Abstract
While the Saaz aromatic variety remains classified as a total-flavanoid-rich cultivar, no inverse correlation was found between total flavanoids and the α-acid content when the dual-purpose varieties Citra, CTZ, Amarillo, Eureka!, Mandarina Bavaria, Mosaic, Polaris, and Sabro were considered. The levels of hop flavan-3-ol monomers, dimers, and trimers (quantitated by HPLC-MS/MS) appeared strongly influenced by variety and harvest year. On the other hand, the catechin/epicatechin ratio (and B3/B2 ratio) proved stable within the same variety through two successive harvest years. Among the nine herein-investigated varieties, Citra and Saaz displayed notable catechin/epicatechin ratios (>3.7 compared to <1.6 for the others), whereas Polaris exhibited the lowest monomer content (less than 800 mg/kg). These distinctive profiles could impact the colloidal and color stability of hop-forward beers.
1. Introduction
In beer, polyphenols are known to contribute to several product characteristics, mainly flavor, astringency, haze, body, and fullness [,,]. Hops usually contribute about 30% of the polyphenols found in beers (the remainder comes from malt) []. Yet, with the extra addition of hops in late and dry-hopped beers, this contribution and thus the total content usually increase, and this often leads to unwanted colloidal haze and color modification during aging [,,]. Better knowledge of hop polyphenol profiles could help predict these aspects and enable the selection of hop varieties according to the expected attributes of the beer.
The polyphenols involved in beer haze and color are mainly oxidation products of mono-, di- and trimers of flavan-3-ols (various combinations of catechins and epicatechin, as illustrated in Figure 1), a subgroup of flavanoids (i.e., flavonoids with a flavan-heterocycle) with an OH in position 3 [,,,]. Recent studies have shown that dehydrodi(epi)catechin A (from (epi)catechin monomer oxidation) can modify beer color []. On the other hand, complexes resulting from interactions between A-type dimers (from procyanidin oxidation, requiring epicatechin as the first subunit) or oligomers and malt hordein are responsible for colloidal haze [,,,]. While early hopping will enhance depolymerization and thus increase monomer concentrations, causing a color intensity increase during storage, late and dry hopping, through simple solubilization, will rather increase dimer and longer oligomer levels, creating colloidal instability in aged beers [,]. In non-alcoholic and low-alcoholic beers (NABLABs), among which hop-forward products have become famous thanks to the masking of the worty off-flavor, the lack of ethanol leads to premature oxidation. Hence, fresh dry-hopped NABLABs often exhibit the same flavan-3-ol profile as aged regular dry-hopped beers, strongly correlating with chill haze [].
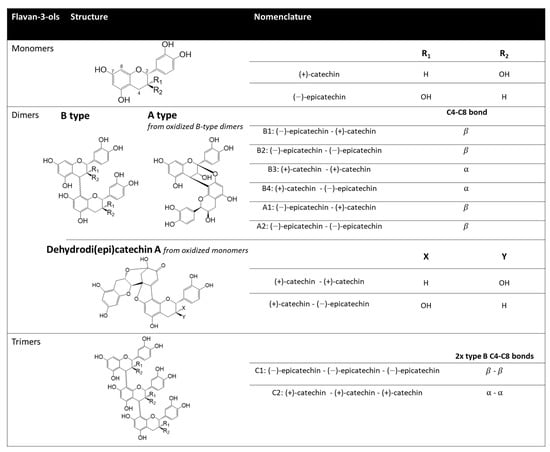
Figure 1.
Structure and nomenclature of mono-, di-, and tri-mers of flavan-3-ols.
In the last two decades, there has been a huge development of novel dual-purpose hop varieties (abundant in both α-acids and essential oils). These new hops were specifically created to meet the increasing demand from craft brewers, who sought varieties enhancing late and dry hopping flavors []. Hop growers in the United States introduced many cultivars with distinctive and unique citrus/passion fruit aroma characteristics (e.g., Amarillo, Citra, Mosaic, Sorachi Ace). German and other hop growers then launched their own dual varieties (e.g., Hallertau Blanc, Polaris, and Mandarina Bavaria from Germany; Nelson Sauvin from New Zealand) []. In 2022, 82% of Pacific Northwest acreage was used to produce these flavor hops [].
Many recent studies have investigated the essential oil composition of these hop varieties [,]. Kankolongo et al. has shown that linalool, geraniol, and β-citronellol can be used to distinguish dual-purpose hop varieties from others []. Yet, the most attractive feature of these varieties appears to be their polyfunctional thiol (PFT) profile, with both a wide variety of compounds (up to forty-one free PFTs identified) and a huge amount of free but mostly bound forms (up to 100 mg/kg glutathionylated 3-sulfanylhexanol [,]). The resulting late- or dry-hopped beers usually end up exhibiting strong citrusy or exotic fruity notes [].
Regarding the non-volatile fraction of these dual hops, which includes polyphenols, the literature remains scarce. In traditional aromatic and bitter hops, the lower the level of α-acids (source of bitterness), the higher the total flavan-3-ol [] and resveratrol [] contents. The opposite is observed for prenylchalcones such as xanthohumol []. The hop polyphenol content is also strongly influenced by several factors: climate conditions, variety and age, harvest time and year, and conditioning techniques [,,,]. (+)-Catechin, procyanidin B3, and procyanidin C2, respectively, have been found at up to 2500, 1400, and 800 mg/kg in aromatic varieties, including Saaz [,]. In barley malt beers, hop is also the only source of epicatechin and its oligomers. As depicted in Figure 2, the occurrence of (−)-epicatechin indicates the efficiency of anthocyanidin reductase (ANR) in a hop, while only leucoanthocyanidin reductase (LAR) is required to convert 3,4-flavandiol to (+)-catechin [].
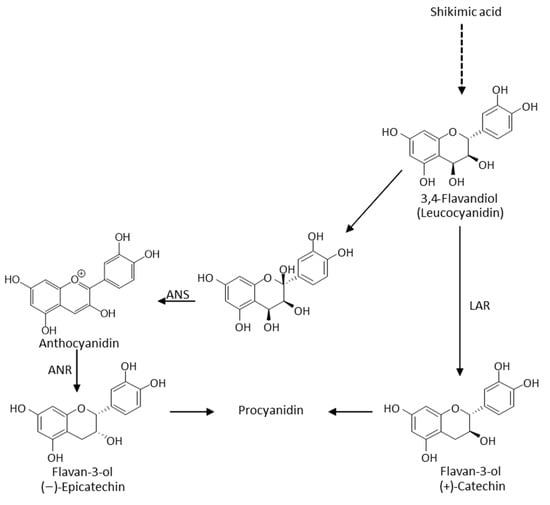
Figure 2.
Simplified catechin and epicatechin biosynthetic pathways.
The present work aimed to investigate the so far unknown flavan-3-ol profiles of eight dual-purpose hop varieties (Citra, CTZ, Sabro, Polaris, Mosaic, Mandarina Bavaria, Amarillo, Eureka!) from two different crop years, as compared to the iconic Saaz aromatic variety. These results will allow discriminating cultivars with atypical contents that could if properly used, help mitigate the unwanted colloidal haze and color modification issues met in hop-forward beers.
Based on already published beer polyphenol analyses [], flavan-3-ol extracts were purified on Sephadex LH-20 resin (a well-known resin used for high polyphenol recovery []), yet after hop delipidation and solid-phase extraction steps (adapted from Jerkovic et al. and Li et al. [,]). The purified extracts were analyzed by Reverse Phase High-Performance Liquid Chromatography (RP-HPLC) with ESI(-)-MS/MS detection []. The total flavanoid assay (reaction with p-dimethylaminocinnamaldehyde) was also applied [] in order to assess whether, in dual-purpose hop varieties, an inverse correlation would still appear between the amount of total flavanoids and that of α-acids.
2. Materials and Methods
2.1. Chemicals
Acetic acid, acetone, acetonitrile, cyclohexane, 99–100% formic acid, 37% hydrochloric acid, methanol, and toluene were purchased from VWR International (Leuven, Belgium). p-Dimethylaminocinnamaldehyde was purchased from Sigma-Aldrich (St-Louis, MO, USA). (±)-Catechin hydrate (purity superior to 98%) and (-)-epicatechin (purity superior to 90%) standards were also purchased from Sigma-Aldrich (Overijse, Belgium). Procyanidin B2 (purity superior to 90%), B3 (purity superior to 95%), and C1 (purity superior to 90%) and (+)-taxifolin (purity superior to 99.9%) standards were from Extrasynthèse (Genay, France). Milli-Q water was used (Millipore, Bedford, MA, USA).
2.2. Hop Samples
All the hops analyzed here were cropped in 2019 and 2020. Eureka!, CTZ, Mandarina Bavaria, Polaris, and Saaz were kindly provided by Hopsteiner (Mainburg, Germany) and Amarillo, Sabro, Citra, and Mosaic by Yakima Chief (Louvain-la-Neuve, Belgium).
2.3. Hop α-Acid Content
The EBC 7.4 method (hop) [] lead conductance values were applied to hop samples to determine the α-acid content.
2.4. Total Flavanoid Analysis
The EBC 9.12 method (beer) [] was adapted to the hop matrix. Hop pellets (500 mg) were finely ground, and three successive solid/liquid extractions (stirring for 10 min at room temperature and then centrifuging for 10 min at 3000× g) were performed with 25 mL acetone/water/acetic acid (70:28:2, v/v/v). The supernatant was collected, and the volume was adjusted to 100 mL with the same solution. Next, 100 μL of this extract was added to a test tube with 3 mL chromogenic solution (p-dimethylaminocinnamaldehyde, p-DMAC, 1 g/L). The mixture was vortexed, and after 10 min, the absorbance was measured at 640 nm with a UV spectrophotometer (UV-240, Shimadzu Benelux, Brussels, Belgium). This protocol was repeated 3 times on each ground hop sample spiked with catechin (+20, +40, and +60 ppm—calibration curve with standard addition). A double blank was used to account for the intrinsic absorbances of the polyphenolic extract and chromogenic solution. First, the absorbance of a solution containing 100 μL polyphenolic extract with 3 mL Milli-Q water was measured (Absblank-mQ). Secondly, the absorbance of 100 μL acetone/water/acetic acid solution (70:28:2, v/v/v) with 3 mL chromogenic solution was measured (Absblank-chromo). To obtain the total flavanoid content in hop, the following equation was used:
where FD is the dilution factor (=6200), S is the slope of the calibration curve obtained by standard addition, Abs640 extract is the absorbance of the hop extract with chromogenic solution, and Abs640 blank is the sum of Absblank-mQ and Absblank-chromo.
2.5. Optimization of Flavan-3-ol Extraction from Hops
2.5.1. Hop Delipidation (Removal of Hydrophobic Compounds)
Milled hop pellets (1.0 g) with 1000 mg/kg IST ((+)-taxifolin) were introduced into a centrifuge vial. To eliminate resins and lipids, a series of 10 min liquid–solid extractions were carried out at room temperature with gentle stirring, first three times with 50 mL toluene and then three times with 50 mL cyclohexane. At the end of the extraction, the sample underwent centrifugation at 3000× g for a duration of 10 min to facilitate solvent removal. After extraction, the solid hop residue was dried under a vacuum.
2.5.2. Solid-Phase Extraction of Hop Flavan-3-ols
Dry delipidated hop residue underwent a series of three extractions using 40 mL of acetone/water/acetic acid (70:28:2, v/v/v) each time. The extractions were carried out at room temperature under gentle stirring for 10 min. After each extraction, the sample was subjected to centrifugation at 3000× g for 10 min, and the resulting supernatant was collected. The combined supernatants were then filtered and concentrated to dryness using vacuum rotary evaporation (at 35 °C). The resulting dry residue was dissolved in 50 mL of a 70:30 (v/v) mixture of water/methanol and filtered again through glass fiber.
2.5.3. Extract Purification on Sephadex
The hop flavan-3-ol extract, including monomers, dimers, and trimers, was purified following the procedure outlined by Callemien and Collin [,]. A 12-mL SPE filtration tube containing 3 g of Sephadex LH-20 resin (Sigma-Aldrich, St. Louis, MO, USA) with a polyethylene frit (Supelco, Bellefonte, P, USA) was used for this purpose. Prior to loading the sample, the resin was preconditioned with methanol/water (30:70, v/v) for 4 h while maintaining a flux rate of 0.5 mL/min. Subsequently, 50 mL of hop extract was introduced into the column. A subsequent washing step was performed with 40 mL of methanol/water (30:70, v/v) to remove undesired components. The flavan-3-ols were then recovered using 70 mL of acetone/water (70:30, v/v). The eluate was subjected to vacuum rotary evaporation at 35 °C to concentrate it to dryness. The resulting residue was then dissolved in 2 mL of acetonitrile/water (30:70, v/v). The purified extracts were finally stored at −80 °C until analysis.
2.6. RP-HPLC-ESI(-)-MS/MS Analyses of Flavan-3-ols
An analytical SpectraSystem (Finnigan MAT, San Jose, CA, USA) was used for the study. The system was equipped with an SCM degasser, an AS3000 autosampler, and a P4000 quaternary pump. Chromatographic separation was achieved using a 150 × 2.1 mm, 3 µm C18 Prevail column (HICHROM, Deerfield, IL, USA) with a flow rate set at 0.2 mL/min. The elution process employed a multilinear gradient of water containing 0.1% formic acid (A) and acetonitrile containing 0.1% formic acid (B). The gradient elution sequence was as follows: 97–91% A from 0 to 5 min, 91–85% A from 5 to 30 min, 85–67% A from 30 to 60 min, 67–0% A from 60 to 70 min, 0–97% A from 70 to 75 min, and finally, a 15 min stabilization at the initial conditions. The column temperature was maintained at 25 °C, and 10 microliters of hop extract were injected. Mass spectra were acquired using an LCQ Duo ion trap mass spectrometer equipped with an ESI source. Collision-induced dissociation spectra were recorded at relative collision energies of 30, 35, and 40% for singly charged ions [M-H]−1 of monomers (m/z = 289), dimers (m/z = 577), and trimers (m/z = 865), respectively. The ESI (negative mode) inlet conditions applied were as follows: source voltage, 4.5 kV; capillary voltage, −4 V; capillary temperature, 250 °C; sheath gas, 50 arbitrary units. The Xcalibur software, version 1.2, was used to control the system. Identification of monomers B2, B3, and C1 was confirmed by injecting commercial standards, while procyanidins B1, B4, and C2 were identified based on mass spectra and following the method outlined by Callemien and Collin []. Chromatograms with identified peaks are illustrated in Figure 3.
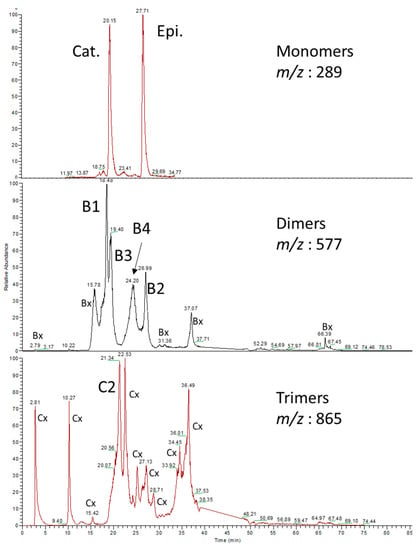
Figure 3.
HPLC-MS/MS chromatograms (m/z = 289 for monomers, m/z = 577 for dimers, and m/z = 865 for trimers) of Amarillo 2019 Sephadex extract. Bx and Cx, observed in most of the hop extracts, are suspected of being oligomers issued from oxidation and epimerization, in agreement with recent data published on processed cocoa [].
2.7. Quantitation
Quantitation was performed with calibration curves (at 25, 50, 75, 100 mg/L) of (±)-catechin hydrate, (-)-epicatechin, procyanidin B3 (also used for B1, B2, and B4), and procyanidin C1 (used for C2). A relative recovery value of 1 was established between the compounds and the internal standard. The concentrations given are means of duplicates (coefficient variation below 15% for each sample).
3. Results and Discussion
3.1. Total Flavanoids vs α-Acids
Dual-purpose hop varieties have the particularity of covering a wider range of alpha acid content (from 7.1 to 20.1% for the here studied cultivars), in contrast to aromatic and bittering hops, which might influence the relationship with total flavanoids.
The here exposed total flavanoid contents (from 4000 to 14,000 mg/kg catechin eq.) are in line with previous studies (e.g., 9200 mg/kg catechin eq. already found in Saaz []). The highest total flavanoid values (11,500–14,000 mg/kg catechin eq.) were again obtained for the Saaz aromatic variety (3.1–3.3% α-acids), where less malonyl CoA competition occurs between humulone and flavan-3-ol biosyntheses []. Yet, in contrast to previously published results obtained with aromatic and bitter hops [,], no strong correlation (R2 < 0.5) emerged here between total flavanoids and α-acids for the nine investigated cultivars (eight of which classified as dual-purpose hops) from the 2019 and 2020 harvest years (Figure 4).
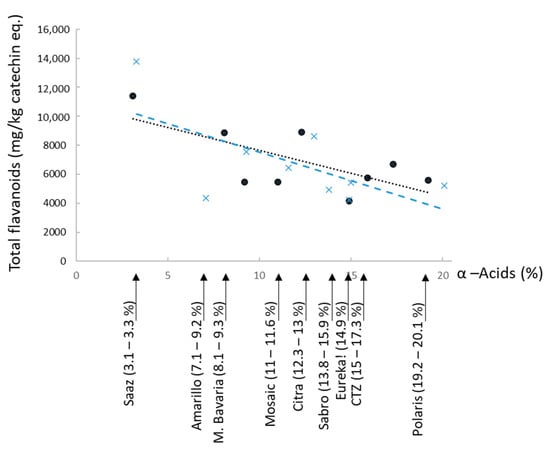
Figure 4.
Relationship between α-acids content and total flavanoids (in catechin equivalents) in Saaz and dual-purpose hops from 2019 (●) and 2020 (×) harvests (R2 = 0.47 and 0.41, respectively).
As phytoalexins, the flavanoids are known to be highly pedoclimatic-dependent [,], partly explaining some variations among harvest years. The freshness of the analyzed samples could also have impacted some data found in the literature, as some cultivars, such as Amarillo, are known to be particularly prone to oxidation [].
Note that this empiric method detects only monomers and the first subunit of each oligomer. This manner of counting is useful, however, as these are the units liable to interact later with oxidized flavan-3-ols.
3.2. Flavan-3-ol Monomers, Dimers, and Trimers
The monomer, dimer, and trimer levels of the nine selected hop cultivars, as determined by HPLC-MS/MS, are depicted in Figure 5a,b.
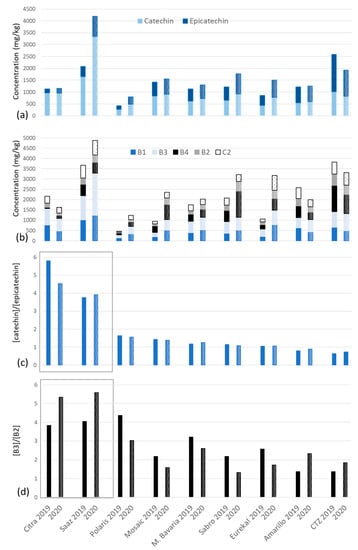
Figure 5.
Levels of (a) monomers and (b) dimers and trimer C2; (c) the catechin/epicatechin ratio, and (d) B3/B2 ratio in hops from 2019 (solid fill) and 2020 (doted) harvests.
The total monomer content, ranging from 427 to 4196 mg/kg (in Polaris 2019 and Saaz 2020, respectively), is consistent with previously reported values (from 300 to 4500 mg/kg [,,,]). As expected, given the total flavanoid contents presented above and previous research on aromatic cultivars, Saaz appeared as the most abundant sample in monomers. In a recent study involving some of the German hop varieties selected here (Mandarina Bavaria, Eureka!, and Polaris), among 32 cultivars all from the 2021 crop year, remarkably similar results were observed, confirming Polaris as the least abundant in flavan-3-ol monomers (297 mg/kg) [].
A rather unexpected finding is the CTZ monomer content, ranking as the second richest sample with up to 2581 mg/kg, despite its 15–17.3% α-acids.
Regarding the relative catechin and epicatechin proportions (illustrated as a catechin/epicatechin ratio in Figure 5c), Citra and Saaz stood out from the other varieties with a ratio above 3.7 (vs. <1.6). Other studies have already shown highly distinctive ratios (from 6.2 for the aromatic Willamette to 0.7 for the bittering Target) [,]. Of a study involving 32 hop varieties, 20 (including Saaz) exhibited a majority of catechin, 6 had a ratio close to 1 (Cascade, Eureka!, Hallertau Blanc, Mandarina Bavaria, Polaris, and Sultana), and the remaining 6 (Bravo, Centennial, Galaxy, Lotus, Solero, Hallertauer Taurus) showed a predominance of epicatechin [].
In regard to dimers and trimers (from 1040 to 4180 and 210 to 710 mg/kg, respectively), similar conclusions can be drawn: values fall within the range of previously reported ones [,], with Saaz and CTZ displaying the highest concentrations and Polaris the lowest. As with the catechin/epicatechin ratio, the Citra and Saaz B3/B2 dimer ratios (Figure 5d) are notably high, above 3.8 (also the case here for Polaris 2019), resulting from much less inserted epicatechin. Interestingly, while in all cases (except for Mosaic 2019), the total amount of dimers consistently surpasses that of total monomers (dimers at 100 to 160% of monomer levels), the catechin content alone still exceeds that of B3 dimer.
When looking closely at dimer proportions, B2 (two (-)-epicatechin subunits) was consistently found at the lowest amount. B3 (two (+)-catechin subunits) was the most representative one, followed (closely) by B1 and B4 in Citra, Saaz, Polaris, Mandarina Bavaria, Eureka! and Amarillo (from both years except Amarillo 2020), while B4 was exceeding B3 and B1 in Mosaïc, CTZ, and Sabro. These new data offer valuable insights and context to the results reported by McMurrough et al. in 1981 (relative amounts of B1, B2, B3, and B4 found at 3:0.5:10:5 in Challenger hop) []. In a more recent study by Schmidt and Biendl, 19 out of the 32 samples exhibited a B3 > B2 ≥ B1 proportion profile, while a B3 > B1 > B2 pattern was observed for the remaining samples (B3 = B2 > B1 being a one-off exception). Yet, this last comparison is biased by the lack of B4 quantitation [].
Even on two successive years (2019 and 2020 harvest years), major variations were observed within the same variety, especially in the Saaz, Eureka!, and Polaris samples (up to 40%). Surprisingly, the catechin/epicatechin ratio showed much less variation according to the harvest year for the same cultivar (<10% except for Citra, with 17%) (Figure 5c). The same applies when including 2021 data from another group []. These results suggest that whatever the (a)biotic stresses [], the relative LAR/ANR enzymatic efficiency remains stable in a variety through successive harvest years.
The sum of the here quantitated mono-, di-, and trimers of flavan-3-ols, ranging from 881 to 9079 mg/kg, are prompting comparison with other matrices rich in flavan-3-ols. Among the extensive selection of beverages and foodstuffs analyzed [], sorghum, another raw material used in the brewing industry, can be emphasized with its total mono-, di- and trimer content of up to 3290 mg/kg [,]. Flavan-3-ol profiles of red and white sorghum highly differed from each other, with red sorghum being richer with a majority of catechin and B1 dimer, while the catechin/epicatechin ratio tended towards 0.5–1 in white sorghum []. In fresh cocoa, another exceptional source of flavane-3-ols, up to 30,600 mg/kg of mono-, di- and trimers, has been mentioned [,]. Among the monomers, (-)-epicatechin is by far the most represented, whatever the variety. Dimers (B2 and B5) and trimers (C1) built from (-)-epicatechin subunits are always the main oligomers [].
4. Conclusions
As already known for other phytoalexins, levels of hop flavan-3-ol monomers, dimers, and trimers (HPLC-MS/MS quantitations) appeared strongly influenced by the variety and harvest year. On the other hand, the relative LAR/ANR enzymatic efficiency appeared stable through two successive harvest years, as depicted by stable catechin/epicatechin and B3/B2 ratios. While the Saaz aromatic variety remains classified as a total-flavanoid-rich cultivar whatever the harvest year, there appeared no inverse correlation between total flavanoids and the α-acid content when the dual-purpose varieties Citra, CTZ, Amarillo, Eureka!, Mandarina Bavaria, Mosaic, Polaris, and Sabro were considered.
Based on the results, Citra and Saaz stood out from the others with a distinctive catechin/epicatechin ratio (>3.7 compared to <1.6 for the other seven varieties). Polaris, on the other hand, exhibited the lowest monomer content (under 800 mg/kg). Since oligomer procyanidins enriched in epicatechin have already been identified in beer as significant contributors to colloidal haze, while monomers are more involved in color instability, various single-hop well-controlled pilot beer productions should now be investigated. Measurements of their haze and color through aging could confirm the potential beneficial effect of using hop varieties with either few monomers or higher catechin/epicatechin ratios.
Our unexpected data here highlighting for the first time a relatively stable catechin/epicatechin ratio through successive harvest years should also be confirmed by analyzing more samples of both hops and other flavan-3-ol-rich plants, fruits, and seeds like sorghum and cocoa.
Author Contributions
Conceptualization, C.C. and S.C.; methodology, A.D. and S.C.; software, M.S.; validation, C.C., M.S. and S.C.; formal analysis, C.C.; investigation, M.S. and A.D.; resources, S.C.; data curation, M.S. and A.D.; writing—original draft preparation, C.C.; writing—review and editing, C.C. and S.C.; visualization, C.C.; supervision, S.C.; project administration, S.C.; funding acquisition, S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
ANR: anthocyanidin reductase; ESI: Electrospray Ionization; IST: internal standard; LAR: leucoanthocyanidin reductase; NABLABs: non-alcoholic and low-alcoholic beers; RP-HPLC-MS/MS: Reversed-Phase High-Pressure Liquid Chromatography with mass spectrometer detector operating in MS/MS mode.
References
- Habschied, K.; Košir, I.J.; Krstanović, V.; Kumrić, G.; Mastanjević, K. Beer Polyphenols—Bitterness, Astringency, and Off-Flavors. Beverages 2021, 7, 38. [Google Scholar] [CrossRef]
- Luck, G.; Liao, H.; Murray, N.J.; Grimmer, H.R.; Warminski, E.E.; Williamson, M.P.; Lilley, T.H.; Haslam, E. Polyphenols, Astringency and Proline-Rich Proteins. Phytochemistry 1994, 37, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Goiris, K.; Jaskula-Goiris, B.; Syryn, E.; Van Opstaele, F.; De Rouck, G.; Aerts, G.; De Cooman, L. The Flavoring Potential of Hop Polyphenols in Beer. J. Am. Soc. Brew. Chem. 2014, 72, 135–142. [Google Scholar] [CrossRef]
- Almaguer, C.; Schönberger, C.; Gastl, M.; Arendt, E.K.; Becker, T. Humulus lupulus—A Story That Begs to Be Told. A Review. J. Inst. Brew. 2014, 120, 289–314. [Google Scholar] [CrossRef]
- Oladokun, O.; James, S.; Cowley, T.; Smart, K.; Hort, J.; Cook, D. Dry-Hopping: The Effects of Temperature and Hop Variety on the Bittering Profiles and Properties of Resultant Beers. BrewingScience 2017, 70, 187–196. [Google Scholar]
- Parkin, E.; Shellhammer, T. Toward Understanding the Bitterness of Dry-Hopped Beer. J. Am. Soc. Brew. Chem. 2017, 75, 363–368. [Google Scholar] [CrossRef]
- Jongberg, S.; Andersen, M.L.; Lund, M.N. Covalent Protein-Polyphenol Bonding as Initial Steps of Haze Formation in Beer. J. Am. Soc. Brew. Chem. 2020, 78, 153–164. [Google Scholar] [CrossRef]
- Bamforth, C.W. Beer Haze. J. Am. Soc. Brew. Chem. 1999, 57, 81–90. [Google Scholar] [CrossRef]
- Vanderhaegen, B.; Neven, H.; Coghe, S.; Verstrepen, K.J.; Verachtert, H.; Derdelinckx, G. Evolution of Chemical and Sensory Properties during Aging of Top-Fermented Beer. J. Agric. Food Chem. 2003, 51, 6782–6790. [Google Scholar] [CrossRef]
- McMurrough, I.; Madigan, D.; Kelly, R.J. The Role of Flavanoid Polyphenols in Beer Stability. J. Am. Soc. Brew. Chem. 1996, 54, 141–148. [Google Scholar] [CrossRef]
- Moll, M.; Fonknechten, G.; Carnielo, M.; Flayeux, R. Changes in Polyphenols from Raw Materials to Finished Beer. MBAA Tech. Q 1984, 21, 79–87. [Google Scholar]
- Silva Ferreira, C.; Simon, M.; Collin, S. Why Catechin and Epicatechin from Early Hopping Impact the Color of Aged Dry-Hopped Beers While Flavan-3-ol Oligomers from Late and Dry Hopping Increase Colloidal Instability. J. Am. Soc. Brew. Chem. 2022, 81, 255–264. [Google Scholar] [CrossRef]
- Aron, P.M.; Shellhammer, T.H. A Discussion of Polyphenols in Beer Physical and Flavour Stability. J. Inst. Brew. 2010, 116, 369–380. [Google Scholar] [CrossRef]
- Asano, K.; Ohtsu, K.; Shinagawa, K.; Hashimoto, N. Affinity of Proanthocyanidins and Their Oxidation Products for Haze-Forming Proteins of Beer and the Formation of Chill Haze. Agric. Biol. Chem. 1984, 48, 1139–1146. [Google Scholar] [CrossRef]
- McGuinness, J.D.; Eastmond, R.; Laws, D.R.J.; Gardner, R.J. The Use of 14c-Labelled Polyphenols to Study Haze Formation in Beer. J. Inst. Brew. 1975, 81, 287–292. [Google Scholar] [CrossRef]
- Millet, M.; Poupard, P.; Guilois-Dubois, S.; Zanchi, D.; Guyot, S. Self-Aggregation of Oxidized Procyanidins Contributes to the Formation of Heat-Reversible Haze in Apple-Based Liqueur Wine. Food Chem. 2019, 276, 797–805. [Google Scholar] [CrossRef]
- Aron, P.M.; Shellhammer, T.H. Profiling of Hop-Derived Flavan-3-ols from Lager Beer in Relation to Hopping Technology. J. Am. Soc. Brew. Chem. 2017, 75, 276–282. [Google Scholar] [CrossRef]
- Simon, M.; Collin, S. Why Oxidation Should Be Still More Feared in NABLABs: Fate of Polyphenols and Bitter Compounds. Beverages 2022, 8, 61. [Google Scholar] [CrossRef]
- Habschied, K.; Krstanović, V.; Lukinac, J.; Jukić, M.; Lučan, M.; Mastanjević, K. Craft Brewing—Is It Really about the Sensory Revolution? Kvasny Prumysl 2019, 65, 13–16. [Google Scholar] [CrossRef]
- Lutz, A.; Kneidl, J.; Kammhuber, K.; Seigner, E. Breeding of Special Flavor Hops to Pave the Way to the Craft Brewers. In Proceedings of the Science Comission, Kiev, Ukraine, 1 June 2013; pp. 21–24. [Google Scholar]
- Hop Growers of America, Inc. 2022 Statistical report. Available online: https://www.usahops.org/img/blog_pdf/435.pdf (accessed on 9 August 2023).
- Duarte, L.M.; Amorim, T.L.; Grazul, R.M.; de Oliveira, M.A.L. Differentiation of Aromatic, Bittering and Dual-Purpose Commercial Hops from Their Terpenic Profiles: An Approach Involving Batch Extraction, GC–MS and Multivariate Analysis. Food Res. Int. 2020, 138, 109768. [Google Scholar] [CrossRef]
- Vollmer, D.M.; Shellhammer, T.H. Influence of Hop Oil Content and Composition on Hop Aroma Intensity in Dry-Hopped Beer. J. Am. Soc. Brew. Chem. 2016, 74, 242–249. [Google Scholar] [CrossRef]
- Kankolongo, M.-L.; Gros, J.; Nizet, S.; Collin, S. Quantitation of Selected Terpenoids and Mercaptans in the Dual-Purpose Hop Varieties Amarillo, Citra, Hallertau Blanc, Mosaic, and Sorachi Ace. J. Agric. Food Chem. 2015, 63, 3022–3030. [Google Scholar] [CrossRef] [PubMed]
- Bonnaffoux, H.; Roland, A.; Schneider, R.; Cavelier, F. Spotlight on Release Mechanisms of Volatile Thiols in Beverages. Food Chem. 2021, 339, 127628. [Google Scholar] [CrossRef] [PubMed]
- Roland, A.; Viel, C.; Reillon, F.; Delpech, S.; Boivin, P.; Schneider, R.; Dagan, L. First Identification and Quantification of Glutathionylated and Cysteinylated Precursors of 3-Mercaptohexan-1-ol and 4-Methyl-4-Mercaptopentan-2-one in Hops (Humulus lupulus). Flavour Fragr. J. 2016, 31, 455–463. [Google Scholar] [CrossRef]
- Takazumi, K.; Takoi, K.; Koie, K.; Tuchiya, Y. Quantitation Method for Polyfunctional Thiols in Hops (Humulus lupulus L.) and Beer Using Specific Extraction of Thiols and Gas Chromatography-Tandem Mass Spectrometry. Anal. Chem. 2017, 89, 11598–11604. [Google Scholar] [CrossRef] [PubMed]
- Elrod, S.M.; Langley, C.; Greenspan, P.; Hofmeister, E. Relationship between Phenolic and Antioxidant Concentration of Humulus lupulus and Alpha Acid Content. J. Am. Soc. Brew. Chem. 2019, 77, 134–139. [Google Scholar] [CrossRef]
- Jerkovic, V.; Collin, S. Occurrence of Resveratrol and Piceid in American and European Hop Cones. J. Agric. Food Chem. 2007, 55, 8754–8758. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.F.; Page, J.E. Xanthohumol and Related Prenylflavonoids from Hops and Beer: To Your Good Health! Phytochemistry 2004, 65, 1317–1330. [Google Scholar] [CrossRef]
- Inui, T.; Okumura, K.; Matsui, H.; Hosoya, T.; Kumazawa, S. Effect of Harvest Time on Some in Vitro Functional Properties of Hop Polyphenols. Food Chem. 2017, 225, 69–76. [Google Scholar] [CrossRef]
- Biendl, M.; Ritter, S.; Schmidt, C. Monitoring of Glycosidically Bound Polyphenols in Hops and Hop Products Using LC-MS/MS Technique. J. Am. Soc. Brew. Chem. 2023, 81, 45–53. [Google Scholar] [CrossRef]
- Li, H.-J.; Deinzer, M.L. Structural Identification and Distribution of Proanthocyanidins in 13 Different Hops. J. Agric. Food Chem. 2006, 54, 4048–4056. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.F.; Miranda, C.L.; Wolthers, K.R.; Schimerlik, M.; Deinzer, M.L.; Buhler, D.R. Identification and in Vitro Biological Activities of Hop Proanthocyanidins: Inhibition of NNOS Activity and Scavenging of Reactive Nitrogen Species. J. Agric. Food Chem. 2002, 50, 3435–3443. [Google Scholar] [CrossRef] [PubMed]
- Jerumanis, J. Quantitative Analysis of Flavanoids in Barley, Hops and Beer by High-Performance Liquid Chromatography (Hplc). J. Inst. Brew. 1985, 91, 250–252. [Google Scholar] [CrossRef]
- Li, H.G.; Deinzer, M.L. Proanthocyanidins in Hops. In Beer in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2008; pp. 333–348. [Google Scholar] [CrossRef]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The Flavonoid Biosynthesis Network in Plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Prior, R.L. Screening of Foods Containing Proanthocyanidins and Their Structural Characterization Using LC-MS/MS and Thiolytic Degradation. J. Agric. Food Chem. 2003, 51, 7513–7521. [Google Scholar] [CrossRef] [PubMed]
- Jerkovic, V.; Callemien, D.; Collin, S. Determination of Stilbenes in Hop Pellets from Different Cultivars. J. Agric. Food Chem. 2005, 53, 4202–4206. [Google Scholar] [CrossRef] [PubMed]
- Delcour, J.A.; Varebeke, D.J. de A New Colourimetric Assay for Flavanoids in Pilsner Beers. J. Inst. Brew. 1985, 91, 37–40. [Google Scholar] [CrossRef]
- EBC Analysis Committee. Analytica-EBC; Verlag Hans Carl: Nurnberg, Germany, 2008. [Google Scholar]
- De Taeye, C.; Kankolongo Cibaka, M.-L.; Jerkovic, V.; Collin, S. Degradation of (−)-Epicatechin and Procyanidin B2 in Aqueous and Lipidic Model Systems. First Evidence of “Chemical” Flavan-3-ol Oligomers in Processed Cocoa. J. Agric. Food Chem. 2014, 62, 9002–9016. [Google Scholar] [CrossRef] [PubMed]
- Lermusieau, G.; Liégeois, C.; Collin, S. Reducing Power of Hop Cultivars and Beer Ageing. Food Chem. 2001, 72, 413–418. [Google Scholar] [CrossRef]
- Goese, M.; Kammhuber, K.; Bacher, A.; Zenk, M.H.; Eisenreich, W. Biosynthesis of Bitter Acids in Hops. Eur. J. Biochem. 1999, 263, 447–454. [Google Scholar] [CrossRef]
- Karabin, M.; Hudcova, T.; Jelinek, L.; Dostalek, P. Biotransformations and Biological Activities of Hop Flavonoids. Biotechnol. Adv. 2015, 33, 1063–1090. [Google Scholar] [CrossRef] [PubMed]
- Callemien, D.; Guyot, S.; Collin, S. Use of Thiolysis Hyphenated to RP-HPLC-ESI(-)-MS/MS for the Analysis of Flavanoids in Fresh Lager Beers. Food Chem. 2008, 110, 1012–1018. [Google Scholar] [CrossRef]
- McMurrough, I. High-Performance Liquid Chromatography of Flavonoids in Barley and Hops. J. Chromatogr. A 1981, 218, 683–693. [Google Scholar] [CrossRef]
- Schmidt, C.; Biendl, M. Quantitative Analysis of a Large Spectrum of Hop Phenolic Compounds by LC-MS/MS. BrewingScience 2023, 76, 38–47. [Google Scholar]
- De Keukeleire, D.; De Cooman, L.; Rong, H.; Heyerick, A.; Kalita, J.; Milligan, S.R. Functional Properties of Hop Polyphenols. Basic Life Sci. 1999, 66, 739–760. [Google Scholar] [CrossRef]
- Aron, P.M.; Kennedy, J.A. Flavan-3-ols: Nature, Occurrence and Biological Activity. Mol. Nutr. Food Res. 2008, 52, 79–104. [Google Scholar] [CrossRef]
- Awika, J.M.; Rooney, L.W. Sorghum Phytochemicals and Their Potential Impact on Human Health. Phytochemistry 2004, 65, 1199–1221. [Google Scholar] [CrossRef] [PubMed]
- Dicko, M.H.; Gruppen, H.; Traore, A.S.; van Berkel, W.J.H.; Voragen, A.G.J. Evaluation of the Effect of Germination on Phenolic Compounds and Antioxidant Activities in Sorghum Varieties. J. Agric. Food Chem. 2005, 53, 2581–2588. [Google Scholar] [CrossRef]
- Gupta, R.K.; Haslam, E. Plant Proanthocyanidins. Part 5. Sorghum Polyphenols. J. Chem. Soc. Perkin 1 1978, 1, 892–896. [Google Scholar] [CrossRef]
- Gu, L.; Kelm, M.; Hammerstone, J.F.; Beecher, G.; Cunningham, D.; Vannozzi, S.; Prior, R.L. Fractionation of Polymeric Procyanidins from Lowbush Blueberry and Quantification of Procyanidins in Selected Foods with an Optimized Normal-Phase HPLC-MS Fluorescent Detection Method. J. Agric. Food Chem. 2002, 50, 4852–4860. [Google Scholar] [CrossRef]
- Awika, J.M.; Dykes, L.; Gu, L.; Rooney, L.W.; Prior, R.L. Processing of Sorghum (Sorghum Bicolor) and Sorghum Products Alters Procyanidin Oligomer and Polymer Distribution and Content. J. Agric. Food Chem. 2003, 51, 5516–5521. [Google Scholar] [CrossRef] [PubMed]
- Cooper, K.A.; Campos-Giménez, E.; Jiménez Alvarez, D.; Nagy, K.; Donovan, J.L.; Williamson, G. Rapid Reversed Phase Ultra-Performance Liquid Chromatography Analysis of the Major Cocoa Polyphenols and Inter-Relationships of Their Concentrations in Chocolate. J. Agric. Food Chem. 2007, 55, 2841–2847. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).