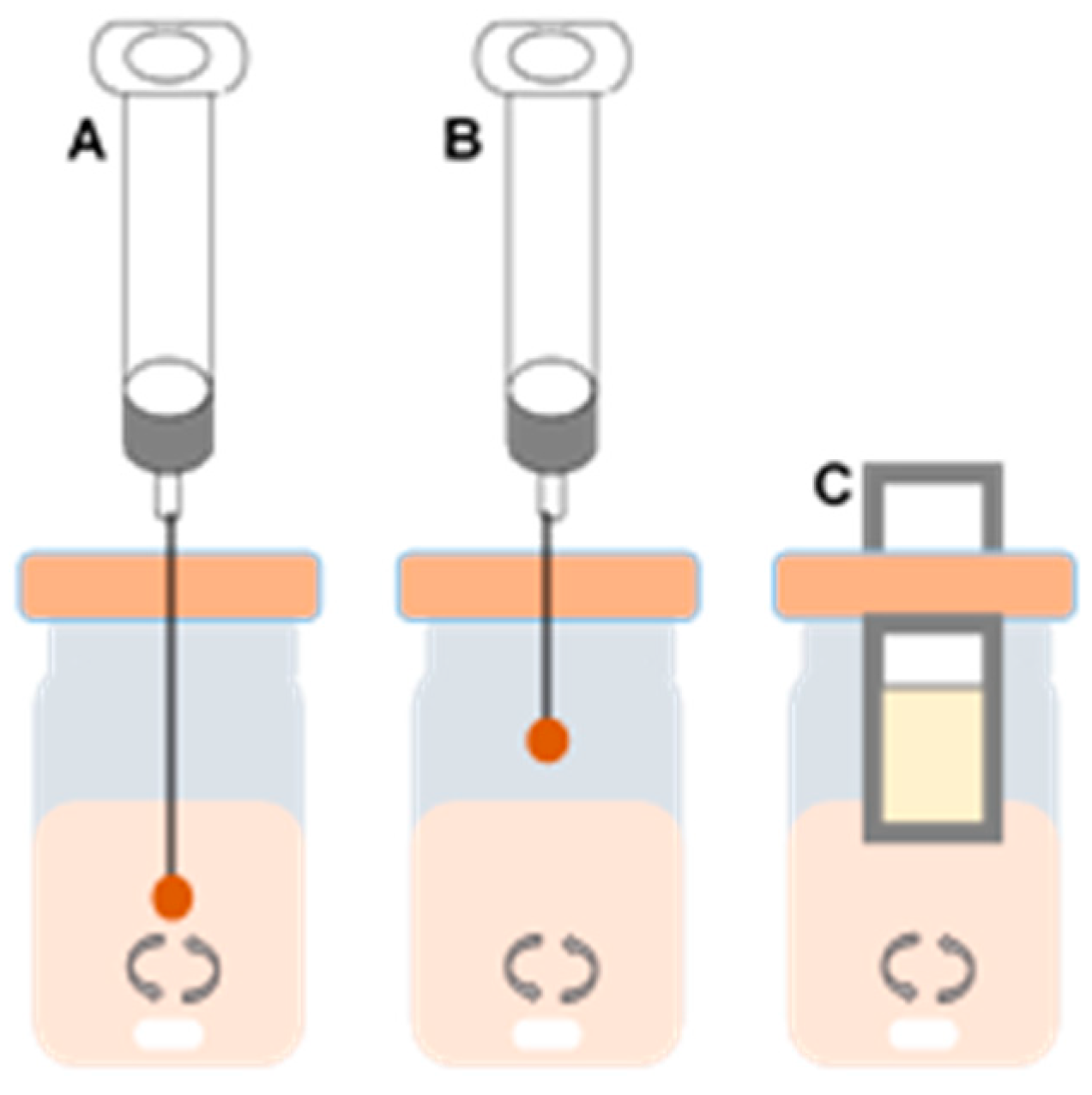
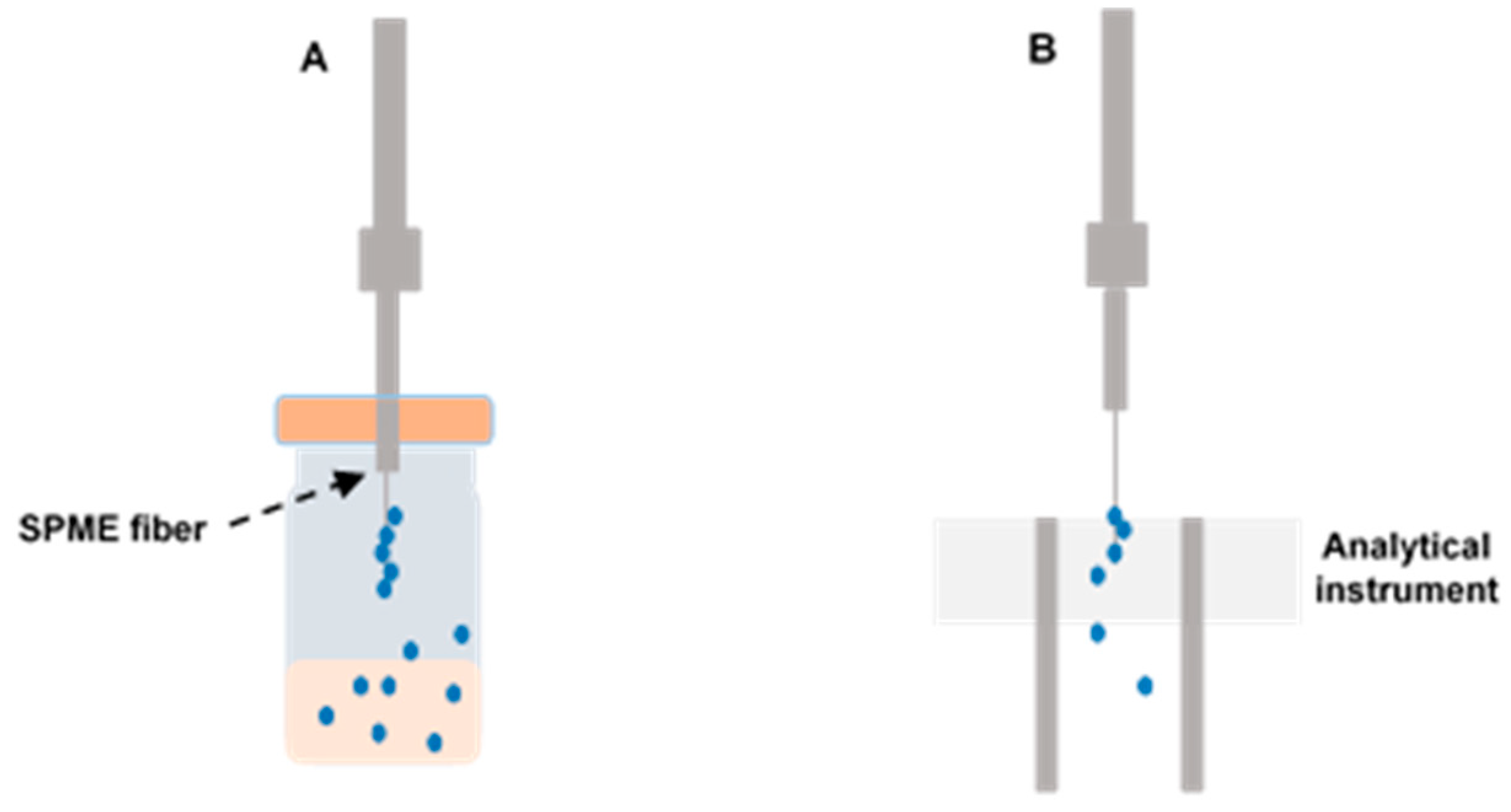
3.2. Solid-Phase Microextraction (SPME)
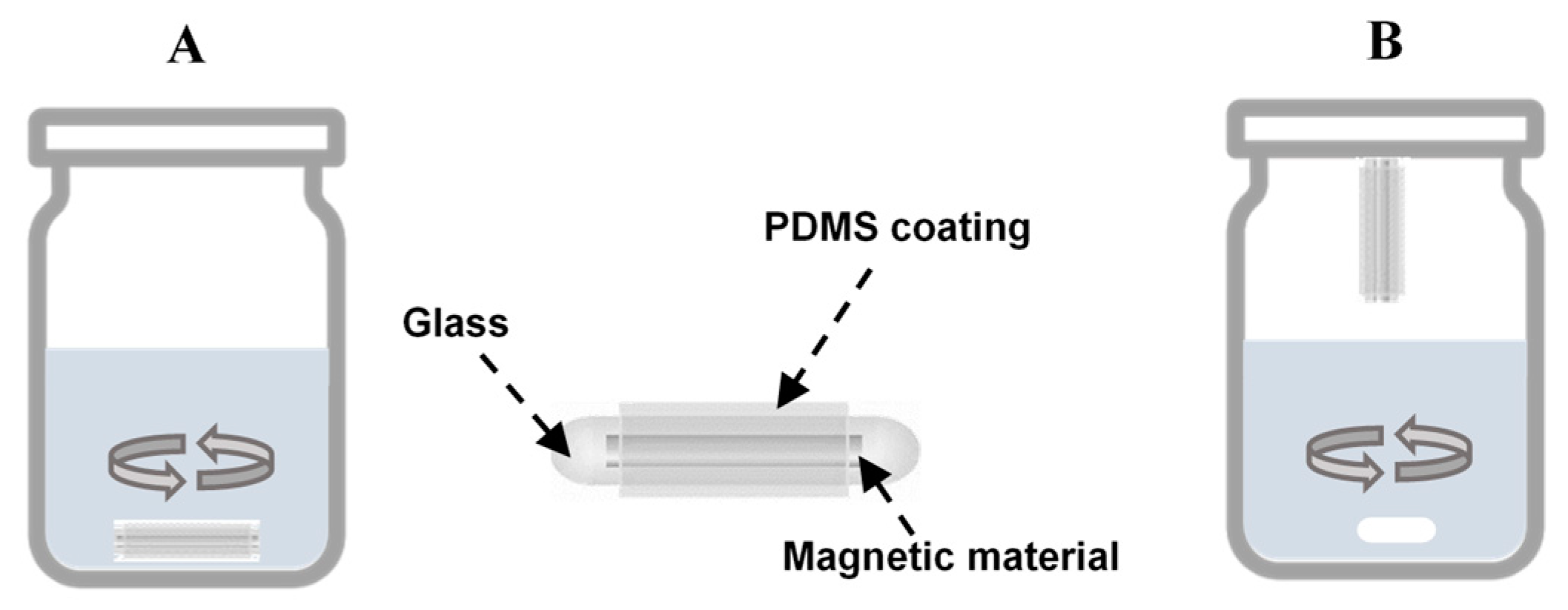
3.3. Stir Bar Sorptive Extraction (SBSE)
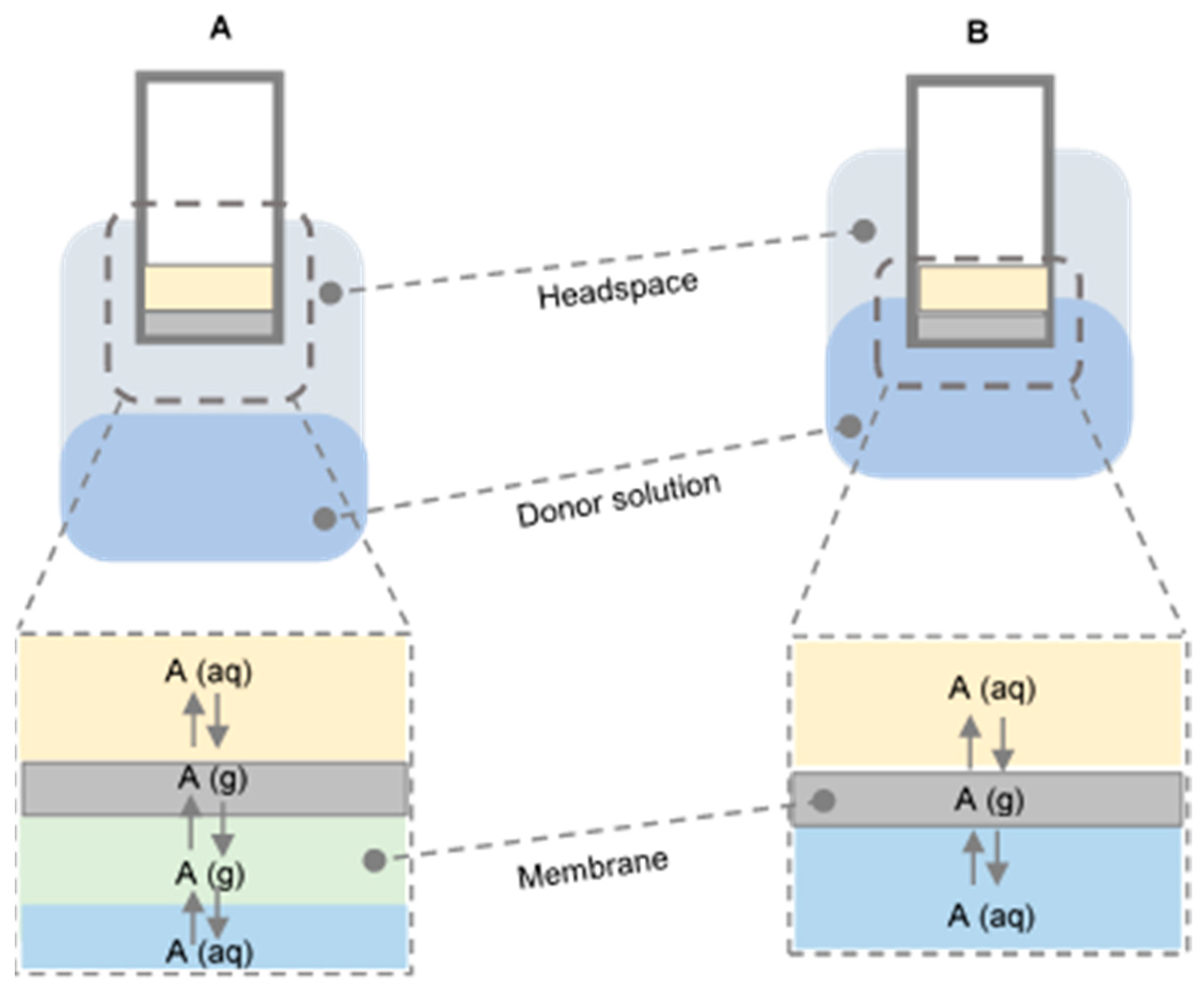
4. Membrane-Assisted Extraction Techniques
Gas Diffusion Microextraction (GDME)
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Smith, R.M. Before the injection—Modern methods of sample preparation for separation techniques. J. Chromatogr. A 2003, 1000, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guo, Z.; Wang, X.; Qiu, C. Sample preparation. J. Chromatogr. A 2008, 1184, 191–219. [Google Scholar] [CrossRef] [PubMed]
- Demeestere, K.; Dewulf, J.; De Witte, B.; Van Langenhove, H. Sample preparation for the analysis of volatile organic compounds in air and water matrices. J. Chromatogr. A 2007, 1153, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Hyötyläinen, T. Critical evaluation of sample pretreatment techniques. Anal. Bioanal. Chem. 2009, 394, 743–758. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.M.C.; da Silva, M.D.R.G.; Cabrita, M.J. Sampling techniques for the determination of volatile components in grape juice, wine and alcoholic beverages. In Comprehensive Sampling and Sample Preparation; Pawliszyn, J., Ed.; Elsevier; Academic Press: Oxford, UK, 2012. [Google Scholar]
- Pawliszyn, J. Sample preparation: Quo vadis? Anal. Chem. 2003, 75, 2543–2558. [Google Scholar] [CrossRef]
- Moreira, N.; Meireles, S.; Brandão, T.; de Pinho, P.G. Optimization of the HS-SPME-GC-IT/MS method using a central composite design for volatile carbonyl compounds determination in beers. Talanta 2013, 117, 523–531. [Google Scholar] [CrossRef]
- Lehnhardt, F.; Gastl, M.; Becker, T. Forced into aging: Analytical prediction of the flavor-stability of lager beer. A review. Crit. Rev. Food Sci. Nutr. 2018, 59, 2642–2653. [Google Scholar] [CrossRef]
- Guedes de Pinho, M.P.; Silva Ferreira, A.C. Role of Strecker aldehydes on beer flavour stability. Dev. Food Sci. 2006, 43, 529–532. [Google Scholar]
- Soares da Costa, M.; Gonçalves, C.; Ibsen, C.; de Pinho, P.G.; Ferreira, A.C.S. Further Insights into the Role of Methional and Phenylacetaldehyde in Lager Beer Flavor Stability. J. Agric. Food Chem. 2004, 52, 7911–7917. [Google Scholar] [CrossRef]
- Tankeviciute, A.; Kazlauskas, R.; Vickackaite, V. Headspace extraction of alcohols into a single drop. Analyst 2001, 126, 1674–1677. [Google Scholar] [CrossRef]
- Saraji, M. Dynamic headspace liquid-phase microextraction of alcohols. J. Chromatogr. A 2005, 1062, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.; Klotzbücher, B.; Wurzbacher, M.; Hanke, S.; Kattein, U.; Back, W.; Becker, T.; Krottenthaler, M. A new validation of relevant substances for the evaluation of beer aging depending on the employed boiling system. J. Inst. Brew. 2010, 116, 41–48. [Google Scholar] [CrossRef]
- Noel, S.C.; Liégeois, C.; Lermusieau, G.; Bodart, E.; Badot, C.; Collin, S. Release of deuterated nonenal during beer aging from labeled precursors synthesized in the boiling kettle. J. Agric. Food Chem. 1999, 47, 4323–4326. [Google Scholar] [CrossRef] [PubMed]
- Guido, L.F.; Carneiro, J.R.; Santos, J.R.; Almeida, P.J.; Rodrigues, J.A.; Barros, A.A. Simultaneous determination of E-2-nonenal and β-damascenone in beer by reversed-phase liquid chromatography with UV detection. J. Chromatogr. A 2004, 1032, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Schieberle, P.; Komarek, D. Changes in key aroma compounds during natural beer aging. ACS Symp. Ser. 2002, 836, 70–79. [Google Scholar]
- Vesely, P.; Lusk, L.; Basarova, G.; Seabrooks, J.; Ryder, D. Analysis of Aldehydes in Beer Using Solid-Phase Microextraction with On-Fiber Derivatization and Gas Chromatography/Mass Spectrometry. J. Agric. Food Chem. 2003, 51, 6941–6944. [Google Scholar] [CrossRef] [PubMed]
- Saison, D.; De Schutter, D.P.; Delvaux, F.; Delvaux, F.R. Determination of carbonyl compounds in beer by derivatisation and headspace solid-phase microextraction in combination with gas chromatography and mass spectrometry. J. Chromatogr. A 2009, 1216, 5061–5068. [Google Scholar] [CrossRef]
- Pizarro, C.; Perez-del-Notario, N.; Gonzalez-Saiz, J.M. Optimisation of a simple and reliable method based on headspace solid-phase microextraction for the determination of volatile phenols in beer. J. Chromatogr. A 2010, 1217, 6013–6021. [Google Scholar] [CrossRef]
- Andres-Iglesias, C.; Blanco, C.A.; García-Serna, J.; Pando, V.; Montero, O. Volatile Compound Profiling in Commercial Lager Regular Beers and Derived Alcohol-Free Beers after Dealcoholization by Vacuum Distillation. Food Anal. Methods 2016, 9, 3230–3241. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, Z.; Xiong, B. Preparation of novel solid-phase microextraction fibers by sol–gel technology for headspace solid-phase microextraction-gas chromatographic analysis of aroma compounds in beer. J. Chromatogr. A 2005, 1065, 287–299. [Google Scholar] [CrossRef]
- Saison, D.; De Schutter, D.P.; Uyttenhove, B.; Delvaux, F.; Delvaux, F.R. Contribution of staling compounds to the aged flavour of lager beer by studying their flavour thresholds. Food Chem. 2009, 114, 1206–1215. [Google Scholar] [CrossRef]
- Saison, D.; Vanbeneden, N.; De Schutter, D.P.; Daenen, L.; Mertens, T.; Delvaux, F.; Delvaux, F.R. Characterisation of the flavour and the chemical composition of lager beer after ageing in varying conditions. Brew. Sci. 2010, 63, 41–53. [Google Scholar]
- Malfliet, S.; Goiris, K.; Aerts, G.; Cooman, L. Analytical-Sensory Determination of Potential Flavour Deficiencies of Light Beers. J. Inst. Brew. 2009, 115, 49–63. [Google Scholar] [CrossRef]
- Ortiz, R.M. Analysis of Selected Aldehydes in Packaged Beer by Solid-Phase Microextraction (SPME)-Gas Chromatography (GC)-Negative Chemical Ionization Mass Spectrometry (NCIMS). J. Am. Soc. Brew. Chem. 2015, 73, 266–274. [Google Scholar] [CrossRef]
- Dennenlöhr, J.; Thörner, S.; Maxminer, J.; Rettberg, N. Analysis of selected staling aldehydes in wort and beer by GC-EI-MS/MS using HS-SPME with on-fiber derivatization. J. Am. Soc. Brew. Chem. 2020, 78, 284–298. [Google Scholar] [CrossRef]
- Lehnhardt, F.; Steiner, J.; Gastl, M.; Becker, T. Prediction Power and Accuracy of Forced Ageing—Matching Sensory and Analytical Results for Lager Beer. Brew. Sci. 2018, 71, 39–48. [Google Scholar]
- Carrillo, G.; Bravo, A.; Zufall, C. Application of Factorial Designs to Study Factors Involved in the Determination of Aldehydes Present in Beer by On-Fiber Derivatization in Combination with Gas Chromatography and Mass Spectrometry. J. Agric. Food Chem. 2011, 59, 4403–4411. [Google Scholar] [CrossRef]
- Sohrabvandi, S.; Mortazavian, A.M.; Rezaei, K. Advanced Analytical Methods for the Analysis of Chemical and Microbiological Properties of Beer. J. Food Drug Anal. 2011, 19, 202–222. [Google Scholar] [CrossRef]
- Ochiai, N.; Sasamoto, K.; Daishima, S.; Heiden, A.; Hoffmann, A. Determination of stale-flavor carbonyl compounds in beer by stir bar sorptive extraction with in-situ derivatization and thermal desorption–gas chromatography–mass spectrometry. J. Chromatogr. A 2003, 986, 101–110. [Google Scholar] [CrossRef]
- McCarthy, S.L. Analysis of Diacetyl and 2-3-pentanedione in beer by HPLC with fluorimetric detection. J. Am. Soc. Brew. Chem. 1995, 53, 178–181. [Google Scholar]
- Loukou, Z.; Zotou, A. Determination of biogenic amines as dansyl derivatives in alcoholic beverages by high-performance liquid chromatography with fluorimetric detection and characterization of the dansylated amines by liquid chromatography–atmospheric pressure chemical ionization mass spectrometry. J. Chromatogr. A 2003, 996, 103–113. [Google Scholar] [PubMed]
- Molins-Legua, C.; Campins-Falcó, P. Solid phase extraction of amines. J. Anal. Chim. Acta 2005, 546, 206–220. [Google Scholar] [CrossRef]
- Slomkowska, A.; Ambroziak, W. Biogenic amine profile of the most popular Polish beers. Eur. Food Res. Technol. 2002, 215, 380–383. [Google Scholar] [CrossRef]
- Li, M.; Yang, Z.; Yang, M.; Shan, L.; Dong, J. Determination of Furfural in Beer by High-Performance Liquid Chromatography with Solid-Phase Extraction. J. Inst. Brew. 2009, 115, 226–231. [Google Scholar] [CrossRef]
- Santos, J.R.; Carneiro, J.; Guido, L.; Almeida, P.; Rodrigues, J.; Barros, A. Determination of E-2-nonenal by high-performance liquid chromatography with UV detection: Assay for the evaluation of beer ageing. J. Chromatogr. A 2003, 985, 395–402. [Google Scholar] [CrossRef]
- Ojala, M.; Kotiaho, T.; Siirilä, J.; Sihvonen, M.-L. Analysis of aldehydes and ketones from beer as O-(2, 3, 4, 5, 6-pentafluorobenzyl) hydroxylamine derivatives. Talanta 1994, 41, 1297–1309. [Google Scholar] [CrossRef]
- Yamada, H.; Somiya, I. The determination of carbonyl compounds in ozonated water by the PFBOA method. Ozone Sci. Eng. 1989, 11, 127–141. [Google Scholar] [CrossRef]
- Andrés-Iglesias, C.; Montero, O.; Sancho, D.; Blanco, C.A. New trends in beer flavour compound analysis. J. Sci. Food Agric. 2015, 95, 1571–1576. [Google Scholar] [CrossRef]
- Stashenko, E.E.; Martínez, J.R. Derivatization and solid-phase microextraction. Trends Anal. Chem. 2004, 23, 553–561. [Google Scholar] [CrossRef]
- Lin, Y.L.; Wang, P.-Y.; Hsieh, L.-L.; Ku, K.-H.; Yeh, Y.-T.; Wu, C.-H. Determination of linear aliphatic aldehydes in heavy metal containing waters by high-performance liquid chromatography using 2,4-dinitrophenylhydrazine derivatization. J. Chromatogr. A 2009, 1216, 6377–6381. [Google Scholar] [CrossRef]
- Guido, L.F.; Fortunato, N.A.; Rodrigues, J.A.; Barros, A.A. Voltammetric assay for the aging of beer. J. Agric. Food Chem. 2003, 51, 3911–3915. [Google Scholar] [CrossRef]
- de Lima, L.F.; Brandao, P.F.; Donegatti, T.A.; Ramos, R.M.; Goncalves, L.M.; Cardoso, A.A.; Pereira, E.A.; Rodrigues, J.A. 4-hydrazinobenzoic acid as a derivatizing agent for aldehyde analysis by HPLC-UV and CE-DAD. Talanta 2018, 187, 113–119. [Google Scholar]
- Ferreira, I.M.; Carvalho, D.O.; da Silva, M.G.; Guido, L.F. Gas-Diffusion Microextraction (GDME) Combined with Derivatization for Assessing Beer Staling Aldehydes: Validation and Application. Foods 2021, 10, 1704. [Google Scholar] [CrossRef]
- Ribeiro, L.H.; Freitas, A.M.C.; da Silva, M.D.R.G. The use of headspace solid phase microextraction for the characterization of volatile compounds in olive oil matrices. Talanta 2008, 77, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Kun-Farkas, G.; Kiss, Z. Quantitative Analysis of Flavor Volatiles in Beer Using Headspace Solid-Phase Microextraction and Gas Chromatography–Flame Ionization Detection (HS-SPME-GC-FID). J. Am. Soc. Brew. Chem. 2015, 73, 261–265. [Google Scholar] [CrossRef]
- Al-Bukhaiti, W.Q.; Noman, A.; Qasim, A.S.; Al-Farga, A. Gas chromatography: Principles, advantages and applications in food analysis. Int. J. Agric. Innov. Res. 2017, 6, 123–128. [Google Scholar]
- Cramer, A.C.J.; Mattinson, D.S.; Fellman, J.K.; Baik, B.-K. Analysis of volatile compounds from various types of barley cultivars. J. Agric. Food Chem. 2005, 53, 7526–7531. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, Z.; Mikulíková, R.; Běláková, S.; Benešová, K.; Márová, I.; Nesvadba, Z. Optimization of modern analytical SPME and SPDE methods for determination of trans-2-nonenal in barley, malt and beer. Chromatographia 2011, 73, 157–161. [Google Scholar] [CrossRef]
- Li, H.; Li, H.; Liu, X.; Chen, B. Analysis of volatile flavor compounds in top fermented wheat beer by headspace sampling-gas chromatography. Int. J. Agric. Biol. Eng. 2012, 5, 67–75. [Google Scholar]
- Hrivňák, J.; Šmogrovičová, D.; Nádaský, P.; Lakatošová, J. Determination of beer aroma compounds using headspace solid-phase microcolumn extraction. Talanta 2010, 83, 294–296. [Google Scholar] [CrossRef]
- Ma, C.; He, Y.; Cao, Y.; Bai, X.; Li, H. Analysis of flavour compounds in beer with extruded sorghum as an adjunct using headspace solid-phase micro-extraction and gas chromatography–mass spectrometry. J. Inst. Brew. 2016, 122, 251–260. [Google Scholar]
- Hawthorne, D.B.; Kavanagh, T.E.; Clarke, B.J. Determination of low molecular weight organic compounds in beer using capillary gas chromatography. J. Am. Soc. Brew. Chem. 1987, 45, 23–27. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, D.; Li, G.; Hao, J.; Jiang, W.; Liu, Z.; Qin, Q. Flavor contribution of esters in lager beers and an analysis of their flavor thresholds. J. Am. Soc. Brew. Chem. 2017, 75, 201–206. [Google Scholar] [CrossRef]
- Tressl, R.; Friese, L.; Fendesack, F.; Koeppler, H. Gas chromatographic-mass spectrometric investigation of hop aroma constituents in beer. J. Agric. Food Chem. 1978, 26, 1422–1426. [Google Scholar] [CrossRef]
- Rodriguez-Bencomo, J.J.; Muñoz-González, C.; Martín-Álvarez, P.J.; Lázaro, E.; Mancebo, R.; Castañé, X.; Pozo-Bayón, M.A. Optimization of a HS-SPME-GC-MS procedure for beer volatile profiling using response surface methodology: Application to follow aroma stability of beers under different storage conditions. Food Anal. Methods 2012, 5, 1386–1397. [Google Scholar]
- da Silva, G.C.; da Silva, A.A.; da Silva, L.S.; Godoy, R.L.D.O.; Nogueira, L.C.; Quitério, S.L.; Raices, R.S. Method development by GC–ECD and HS-SPME–GC–MS for beer volatile analysis. Food Chem. 2015, 167, 71–77. [Google Scholar] [PubMed]
- Ruvalcaba, J.E.; Durán-Guerrero, E.; Barroso, C.G.; Castro, R. Development of a stir bar sorptive extraction method to study different beer styles volatile profiles. Food Res. Int. 2019, 126, 108680. [Google Scholar] [PubMed]
- Charry-Parra, G.; Dejesus-Echevarria, M.; Perez, F.J. Beer volatile analysis: Optimization of HS/SPME coupled to GC/MS/FID. J. Food Sci. 2011, 76, C205–C211. [Google Scholar] [CrossRef] [PubMed]
- Horák, T.; Čulík, J.; Kellner, V.; Jurková, M.; Čejka, P.; Hašková, D.; Dvořák, J. Analysis of selected esters in beer: Comparison of solid-phase microextraction and stir bar sorptive extraction. J. Inst. Brew. 2010, 116, 81–85. [Google Scholar] [CrossRef]
- Rodrigues, J.A.; Barros, A.S.; Carvalho, B.; Brandão, T.; Gil, A.M.; Ferreira, A.C.S. Evaluation of beer deterioration by gas chromatography–mass spectrometry/multivariate analysis: A rapid tool for assessing beer composition. J. Chromatogr. A 2011, 1218, 990–996. [Google Scholar]
- Coelho, E.; Lemos, M.; Genisheva, Z.; Domingues, L.; Vilanova, M.; Oliveira, J.M. Validation of a LLME/GC-MS methodology for quantification of volatile compounds in fermented beverages. Molecules 2020, 25, 621. [Google Scholar]
- Pejin, J.D.; Grujic, O.; Marjanovic, N.; Vujic, D.; Kocic-Tanackov, S. Determina tion of diacetyl and 2, 3-pentanedione in beer by gc/ms using solid-phase extraction columns. Acta Period. Technol. 2002, 33, 45–54. [Google Scholar] [CrossRef]
- Ferreira, I.M.; Freitas, F.; Pinheiro, S.; Mourão, M.F.; Guido, L.F.; da Silva, M.G. Impact of temperature during beer storage on beer chemical profile. LWT-Food Sci. Technol. 2022, 154, 112688. [Google Scholar]
- Lermusieau, G.; Bulens, M.; Collin, S. Use of GC−olfactometry to identify the hop aromatic compounds in beer. J. Agric. Food Chem. 2001, 49, 3867–3874. [Google Scholar] [CrossRef]
- Murakami, A.A.; Goldstein, H.; Navarro, A.; Seabrooks, J.R.; Ryder, D.S. Investigation of beer flavor by gas chromatography-olfactometry. J. Am. Soc. Brew. Chem. 2003, 61, 23–32. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Urpi-Sarda, M.; Rotchés-Ribalta, M.; Zamora-Ros, R.; Llorach, R.; Lamuela-Raventós, R.M.; Estruch, R.; Andrés-Lacueva, C. Determination of resveratrol and piceid in beer matrices by solid-phase extraction and liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.F.; Taylor, A.W.; Deinzer, M.L. Quantitative analysis of xanthohumol and related prenylflavonoids in hops and beer by liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 1999, 832, 97–107. [Google Scholar] [CrossRef]
- Gonçalves, L.M.; Magalhães, P.J.; Valente, I.M.; Pacheco, J.G.; Dostálek, P.; Sýkora, D.; Rodrigues, J.A.; Barros, A.A. Analysis of aldehydes in beer by gas-diffusion microextraction: Characterization by high-performance liquid chromatography–diode-array detection–atmospheric pressure chemical ionization–mass spectrometry. J. Chromatogr. A 2010, 1217, 3717–3722. [Google Scholar]
- Hellwig, M.; Witte, S.; Henle, T. Free and Protein-Bound Maillard Reaction Products in Beer: Method Development and a Survey of Different Beer Types. J. Agric. Food Chem. 2016, 64, 7234–7243. [Google Scholar] [CrossRef]
- Cheiran, K.P.; Raimundo, V.P.; Manfroi, V.; Anzanello, M.J.; Kahmann, A.; Rodrigues, E.; Frazzon, J. Simultaneous identification of low-molecular weight phenolic and nitrogen compounds in craft beers by HPLC-ESI-MS/MS. Food Chem. 2019, 286, 113–122. [Google Scholar]
- Plutowska, B.; Wardencki, W. Application of gas chromatography–olfactometry (GC–O) in analysis and quality assessment of alcoholic beverages—A review. Food Chem. 2008, 107, 449–463. [Google Scholar] [CrossRef]
- Delahunty, C.M.; Eyres, G.; Dufour, J.P. Gas chromatography-olfactometry. J. Sep. Sci. 2006, 29, 2107–2125. [Google Scholar] [CrossRef]
- Jandera, P. Methods for the HPLC Analysis of Phenolic Compounds and Flavonoids in Beer. In Beer in Health and Disease Prevention; Preedy, V.R., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2009; pp. 1003–1014. [Google Scholar]
- Watson, J.T.; Sparkman, O.D. Liquid Chromatography/Maas spectrometry. In Introduction to Mass Spectrometry: Instrumentation, Applications, and Strategies for Data Interpretation; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Nickerson, B.; Colón, I. Liquid–liquid and solid-phase extraction techniques. In Sample Preparation of Pharmaceutical Dosage Forms; Springer: Berlin/Heidelberg, Germany, 2011; pp. 63–92. [Google Scholar]
- Cantwell, F.F.; Losier, M. Liquid-Liquid Extraction in Sampling and Sample Preparation in Field and Laboratory: Fundamentals and New Directions in Sample Preparation; Pawliszyn, J., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2002; pp. 297–474. [Google Scholar]
- Ötles, S.; Kartal, C. Solid-Phase Extraction (SPE): Principles and applications in food samples. Acta Sci. Pol. Technol. Aliment. 2016, 15, 5–15. [Google Scholar] [CrossRef]
- Liu, H.; Dasgupta, P.K. Analytical chemistry in a drop. Solvent extraction in a microdrop. Anal. Chem. 1996, 68, 1817–1821. [Google Scholar] [CrossRef] [PubMed]
- Padrón, M.; Afonso-Olivares, C.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Microextraction techniques coupled to liquid chromatography with mass spectrometry for the determination of organic micropollutants in environmental water samples. Molecules 2014, 19, 10320–10349. [Google Scholar] [CrossRef]
- Sarafraz-Yazdi, A.; Amiri, A. Liquid-phase microextraction. Trends Anal. Chem. 2010, 29, 1–14. [Google Scholar] [CrossRef]
- Zhang, J.; Su, T.; Lee, H.K. Headspace water-based liquid-phase microextraction. Anal. Chem. 2005, 77, 1988–1992. [Google Scholar] [CrossRef]
- Lee, J.; Lee, H.K.; Rasmussen, K.E.; Pedersen-Bjergaard, S. Environmental and bioanalytical applications of hollow fiber membrane liquid-phase microextraction: A review. Anal. Chim. Acta 2008, 624, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.M.L.; Ríos, A.; Valcárcel, M. Automatic determination of total aliphatic amines by on-line photometric liquid-liquid microextraction. J. Anal. Chem. 1996, 356, 49–51. [Google Scholar] [CrossRef]
- Xiao, Q.; Yu, C.; Xing, J.; Hu, B. Comparison of headspace and direct single-drop microextraction and headspace solid-phase microextraction for the measurement of volatile sulfur compounds in beer and beverage by gas chromatography with flame photometric detection. J. Chromatogr. A 2006, 1125, 133–137. [Google Scholar] [CrossRef]
- Bolaños, P.P.; Romero-González, R.; Frenich, A.G.; Vidal, J.L.M. Application of hollow fibre liquid phase microextraction for the multiresidue determination of pesticides in alcoholic beverages by ultra-high pressure liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. A 2008, 1208, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Poole, C.F. New trends in solid-phase extraction. Trends Anal. Chem. 2003, 22, 362–373. [Google Scholar] [CrossRef]
- Buldini, P.L.; Ricci, L.; Sharma, J.L. Recent applications of sample preparation techniques in food analysis. J. Chromatogr. A 2002, 975, 47–70. [Google Scholar] [CrossRef] [PubMed]
- Camel, V. Solid phase extraction of trace elements. Spectrochim. Acta Part B At. Spectrosc. 2003, 58, 1177–1233. [Google Scholar] [CrossRef]
- Mills, M.S.; Thurman, E.M. Solid-Phase Extraction: Principles and Practice; Wiley: New York, NY, USA, 1998. [Google Scholar]
- Prosen, H.; Zupančič-Kralj, L. Solid-phase microextraction. Trends Anal. Chem. 1999, 18, 272–282. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Szczepańska, N.; de la Guardia, M.; Namieśnik, J. Miniaturized solid-phase extraction techniques. Trends Anal. Chem. 2015, 73, 19–38. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, M.J.; Pawliszyn, J. Solid-phase microextraction. A solvent-free alternative for sample preparation. Anal. Chem. 1994, 66, 844A–853A. [Google Scholar] [CrossRef]
- Lord, H.; Pawliszyn, J. Evolution of solid-phase microextraction technology. J. Chromatogr. A 2000, 885, 153–193. [Google Scholar] [CrossRef]
- Pawliszyn, J. Theory of solid-phase microextraction. In Handbook of Solid Phase Microextraction; Elsevier: Amsterdam, The Netherlands, 2012; pp. 13–59. [Google Scholar]
- Eisert, R.; Pawliszyn, J. New trends in solid-phase microextraction. Crit. Rev. Anal. Chem. 1997, 27, 103–135. [Google Scholar] [CrossRef]
- Wieczorek, M.N.; Zhou, W.; Pawliszyn, J. Sequential thin film-solid phase microextraction as a new strategy for addressing displacement and saturation effects in food analysis. Food Chem. 2022, 389, 133038. [Google Scholar] [CrossRef]
- Hernandes, K.C.; Souza-Silva, A.; Assumpção, C.F.; Zini, C.A.; Welke, J.E. Validation of an analytical method using HS-SPME-GC/MS-SIM to assess the exposure risk to carbonyl compounds and furan derivatives through beer consumption. Food Addit. Contam. Part A-Chem. Anal. Control Expo. Risk Assess. 2019, 36, 1808–1821. [Google Scholar] [CrossRef] [PubMed]
- Peña, R.M.; Barciela, J.; Herrero, C.; García-Martín, S. Optimization of solid-phase microextraction methods for GC-MS determination of terpenes in wine. J. Sci. Food Agric. 2005, 85, 1227–1234. [Google Scholar] [CrossRef]
- Boutou, S.; Chatonnet, P. Rapid headspace solid-phase microextraction/gas chromatographic/mass spectrometric assay for the quantitative determination of some of the main odorants causing off-flavours in wine. J. Chromatogr. A 2007, 1141, 1–9. [Google Scholar] [CrossRef]
- Gómez-Ariza, J.L.; García-Barrera, T.; Lorenzo, F.; Beltrán, R. Use of multiple headspace solid-phase microextraction and pervaporation for the determination of off-flavours in wine. J. Chromatogr. A 2006, 1112, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Jeleñ, H.H.; Dabrowska, A.; Klensporf, D.; Nawrocki, J.; Wasowicz, E. Determination of C3–C10 alifatic aldehydes using PFBHA derivatization and solid phase microextraction (SPME). Application to the analysis of beer. J. Anal. Chim. Acta 2004, 49, 869. [Google Scholar]
- Rossi, S.; Sileoni, V.; Perretti, G.; Marconi, O. Characterization of the volatile profiles of beer using headspace solid-phase microextraction and gas chromatography–mass spectrometry. J. Sci. Food Agric. 2014, 94, 919–928. [Google Scholar] [CrossRef]
- Filipowska, W.; Jaskula-Goiris, B.; Ditrych, M.; Schlich, J.; De Rouck, G.; Aerts, G.; De Cooman, L. Determination of optimal sample preparation for aldehyde extraction from pale malts and their quantification via headspace solid-phase microextraction followed by gas chromatography and mass spectrometry. J. Chromatogr. A 2020, 1612, 460647. [Google Scholar] [CrossRef]
- Saison, D.; De Schutter, D.P.; Delvaux, F.; Delvaux, F.R. Optimisation of a complete method for the analysis of volatiles involved in the flavour stability of beer by solid-phase microextraction in combination with gas chromatography and mass spectrometry. J. Chromatogr. A 2008, 1190, 342–349. [Google Scholar] [CrossRef]
- Castro, L.F.; Ross, C.F. Determination of flavour compounds in beer using stir-bar sorptive extraction and solid-phase microextraction. J. Inst. Brew. 2015, 121, 197–203. [Google Scholar] [CrossRef]
- Saito, K.; Tokorodani, Y.; Sakamoto, C.; Kataoka, H. Headspace Solid-Phase Microextraction/Gas Chromatography-Mass Spectrometry for the Determination of 2-Nonenal and Its Application to Body Odor Analysis. Molecules 2021, 26, 5739. [Google Scholar] [CrossRef]
- Scherer, R.; Wagner, R.; Kowalski, C.H.; Godoy, H.T. (E)-2-Nonenal determination in brazilian beers using headspace solid-phase microextraction and gas chromatographic coupled mass spectrometry (HS-SPME-GC-MS). J. Food Sci. Technol. 2010, 30, 161–165. [Google Scholar] [CrossRef][Green Version]
- Campillo, N.; Peñalver, R.; López-García, I.; Hernández-Córdoba, M. Headspace solid-phase microextraction for the determination of volatile organic sulphur and selenium compounds in beers, wines and spirits using gas chromatography and atomic emission detection. J. Chromatogr. A 2009, 1216, 6735–6740. [Google Scholar] [CrossRef] [PubMed]
- Nespor, J.; Karabín, M.; Štulíková, K.; Dostálek, P. An HS-SPME-GC-MS Method for Profiling Volatile Compounds as Related to Technology Used in Cider Production. Molecules 2019, 24, 2117. [Google Scholar] [CrossRef] [PubMed]
- Burin, V.M.; Marchand, S.; de Revel, G.; Bordignon-Luiz, M.T. Development and validation of method for heterocyclic compounds in wine: Optimization of HS-SPME conditions applying a response surface methodology. Talanta 2013, 117, 87–93. [Google Scholar] [CrossRef]
- Riu-Aumatell, M.; Miró, P.; Serra-Cayuela, A.; Buxaderas, S.; López-Tamames, E. Assessment of the aroma profiles of low-alcohol beers using HS-SPME–GC-MS. Food Res. Int. 2014, 57, 196–202. [Google Scholar] [CrossRef]
- Giannetti, V.; Mariani, M.B.; Torrelli, P.; Marini, F. Flavour component analysis by HS-SPME/GC–MS and chemometric modeling to characterize Pilsner-style Lager craft beers. Microchem. J. 2019, 149, 103991. [Google Scholar] [CrossRef]
- Riu-Aumatell, M.; Bosch-Fusté, J.; López-Tamames, E.; Buxaderas, S. Development of volatile compounds of cava (Spanish sparkling wine) during long ageing time in contact with lees. Food Chem. 2006, 95, 237–242. [Google Scholar] [CrossRef]
- Alves, V.; Gonçalves, J.; Figueira, J.A.; Ornelas, L.P.; Branco, R.N.; Câmara, J.S.; Pereira, J.A. Beer volatile fingerprinting at different brewing steps. Food Chem. 2020, 326, 126856. [Google Scholar] [CrossRef]
- López, R.; Lapena, A.C.; Cacho, J.; Ferreira, V. Quantitative determination of wine highly volatile sulfur compounds by using automated headspace solid-phase microextraction and gas chromatography-pulsed flame photometric detection: Critical study and optimization of a new procedure. J. Chromatogr. A 2007, 1143, 8–15. [Google Scholar] [CrossRef]
- Fiorini, D.; Caprioli, G.; Sagratini, G.; Maggi, F.; Vittori, S.; Marcantoni, E.; Ballini, R. Quantitative profiling of volatile and phenolic substances in the wine Vernaccia di Serrapetrona by development of an HS-SPME-GC-FID/MS method and HPLC-MS. Food Anal. Methods 2014, 7, 1651–1660. [Google Scholar] [CrossRef]
- Jeleń, H.H.; Wlazły, K.; Wasowicz, E.; Kamiński, E. Solid-phase microextraction for the analysis of some alcohols and esters in beer: Comparison with static headspace method. J. Agric. Food Chem. 1998, 46, 1469–1473. [Google Scholar] [CrossRef]
- Vas, G.Y.; Kõteleky, K.; Farkas, M.; Dobó, A.; Vékey, K. Fast screening method for wine headspace compounds using solid-phase microextraction (SPME) and capillary GC technique. Am. J. Enelogy Vitic. 1998, 49, 100–104. [Google Scholar] [CrossRef]
- Lanças, F.M. The role of the separation sciences in the 21th century. J. Braz. Chem. Soc. 2003, 14, 183–197. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Chai, X.S. Some recent developments in headspace gas chromatography. Curr. Anal. Chem. 2005, 1, 79–83. [Google Scholar] [CrossRef]
- Lochow, E.; Peschmann, P.; Hellwig, C. Determination of beer fermentation by-products via gas chromatography. Brauwelt Int. 2005, 23, 270–271. [Google Scholar]
- Pan, L.; Pawliszyn, J. Derivatization/solid-phase microextraction: New approach to polar analytes. J. Anal. Chem. 1997, 69, 196–205. [Google Scholar] [CrossRef]
- Buchholz, K.D.; Pawliszyn, J. Optimization of solid-phase microextraction conditions for determination of phenols. Anal. Chem. 1994, 66, 160–167. [Google Scholar] [CrossRef]
- Pan, L.; Adams, M.; Pawliszyn, J. Determination of fatty acids using solid phase microextraction. Anal. Chem. 1995, 67, 4396–4403. [Google Scholar] [CrossRef]
- Snow, N.H.; Slack, G.C. Head-space analysis in modern gas chromatography. Trends Anal. Chem. 2002, 21, 608–617. [Google Scholar] [CrossRef]
- Jiao, J.; Ding, N.; Shi, T.; Chai, X.; Cong, P.; Zhu, Z. Study of chromatographic fingerprint of the flavor in beer by HS-SPME-GC. Anal. Lett. 2011, 44, 648–655. [Google Scholar] [CrossRef]
- Hasan, C.K.; Ghiasvand, A.; Lewis, T.W.; Nesterenko, P.N.; Paull, B. Recent advances in stir-bar sorptive extraction: Coatings, technical improvements, and applications. Anal. Chim. Acta 2020, 1139, 222–240. [Google Scholar] [CrossRef]
- David, F.; Sandra, P. Stir bar sorptive extraction for trace analysis. J. Chromatogr. A 2007, 1152, 54–69. [Google Scholar] [CrossRef] [PubMed]
- Paz, A.I.; Blanco, C.A.; Andres-Iglesias, C.; Palacio, L.; Pradanos, P.; Hernandez, A. Aroma recovery of beer flavors by pervaporation through polydimethylsiloxane membranes. J. Food Process Eng. 2017, 40, e12556. [Google Scholar] [CrossRef]
- Camino-Sánchez, F.J.; Rodríguez-Gómez, R.; Zafra-Gómez, A.; Santos-Fandila, A.; Vílchez, J.L. Stir bar sorptive extraction: Recent applications, limitations and future trends. Talanta 2014, 130, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Prieto, A.; Basauri, O.; Rodil, R.; Usobiaga, A.; Fernández, L.; Etxebarria, N.; Zuloaga, O. Stir-bar sorptive extraction: A view on method optimisation, novel applications, limitations and potential solutions. J. Chromatogr. A 2010, 1217, 2642–2666. [Google Scholar] [CrossRef]
- Bicchi, C.; Iori, C.; Rubiolo, P.; Sandra, P. Headspace sorptive extraction (HSSE), stir bar sorptive extraction (SBSE), and solid phase microextraction (SPME) applied to the analysis of roasted Arabica coffee and coffee brew. J. Agric. Food Chem. 2002, 50, 449–459. [Google Scholar] [CrossRef]
- Ruvalcaba, J.E.; Durán-Guerrero, E.; Barroso, C.G.; Castro, R. Development of head space sorptive extraction method for the determination of volatile compounds in beer and comparison with stir bar sorptive extraction. Foods 2020, 9, 255. [Google Scholar] [CrossRef]
- Baltussen, E.; Cramers, C.A.M.G.; Sandra, P. Sorptive sample preparation—A review. Anal. Bioanal. Chem. 2002, 373, 3–22. [Google Scholar] [CrossRef]
- Benanou, D.; Acobas, F.; de Roubin, M.R.; David, F.; Sandra, P. Analysis of off-flavors in the aquatic environment by stir bar sorptive extraction–thermal desorption–capillary GC/MS/olfactometry. Anal. Bioanal. Chem. 2003, 376, 69–77. [Google Scholar] [CrossRef]
- Zhang, P.H.; Carlin, S.; Lotti, C.; Mattivi, F.; Vrhovsek, U. On sample preparation methods for fermented beverage VOCs profiling by GCxGC-TOFMS. Metabolomics 2020, 16, 102. [Google Scholar] [CrossRef]
- Ochiai, N.; Sasamoto, K.; David, F.; Sandra, P. Solvent-assisted stir bar sorptive extraction by using swollen polydimethylsiloxane for enhanced recovery of polar solutes in aqueous samples: Application to aroma compounds in beer and pesticides in wine. J. Chromatogr. A 2016, 1455, 45–56. [Google Scholar] [CrossRef]
- Tsuji, H.; Mizuno, A. Volatile compounds and the changes in their concentration levels during storage in beers containing varying malt concentrations. J. Food Sci. 2010, 75, C79–C84. [Google Scholar] [CrossRef] [PubMed]
- Horák, T.; Čulík, J.; Kellner, V.; Čejka, P.; Hašková, D.; Jurková, M.; Dvořák, J. Determination of selected beer flavours: Comparison of a stir bar sorptive extraction and a steam distillation procedure. J. Inst. Brew. 2011, 117, 617–621. [Google Scholar] [CrossRef]
- Cucu, T.; David, F.; Devos, C.; Sandra, P. Untargeted flavor profiling of lager beers by stir bar sorptive extraction -capillary gas chromatography—time-of-flight mass spectrometry: High analytical performance with a green touch. J. Chromatogr. A 2021, 1647, 462164. [Google Scholar] [CrossRef] [PubMed]
- Kreck, M.; Püschel, S.; Wüst, M.; Mosandl, A. Biogenetic studies in Syringa vulgaris L.: Synthesis and bioconversion of deuterium-labeled precursors into lilac aldehydes and lilac alcohols. J. Agric. Food Chem. 2003, 51, 463–469. [Google Scholar] [CrossRef]
- Diaz, A.B.; Duran-Guerrero, E.; Valiente, S.; Castro, R.; Lasanta, C. Development and Characterization of Probiotic Beers with Saccharomyces boulardii as an Alternative to Conventional Brewer’s Yeast. Foods 2023, 12, 2912. [Google Scholar] [CrossRef]
- Castro, R.; Díaz, A.B.; Durán-Guerrero, E.; Lasanta, C. Influence of different fermentation conditions on the analytical and sensory properties of craft beers: Hopping, fermentation temperature and yeast strain. J. Food Compos. Anal. 2022, 106, 104278. [Google Scholar] [CrossRef]
- Lasanta, C.; Duran-Guerrero, E.; Diaz, A.B.; Castro, R. Influence of fermentation temperature and yeast type on the chemical and sensory profile of handcrafted beers. J. Sci. Food Agric. 2021, 101, 1174–1181. [Google Scholar] [CrossRef]
- Isogai, A.; Utsunomiya, H.; Kanda, R.; Iwata, H. Changes in the aroma compounds of sake during aging. J. Agric. Food Chem. 2005, 53, 4118–4123. [Google Scholar] [CrossRef]
- Ochiai, N.; Sasamoto, K.; Sasaki, T.; David, F.; Sandra, P. Fractionated stir bar sorptive extraction using conventional and solvent-assisted approaches for enhanced identification capabilities of aroma compounds in beverages. J. Chromatogr. A 2020, 1628, 461475. [Google Scholar] [CrossRef]
- Marsili, R.T.; Laskonis, L.C.; Kenaan, C. Evaluation of PDMS-based extraction techniques and GC-TOFMS for the analysis of off-flavor chemicals in beer. J. Am. Soc. Brew. Chem. 2007, 65, 129–137. [Google Scholar] [CrossRef]
- Kishimoto, T.; Wanikawa, A.; Kagami, N.; Kawatsura, K. Analysis of hop-derived terpenoids in beer and evaluation of their behavior using the stir bar−sorptive extraction method with GC-MS. J. Agric. Food Chem. 2005, 53, 4701–4707. [Google Scholar] [CrossRef]
- De Villiers, A.; Vanhoenacker, G.; Lynen, F.; Sandra, P. Stir bar sorptive extraction-liquid desorption applied to the analysis of hop-derived bitter acids in beer by micellar electrokinetic chromatography. Electrophoresis 2004, 25, 664–669. [Google Scholar] [CrossRef]
- Kishimoto, T.; Teramoto, S.; Fujita, A.; Yamada, O. Principal Component Analysis of Hop-Derived Odorants Identified by Stir Bar Sorptive Extraction Method. J. Am. Soc. Brew. Chem. 2021, 79, 272–280. [Google Scholar] [CrossRef]
- Horák, T.; Čulík, J.; Jurková, M.; Čejka, P.; Kellner, V. Determination of free medium-chain fatty acids in beer by stir bar sorptive extraction. J. Chromatogr. A 2008, 1196, 96–99. [Google Scholar] [CrossRef]
- Jönsson, J.Å.; Mathiasson, L. Membrane extraction in analytical chemistry. J. Sep. Sci. 2001, 24, 495–507. [Google Scholar] [CrossRef]
- Jönsson, J.A. Membrane extraction for sample preparation—A practical guide. J. Chromatogr. 2003, 57, S317–S324. [Google Scholar] [CrossRef]
- Jönsson, J.A.; Mathiasson, L. Membrane-based techniques for sample enrichment. J. Chromatogr. A 2000, 902, 205–225. [Google Scholar] [CrossRef] [PubMed]
- Chimuka, L.; Cukrowska, E.; Jönsson, J.Å. Why liquid membrane extraction is an attractive alternative in sample preparation. Pure Appl. Chem. 2004, 76, 707–722. [Google Scholar] [CrossRef]
- Moskvin, L.N.; Nikitina, T.G. Membrane methods of substance separation in analytical chemistry. J. Anal. Chem. 2004, 59, 2–16. [Google Scholar] [CrossRef]
- Bendtsen, A.B.; Jørgensen, S.S. Determination of total and free sulfite in unstabilized beer by flow injection analysis. J. AOAC Int. 1994, 77, 948–951. [Google Scholar] [CrossRef]
- Firnandes, S.M.V.; Rangel, A.O.S.S.; Lima, J.L.F.C. Determination of total sulphur dioxide in beer by flow injection spectrophotometry using gas-diffusion and the merging zones technique. J. Inst. Brew. 1998, 104, 203–205. [Google Scholar] [CrossRef]
- Silvestre, C.I.C.; Santos, J.L.; Lima, J.L.; Zagatto, E.A. Liquid–liquid extraction in flow analysis: A critical review. Anal. Chim. Acta 2009, 652, 54–65. [Google Scholar] [CrossRef]
- Lord, H.; Pawliszyn, J. Microextraction of drugs. J. Chromatogr. A 2000, 902, 17–63. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, J.G.; Valente, I.M.; Gonçalves, L.M.; Rodrigues, J.A.; Barros, A.A. Gas-diffusion microextraction. J. Sep. Sci. 2010, 33, 3207–3212. [Google Scholar] [CrossRef]
- Brandao, P.F.; Ramos, R.M.; Almeida, P.J.; Rodrigues, J.A. Determination of Carbonyl Compounds in Cork Agglomerates by GDME-HPLC-UV: Identification of the Extracted Compounds by HPLC-MS/MS. J. Agric. Food Chem. 2017, 65, 1037–1042. [Google Scholar] [CrossRef]
- Ramos, R.M.; Pacheco, J.G.; Gonçalves, L.M.; Valente, I.M.; Rodrigues, J.A.; Barros, A.A. Determination of free and total diacetyl in wine by HPLC–UV using gas-diffusion microextraction and pre-column derivatization. Food Control 2012, 24, 220–224. [Google Scholar] [CrossRef]
- Cruz, M.P.; Valente, I.M.; Gonçalves, L.M.; Rodrigues, J.A.; Barros, A.A. Application of gas-diffusion microextraction to the analysis of free and bound acetaldehyde in wines by HPLC–UV and characterization of the extracted compounds by MS/MS detection. Anal. Bioanal. Chem. 2012, 403, 1031–1037. [Google Scholar] [CrossRef]

| Extraction Technique | Compounds | Bibliographic References |
|---|---|---|
| Liquid–liquid extraction (LLE) | Aldehydes; alcohols; ketones | [8,9,10] |
| Liquid–liquid extraction (LLE) | Aldehydes | [10] |
| Solvent drop microextraction (SDME) | Alcohols | [11,12] |
| Steam distillation | Aldehydes | [13,14,15] |
| Solvent-assisted flavor evaporation (SAFE) | Aldehydes | [8,16] |
| Headspace solid-phase microextraction (HS-SPME) | Aldehydes; volatile fraction; carbonyl compounds | [9,17,18,19] |
| Headspace solid-phase microextraction (HS-SPME) | Volatile compounds | [20,21] |
| Solid-phase microextraction (SPME, on fiber derivatization with PFBHA) | Aldehydes; ketones; esters; ethers | [7,8,18,22,23,24,25,26,27,28] |
| Stir bar sorptive extraction (SBSE, with derivatization with PFBHA) | Aldehydes; | [29,30] |
| Solid-phase extraction (SPE) | Ketones; amines | [31,32,33] |
| Solid-phase extraction (SPE) | Aldehydes; amines | [33,34,35,36] |
| Derivatization with PFBHA | Carbonyl compounds | [37,38] |
| Extraction Technique | Separation Technique | Compounds | Bibliographic References |
|---|---|---|---|
| HS-SPME | GC-FID | Flavor compounds (aldehydes, ketones, alcohols, esters) | [45,46] |
| HS-SPME SBSE LLME SPE | GC-MS GC-O | Flavor compounds (aldehydes, ketones, alcohols, esters) | [22,39,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64] |
| HS-SPME | GC-O | Flavor compounds (esters, hop aroma compounds) | [54,65,66] |
| SPE | LC-MS | Hop acids, aflatoxins, amines, oligosaccharides, semi-volatile compounds | [39,67,68] |
| GDME (with derivatization) | HPLC-UV | Aldehydes, amines | [43,44,69] |
| SPE | HPLC-MS | Maillard reaction products, polyphenols | [70,71] |
| Fiber Type | Food Samples | Analyte(s) | Bibliographic Reference |
|---|---|---|---|
| PDMS | Beer | Non-polar flavor compounds; carbonyls; furan derivatives | [97,98] |
| Wine | Volatile compounds; terpenes; aldehydes | [99,100,101,102] | |
| PDMS-DVB | Beer | Aldehydes; ketones; esters; alcohols; carboxylic acids; hydrocarbons; E-2-nonenal | [7,9,17,103,104,105,106,107] |
| CAR-PDMS | Beer | Esters; alcohols; aldehydes; ketones; fatty acids; ethers; hydrocarbons; sulfur compounds; aromatic compounds; alicyclic compound; heterocyclic compounds E-2-nonenal | [9,108,109,110] |
| Wine | Heterocyclic compounds | [111] | |
| DVB-CAR-PDMS | Beer | Aldehydes; ketones; alcohols; esters; terpenes; carboxylic acids; volatile compounds fingerprint | [45,56,64,112,113,114,115] |
| Wine | Sulfur compounds; volatile compounds | [116,117] | |
| PA | Beer | Alcohols and esters | [118] |
| Wine | Aroma volatiles | [119] |
| SBSE Extraction | |
|---|---|
| Analyte | Bibliographic References |
| Carbonyl compounds | [30,106,139] |
| Beer flavors | [140,141] |
| Esters | [106,139,142] |
| Aroma compounds | [143,144,145,146,147] |
| Off-flavors in aged beers | [148] |
| Terpenoids | [149] |
| Hop-derived bitter acids | [150,151] |
| Free fatty acids | [152] |
| Three-Phase Membrane Extraction | Two-Phase Membrane Extraction | One-Phase Membrane Extraction | ||||||
|---|---|---|---|---|---|---|---|---|
| SLME * | PME * | MESI * | MIMS * | GD * | MMLLE * | MASE * | Dialysis | |
| Donor | Aqueous | Aqueous/ Organic | Aqueous/ Organic/ Gaseous | Aqueous/ Gaseous | Aqueous | Aqueous | Aqueous | Aqueous |
| Membrane | Organic, liquid | Polymer | Polymer | Non-porous/ Porous | Pores | Solvent | Pores | |
| Acceptor | Aqueous | Aqueous/ Organic | Gas/Sorbent | Vacuum of MS spectrometer | Aqueous | Solvent | Aqueous/ Organic | Aqueous |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, I.M.; Guido, L.F. Advances in Extraction Techniques for Beer Flavor Compounds. Beverages 2023, 9, 71. https://doi.org/10.3390/beverages9030071
Ferreira IM, Guido LF. Advances in Extraction Techniques for Beer Flavor Compounds. Beverages. 2023; 9(3):71. https://doi.org/10.3390/beverages9030071
Chicago/Turabian StyleFerreira, Inês M., and Luis F. Guido. 2023. "Advances in Extraction Techniques for Beer Flavor Compounds" Beverages 9, no. 3: 71. https://doi.org/10.3390/beverages9030071
APA StyleFerreira, I. M., & Guido, L. F. (2023). Advances in Extraction Techniques for Beer Flavor Compounds. Beverages, 9(3), 71. https://doi.org/10.3390/beverages9030071







